Novel Treatments for Diabetic Foot Osteomyelitis: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Review
3.1. Intraoperative Therapies
3.1.1. Bioabsorbable Therapies
3.1.2. Nonabsorbable Therapies
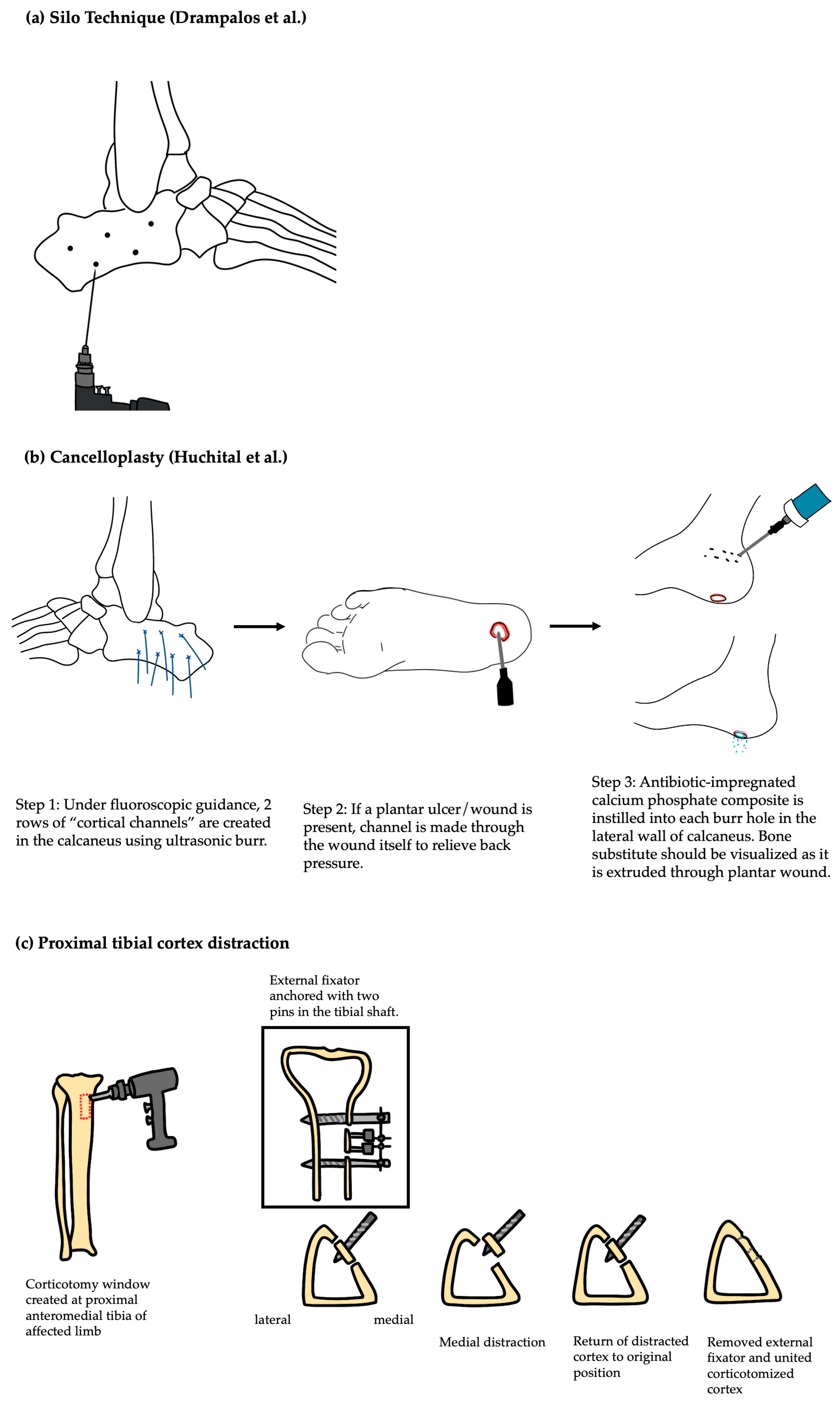
3.1.3. Surgical Technique
3.2. Nonsurgical Therapy
3.2.1. Adjunct Therapy to Antibiotics
3.2.2. Topical and Local Therapy
4. Discussion
4.1. Critical Comparison of Treatment Types
4.2. Clinical Indications and Guidance
4.3. Economic Impact
4.4. Postoperative Quality of Life
4.5. Technical Barriers
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFO | Diabetic foot osteomyelitis |
| LEA | Lower extremity amputation |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| TMA | Transmetatarsal amputation |
References
- Armstrong, D.G.; Tan, T.-W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Y.; Xiang, Y.; Chen, Y.; Shi, Y.; Ge, X.; Zeng, B.; Shen, J. Hyperthermia-Enhanced Immunoregulation Hydrogel for Oxygenation and ROS Neutralization in Diabetic Foot Ulcers. Cell Biomater. 2025, 1, 100020. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Murdoch, D.P.; Peters, E.J.G.; Lipsky, B.A. Validation of the Infectious Diseases Society of America’s Diabetic Foot Infection Classification System. Clin. Infect. Dis. 2007, 44, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Ndosi, M.; Wright-Hughes, A.; Brown, S.; Backhouse, M.; Lipsky, B.A.; Bhogal, M.; Reynolds, C.; Vowden, P.; Jude, E.B.; Nixon, J.; et al. Prognosis of the Infected Diabetic Foot Ulcer: A 12-Month Prospective Observational Study. Diabet. Med. 2018, 35, 78–88. [Google Scholar] [CrossRef]
- Schofield, C.J.; Libby, G.; Brennan, G.M.; MacAlpine, R.R.; Morris, A.D.; Leese, G.P.; DARTS/MEMO Collaboration. Mortality and Hospitalization in Patients after Amputation: A Comparison between Patients with and without Diabetes. Diabetes Care 2006, 29, 2252–2256. [Google Scholar] [CrossRef]
- Lipsky, B.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.; Kono, S.; Lavery, L.; Senneville, É.; Urbančič-Rovan, V.; van Asten, S.; Peters, E.J.G.; et al. IWGDF Guidance on the Diagnosis and Management of Foot Infections in Persons with Diabetes. Diabetes/Metab. Res. Rev. 2015, 32, 45–74. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Uçkay, İ. Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update. Medicina 2021, 57, 339. [Google Scholar] [CrossRef]
- Uçkay, I.; Pires, D.; Agostinho, A.; Guanziroli, N.; Öztürk, M.; Bartolone, P.; Tscholl, P.; Betz, M.; Pittet, D. Enterococci in Orthopaedic Infections: Who Is at Risk Getting Infected? J. Infect. 2017, 75, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Jamei, O.; Gjoni, S.; Zenelaj, B.; Kressmann, B.; Belaieff, W.; Hannouche, D.; Uçkay, I. Which Orthopaedic Patients Are Infected with Gram-Negative Non-Fermenting Rods? J. Bone Jt. Infect. 2017, 2, 73–76. [Google Scholar] [CrossRef]
- Zenelaj, B.; Bouvet, C.; Lipsky, B.A.; Uçkay, I. Do Diabetic Foot Infections with Methicillin-Resistant Staphylococcus aureus Differ from Those with Other Pathogens? Int. J. Low. Extrem. Wounds 2014, 13, 263–272. [Google Scholar] [CrossRef]
- Pitocco, D.; Spanu, T.; Di Leo, M.; Vitiello, R.; Rizzi, A.; Tartaglione, L.; Fiori, B.; Caputo, S.; Tinelli, G.; Zaccardi, F.; et al. Diabetic Foot Infections: A Comprehensive Overview. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Senneville, E.; Robineau, O. Treatment Options for Diabetic Foot Osteomyelitis. Expert Opin. Pharmacother. 2017, 18, 759–765. [Google Scholar] [CrossRef]
- Robineau, O.; Nguyen, S.; Senneville, E. Optimising the Quality and Outcomes of Treatments for Diabetic Foot Infections. Expert Rev. Anti-Infect. Ther. 2016, 14, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.J.G.; Lipsky, B.A.; Berendt, A.R.; Embil, J.M.; Lavery, L.A.; Senneville, E.; Urbančič-Rovan, V.; Bakker, K.; Jeffcoate, W.J. A Systematic Review of the Effectiveness of Interventions in the Management of Infection in the Diabetic Foot. Diabetes/Metab. Res. Rev. 2012, 28, 142–162. [Google Scholar] [CrossRef]
- Faglia, E.; Clerici, G.; Caminiti, M.; Curci, V.; Somalvico, F. Prognostic Difference Between Soft Tissue Abscess and Osteomyelitis of the Foot in Patients with Diabetes: Data from a Consecutive Series of 452 Hospitalized Patients. J. Foot Ankle Surg. 2012, 51, 34–38. [Google Scholar] [CrossRef]
- Gitelis, S.; Brebach, G.T. The Treatment of Chronic Osteomyelitis with a Biodegradable Antibiotic-Impregnated Implant. J. Orthop. Surg. 2002, 10, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.S.; Antoci, V.; Harrison, G.; Patal, P.; Freeman, T.A.; Shapiro, I.M.; Parvizi, J.; Hickok, N.J.; Radin, S.; Ducheyne, P. Controlled Release of Vancomycin from Thin Sol-Gel Films on Implant Surfaces Successfully Controls Osteomyelitis. J. Orthop. Res. 2009, 27, 701–709. [Google Scholar] [CrossRef]
- Neut, D.; van de Belt, H.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Residual Gentamicin-Release from Antibiotic-Loaded Polymethylmethacrylate Beads after 5 Years of Implantation. Biomaterials 2003, 24, 1829–1831. [Google Scholar] [CrossRef]
- Neut, D.; van de Belt, H.; Stokroos, I.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Biomaterial-Associated Infection of Gentamicin-Loaded PMMA Beads in Orthopaedic Revision Surgery. J. Antimicrob. Chemother. 2001, 47, 885–891. [Google Scholar] [CrossRef]
- Krause, F.G.; deVries, G.; Meakin, C.; Kalla, T.P.; Younger, A.S.E. Outcome of Transmetatarsal Amputations in Diabetics Using Antibiotic Beads. Foot Ankle Int. 2009, 30, 486–493. [Google Scholar] [CrossRef]
- Gauland, C. Managing Lower-Extremity Osteomyelitis Locally with Surgical Debridement and Synthetic Calcium Sulfate Antibiotic Tablets. Adv. Ski. Wound Care 2011, 24, 515–523. [Google Scholar] [CrossRef]
- Lavery, L.A.; Reyes, M.C.; Najafi, B.; Coye, T.L.; Sideman, M.; Siah, M.C.; Tarricone, A.N. The Infected Diabetic Foot: Risk Factors for Re-Infection after Treatment for Diabetic Foot Osteomyelitis. Wound Repair Regen. 2025, 33, e13246. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.-H.; Zhou, C.-H.; Song, H.-J.; Cheng, G.-Y.; Zhang, H.-A.; Fang, J.; Tao, R. Infected Bone Resection plus Adjuvant Antibiotic-Impregnated Calcium Sulfate versus Infected Bone Resection Alone in the Treatment of Diabetic Forefoot Osteomyelitis. BMC Musculoskelet. Disord. 2019, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A. Revisiting the Physical and Chemical Nature of the Mineral Component of Bone. Acta Biomater. 2025, 196, 1–16. [Google Scholar] [CrossRef]
- Drampalos, E.; Mohammad, H.R.; Kosmidis, C.; Balal, M.; Wong, J.; Pillai, A. Single Stage Treatment of Diabetic Calcaneal Osteomyelitis with an Absorbable Gentamicin-Loaded Calcium Sulphate/Hydroxyapatite Biocomposite: The Silo Technique. Foot 2018, 34, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Niazi, N.S.; Drampalos, E.; Morrissey, N.; Jahangir, N.; Wee, A.; Pillai, A. Adjuvant Antibiotic Loaded Bio Composite in the Management of Diabetic Foot Osteomyelitis—A Multicentre Study. Foot 2019, 39, 22–27. [Google Scholar] [CrossRef]
- Chia, C.L.K.; Shelat, V.G.; Low, W.; George, S.; Rao, J. The Use of Collatamp G, Local Gentamicin-Collagen Sponge, in Reducing Wound Infection. Int. Surg. 2014, 99, 565–570. [Google Scholar] [CrossRef]
- Radu, F.; Bause, M.; Knabner, P.; Lee, G.W.; Friess, W.C. Modeling of Drug Release from Collagen Matrices. J. Pharm. Sci. 2002, 91, 964–972. [Google Scholar] [CrossRef]
- Ruszczak, Z.; Friess, W. Collagen as a Carrier for On-Site Delivery of Antibacterial Drugs. Adv. Drug Deliv. Rev. 2003, 55, 1679–1698. [Google Scholar] [CrossRef]
- Varga, M.; Sixta, B.; Bem, R.; Matia, I.; Jirkovska, A.; Adamec, M. Application of Gentamicin-Collagen Sponge Shortened Wound Healing Time after Minor Amputations in Diabetic Patients–A Prospective, Randomised Trial. Arch. Med. Sci. 2014, 10, 283–287. [Google Scholar] [CrossRef]
- Hench, L.; Splinter, R.; Allen, W.; Greenlee, T. Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- De Giglio, R.; Di Vieste, G.; Mondello, T.; Balduzzi, G.; Masserini, B.; Formenti, I.; Lodigiani, S.; Pallavicini, D.; Pintaudi, B.; Mazzone, A. Efficacy and Safety of Bioactive Glass S53P4 as a Treatment for Diabetic Foot Osteomyelitis. J. Foot Ankle Surg. 2021, 60, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, Y.S.; Ferreira, N. The Role of Bioactive Glass in the Management of Chronic Osteomyelitis: A Systematic Review of Literature and Current Evidence. Infect. Dis. 2020, 52, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Iacopi, E.; Pieruzzi, L.; Goretti, C.; Piaggesi, A. Pilot Experience on the Use of S53P4 Bioactive Glass in the Surgical Management of Diabetic Foot Osteomyelitis. Int. J. Low Extrem. Wounds 2022, 21, 57–64. [Google Scholar] [CrossRef]
- Kastrin, M.; Urbančič Rovan, V.; Frangež, I. Possible Advantages of S53P4 Bioactive Glass in the Treatment of Septic Osteoarthritis of the First Metatarsophalangeal Joint in the Diabetic Foot. J. Clin. Med. 2021, 10, 1208. [Google Scholar] [CrossRef]
- McKee, M.D.; Wild, L.M.; Schemitsch, E.H.; Waddell, J.P. The Use of an Antibiotic-Impregnated, Osteoconductive, Bioabsorbable Bone Substitute in the Treatment of Infected Long Bone Defects: Early Results of a Prospective Trial. J. Orthop. Trauma 2002, 16, 622–627. [Google Scholar] [CrossRef]
- Hsieh, P.-H.; Chang, Y.-H.; Chen, S.-H.; Ueng, S.W.N.; Shih, C.-H. High Concentration and Bioactivity of Vancomycin and Aztreonam Eluted from Simplex Cement Spacers in Two-Stage Revision of Infected Hip Implants: A Study of 46 Patients at an Average Follow-up of 107 Days. J. Orthop. Res. 2006, 24, 1615–1621. [Google Scholar] [CrossRef]
- Melamed, E.A.; Peled, E. Antibiotic Impregnated Cement Spacer for Salvage of Diabetic Osteomyelitis. Foot Ankle Int. 2012, 33, 213–219. [Google Scholar] [CrossRef]
- Khury, F.; Karkabi, I.; Mazzawi, E.; Norman, D.; Melamed, E.A.; Peled, E. Revisiting Antibiotic-Impregnated Cement Spacer for Diabetic Osteomyelitis of the Foot. Antibiotics 2024, 13, 1153. [Google Scholar] [CrossRef]
- Huchital, M.J.; Saleh, A.; Patel, R.; Subik, M. Cancelloplasty for Treatment of Osteomyelitis of the Calcaneus: A Novel Technique and Case Report. Foot Ankle Spec. 2021, 14, 255–265. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.L.; García-Madrid, M.; García-Álvarez, Y.; Álvaro-Afonso, F.J.; Sanz-Corbalán, I.; García-Morales, E. Conservative Surgery for Chronic Diabetic Foot Osteomyelitis: Procedures and Recommendations. J. Clin. Orthop. Trauma 2020, 16, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Sánchez, J.; Lipsky, B.A. Modern Management of Diabetic Foot Osteomyelitis. The When, How and Why of Conservative Approaches. Expert. Rev. Anti-Infect. Ther. 2018, 16, 35–50. [Google Scholar] [CrossRef]
- Lázaro Martínez, J.L.; García Álvarez, Y.; Tardáguila-García, A.; García Morales, E. Optimal Management of Diabetic Foot Osteomyelitis: Challenges and Solutions. Diabetes Metab. Syndr. Obes. 2019, 12, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Mackie, K.M.; Sare, J.; Walsh, A.K.M.; Pherwani, A.D. A Novel Approach to the Management of the Diabetic Foot: Metatarsal Excision in the Treatment of Osteomyelitis. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 217–219. [Google Scholar] [CrossRef]
- Schöni, M.; Soldevila-Boixader, L.; Böni, T.; Muñoz Laguna, J.; Uçkay, I.; Waibel, F.W.A. Comparative Efficacy of Conservative Surgery vs Minor Amputation for Diabetic Foot Osteomyelitis. Foot Ankle Int. 2023, 44, 1142–1149. [Google Scholar] [CrossRef]
- Moosa, S.R.; Allan, A.H.; Younes, A.N.; Bakri, F.G.; Younes, N.A. Percutaneous Partial Bone Excision in the Management of Diabetic Toe Osteomyelitis. Foot Ankle Int. 2023, 44, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Ilizarov, G.A. The Tension-Stress Effect on the Genesis and Growth of Tissues. Part 1. The influence of stability of fixation and soft-tissue preservation. Clin. Orthop. Relat. Res. 1989, 238, 249–281. [Google Scholar] [CrossRef]
- Fan, Z.-Q.; Yu, Z.-H.; Zheng, J.-Z.; Yu, B.-F.; Liu, D.-W. Tibial Cortex Transverse Distraction in Treating Diabetic Foot Ulcers: What Are We Concerned About? J. Int. Med. Res. 2020, 48, 0300060520954697. [Google Scholar] [CrossRef]
- Chen, S.; Du, Z.; Yan, M.; Yue, B.; Wang, Y. Morphological Classification of the Femoral Trochlear Groove Based on a Quantitative Measurement of Computed Tomographic Models. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3163–3170. [Google Scholar] [CrossRef]
- Xu, J.; Bai, M.; Peng, C.; Yan, X.; Wei, C.; Lu, J.; Zhao, J.; Shi, N. An Novel and Alternative Treatment Method for Large Heel Ulceration in Diabetic Patients: Proximal Tibial Cortex Transverse Distraction. Int. Wound J. 2023, 20, 732–739. [Google Scholar] [CrossRef]
- Markakis, K.; Faris, A.R.; Sharaf, H.; Faris, B.; Rees, S.; Bowling, F.L. Local Antibiotic Delivery Systems: Current and Future Applications for Diabetic Foot Infections. Int. J. Low Extrem. Wounds 2018, 17, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.M.; Bessesen, M.T.; Doros, G.; Brown, S.T.; Saade, E.; Hermos, J.; Perez, F.; Skalweit, M.; Spellberg, B.; Bonomo, R.A. Adjunctive Rifampin Therapy for Diabetic Foot Osteomyelitis in the Veterans Health Administration. JAMA Netw. Open 2019, 2, e1916003. [Google Scholar] [CrossRef] [PubMed]
- Senneville, E.; Lombart, A.; Beltrand, E.; Valette, M.; Legout, L.; Cazaubiel, M.; Yazdanpanah, Y.; Fontaine, P. Outcome of Diabetic Foot Osteomyelitis Treated Nonsurgically: A Retrospective Cohort Study. Diabetes Care 2008, 31, 637–642. [Google Scholar] [CrossRef]
- Office of Research & Development. CSP #2001–Investigation of Rifampin to Reduce Pedal Amputations for Osteomyelitis in Diabetics (VA Intrepid); VA Office of Research and Development: Boston, MA, USA, 2025.
- Turzańska, K.; Adesanya, O.; Rajagopal, A.; Pryce, M.T.; Fitzgerald Hughes, D. Improving the Management and Treatment of Diabetic Foot Infection: Challenges and Research Opportunities. Int. J. Mol. Sci. 2023, 24, 3913. [Google Scholar] [CrossRef]
- Goldberg, K.; Sarig, H.; Zaknoon, F.; Epand, R.F.; Epand, R.M.; Mor, A. Sensitization of Gram-Negative Bacteria by Targeting the Membrane Potential. FASEB J. 2013, 27, 3818–3826. [Google Scholar] [CrossRef]
- Vargas, A.; Garcia, G.; Rivara, K.; Woodburn, K.; Clemens, L.E.; Simon, S.I. A Designed Host Defense Peptide for the Topical Treatment of MRSA-Infected Diabetic Wounds. Int. J. Mol. Sci. 2023, 24, 2143. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Thakur, J.; Pal, S.; Gupta, R.; Mishra, D.; Kumar, S.; Yadav, K.; Saini, A.; Yavvari, P.S.; Vedantham, M.; et al. Cholic Acid-Peptide Conjugates as Potent Antimicrobials against Interkingdom Polymicrobial Biofilms. Antimicrob. Agents Chemother. 2019, 63, e00520-19. [Google Scholar] [CrossRef]
- Chatupheeraphat, C.; Peamchai, J.; Luk-In, S.; Yainoy, S.; Eiamphungporn, W. Synergistic Effect of Two Antimicrobial Peptides, BP203 and MAP-0403 J-2 with Conventional Antibiotics against Colistin-Resistant Escherichia coli and Klebsiella pneumoniae Clinical Isolates. PLoS ONE 2023, 18, e0294287. [Google Scholar] [CrossRef]
- Luo, X.-Y.; Hu, C.-M.; Yin, Q.; Zhang, X.-M.; Liu, Z.-Z.; Zhou, C.-K.; Zhang, J.-G.; Chen, W.; Yang, Y.-J. Dual-Mechanism Peptide SR25 Has Broad Antimicrobial Activity and Potential Application for Healing Bacteria-Infected Diabetic Wounds. Adv. Sci. 2024, 11, e2401793. [Google Scholar] [CrossRef]
- Gariani, K.; Pham, T.-T.; Kressmann, B.; Jornayvaz, F.R.; Gastaldi, G.; Stafylakis, D.; Philippe, J.; Lipsky, B.A.; Uçkay, İ. Three Weeks Versus Six Weeks of Antibiotic Therapy for Diabetic Foot Osteomyelitis: A Prospective, Randomized, Noninferiority Pilot Trial. Clin. Infect. Dis. 2021, 73, e1539–e1545. [Google Scholar] [CrossRef]
- Senneville, É.; Albalawi, Z.; van Asten, S.A.; Abbas, Z.G.; Allison, G.; Aragón-Sánchez, J.; Embil, J.M.; Lavery, L.A.; Alhasan, M.; Oz, O.; et al. IWGDF/IDSA Guidelines on the Diagnosis and Treatment of Diabetes-Related Foot Infections (IWGDF/IDSA 2023). Clin. Infect. Dis. 2023, 40, ciad527. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.; Kleinman, Y. A Novel Approach to the Treatment of Diabetic Foot Abscesses–a Case Series. J. Wound Care 2014, 23, 394–399. [Google Scholar] [CrossRef]
- Gordillo, G.M.; Roy, S.; Khanna, S.; Schlanger, R.; Khandelwal, S.; Phillips, G.; Sen, C.K. Topical Oxygen Therapy Induces Vascular Endothelial Growth Factor Expression and Improves Closure of Clinically Presented Chronic Wounds. Clin. Exp. Pharmacol. Physiol. 2008, 35, 957–964. [Google Scholar] [CrossRef]
- Blackman, E.; Moore, C.; Hyatt, J.; Railton, R.; Frye, C. Topical Wound Oxygen Therapy in the Treatment of Severe Diabetic Foot Ulcers: A Prospective Controlled Study. Ostomy Wound Manag. 2010, 56, 24–31. [Google Scholar]
- Uçkay, I.; Aragón-Sánchez, J.; Lew, D.; Lipsky, B.A. Diabetic Foot Infections: What Have We Learned in the Last 30 Years? Int. J. Infect. Dis. 2015, 40, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kalliainen, L.K.; Gordillo, G.M.; Schlanger, R.; Sen, C.K. Topical Oxygen as an Adjunct to Wound Healing: A Clinical Case Series. Pathophysiology 2003, 9, 81–87. [Google Scholar] [CrossRef]
- Peters, E.J.G.; Albalawi, Z.; van Asten, S.A.; Abbas, Z.G.; Allison, G.; Aragón-Sánchez, J.; Embil, J.M.; Lavery, L.A.; Alhasan, M.; Oz, O.; et al. Interventions in the Management of Diabetes-Related Foot Infections: A Systematic Review. Diabetes/Metab. Res. Rev. 2024, 40, e3730. [Google Scholar] [CrossRef]
- Nguyen, S.; Wallard, P.; Robineau, O.; Topolinski, H.; Beltrand, E.; Benkanoun, A.; Baranski, D.; Descamps, D.; Senneville, E. Conservative Surgical Treatment for Metatarsal Osteomyelitis in Diabetic Foot: Experience of Two French Centres. Diabetes/Metab. Res. Rev. 2022, 38, e3534. [Google Scholar] [CrossRef]
- Geraghty, T.; LaPorta, G. Current Health and Economic Burden of Chronic Diabetic Osteomyelitis. Expert. Rev. Pharmacoecon. Outcomes Res. 2019, 19, 279–286. [Google Scholar] [CrossRef]
- Wright, B.; Roberts, C.; Seligson, D.; Malkani, A.; Mccabe, S. Cost of Antibiotic Beads Is Justified: A Study of Open Fracture Wounds and Chronic Osteomyelitis. J. Long-Term Eff. Med. Implant. 2007, 17, 181–185. [Google Scholar] [CrossRef]
- Serrier, H.; Huot, L.; Brosset, S.; Batailler, C.; Ferry, T. Cost-Effectiveness of a Bone Substitute Delivering Gentamicin in the Treatment of Chronic Osteomyelitis of Long Bones: Protocol for the CONVICTION Randomized Multicenter Study. Front. Med. 2023, 10, 1116711. [Google Scholar] [CrossRef] [PubMed]
- Bioactive Glass Powder, S53P4, ≥98%, 50–250 Μm Particle Size|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/US/en/product/aldrich/915580?srsltid=AfmBOopGR5zzfN0jhLgVvTDWBGx3kwOlzNPC7a_wgEq0tgvqWlBHbSFu (accessed on 4 July 2025).
- Geurts, J.; van Vugt, T.; Thijssen, E.; Arts, J.J. Cost-Effectiveness Study of One-Stage Treatment of Chronic Osteomyelitis with Bioactive Glass S53P4. Materials 2019, 12, 3209. [Google Scholar] [CrossRef]
- Chan, B.C.-F.; Campbell, K.E. An Economic Evaluation Examining the Cost-effectiveness of Continuous Diffusion of Oxygen Therapy for Individuals with Diabetic Foot Ulcers. Int. Wound J. 2020, 17, 1791–1808. [Google Scholar] [CrossRef]
- Monami, M.; Bordoni, L.; Ragghianti, B.; Silverii, G.A.; Mannucci, E. Efficacy and Safety of a Bio-Absorbable Antibiotic Delivery in Calcium Sulphate Granules for the Treatment of Osteomyelitis in Patients with Diabetic Foot: A Randomized, Double Blinded, Controlled Clinical Study the BIG D-FOOT Study. Diabetes Obes. Metab. 2025, 27, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Berli, M.; Sendi, P.; Lipsky, B.A. Principles and Practice of Antibiotic Stewardship in the Management of Diabetic Foot Infections. Curr. Opin. Infect. Dis. 2019, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Commons, R.J.; Robinson, C.H.; Gawler, D.; Davis, J.S.; Price, R.N. High Burden of Diabetic Foot Infections in the Top End of Australia: An Emerging Health Crisis (DEFINE Study). Diabetes Res. Clin. Pract. 2015, 110, 147–157. [Google Scholar] [CrossRef]
- Clarke, P.; Gray, A.; Holman, R. Estimating Utility Values for Health States of Type 2 Diabetic Patients Using the EQ-5D (UKPDS 62). Med. Decis. Mak. 2002, 22, 340–349. [Google Scholar] [CrossRef]
- Wang, J.-S.; Dunne, N. Bone cement fixation: Acrylic cements. In Joint Replacement Technology; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 212–251. [Google Scholar]
- Nguyen, A.T.; Li, R.A.; Galiano, R.D. Safety and Efficacy of S53P4 Bioactive Glass in Osteomyelitis Management: A Systematic Review and Meta-Analysis. J. Biomed. Mater. Res. B Appl. Biomater. 2025, 113, e35597. [Google Scholar] [CrossRef]
- Gatti, S.D.; Gaddi, D.; Turati, M.; Leone, G.; Arts, J.J.; Pessina, F.; Carminati, M.; Zatti, G.; De Rosa, L.; Bigoni, M. Clinical Outcomes and Complications of S53P4 Bioactive Glass in Chronic Osteomyelitis and Septic Non-Unions: A Retrospective Single-Center Study. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 489–499. [Google Scholar] [CrossRef]
| Intervention | LOE | Mechanism | Clinical Outcomes | Limitations |
|---|---|---|---|---|
| Bioabsorbable Therapy | ||||
| Calcium sulfate beads | III, IV (therapeutic) | Antibiotic-impregnated calcium sulfate beads provide high local antibiotic levels without systemic toxicity and gradually dissolve, eliminating removal while filling dead space and controlling infection in poorly vascularized bone. | Across three key studies of over 380 patients treated with antibiotic-impregnated calcium sulfate beads, clinical resolution was reported in 75% to 90% of cases with no recurrences during follow-up. | Bead implantation remains an invasive adjunct requiring surgical placement, which may not be suitable for all patients. |
| Collagen-based implants | IIa (therapeutic), preclinical | Collagenase degrades the collagen matrix, enabling sustained local gentamicin release directly to the infection site while the implant is resorbed. | Gentamicin-impregnated collagen sponges reduce wound infection risk and speed healing in DFO, with one trial reporting ~2-week healing post-amputation, without affecting hospital stay, revision, or re-amputation rates. | Collagen sponges require surgical placement and may not address dead space as effectively as other local delivery systems. |
| Bioactive glass | IIb, IV (therapeutic), preclinical | BG provides antimicrobial activity through local pH elevation and ion release, while supporting bone regeneration via osteostimulation and osteoconduction. | BG has demonstrated infection eradication rates of 90% to 100% in small DFO cohorts, with no reported recurrences and superior outcomes compared to debridement alone. | BG requires surgical implantation, may be challenging to contour for complex defects, and its resorption rate can be unpredictable, potentially affecting bone healing. |
| Nonabsorbable Therapy | ||||
| Antibiotic-impregnated cement spacer | IV (therapeutic) | ACS provides prolonged local antibiotic delivery while filling dead space and offering temporary structural support following debridement. | ACS has shown infection eradication rates of 58% to 91% in small DFO case series, with some patients requiring additional procedures or amputations. | ACS is nonabsorbable, requiring removal or revision, and poses a risk of biofilm formation if retained; shaping can be challenging in small or irregular defects. |
| Surgical Technique | ||||
| Conservative bone-sparing techniques | IIb, III, IV (therapeutic) | These techniques aim to limit infection through selective removal of infected bone while preserving limb structure and function. | Conservative techniques like metatarsal excision and partial bone removal show mixed outcomes, with good mobility or healing in most cases but notable risks of infection, readmission, and higher revision rates compared to minor amputation. | Outcomes across and within techniques are inconsistent and are further associated with risks of persistent infection, wound complications, and need for revision. |
| Adjunctive regenerative techniques | III, IV (therapeutic) | Cancelloplasty fills dead space with antibiotic-loaded bone substitute to eradicate infection; tibial distraction promotes angiogenesis and tissue regeneration through controlled mechanical strain. | Adjunctive techniques like cancelloplasty and tibial distraction show promise in managing diabetic foot osteomyelitis, with early reports noting full healing without reoperation in isolated cases and improved ulcer healing and wound closure rates without amputation in small series. | Such techniques require specialized expertise and equipment, with risks of pin-site infection (distraction) and limited generalizability. |
| Conservative bone-sparing techniques | IIb, III, IV (therapeutic) | These techniques aim to limit infection through selective removal of infected bone while preserving limb structure and function. | Conservative techniques like metatarsal excision and partial bone removal show mixed outcomes, with good mobility or healing in most cases but notable risks of infection, readmission, and higher revision rates compared to minor amputation. | Outcomes across and within techniques are inconsistent and are further associated with risks of persistent infection, wound complications, and need for revision. |
| Intervention | LOE | Mechanism | Clinical Outcomes | Limitations |
|---|---|---|---|---|
| Adjunct therapy to traditional antibiotic regimens | ||||
| Rifampicin | I (ongoing), III (therapeutic) | Rifampicin provides broad-spectrum antimicrobial activity with strong bone penetration and biofilm-disrupting properties, enhancing bacterial eradication in osteomyelitis. | Retrospective studies report that adding rifampicin to standard antimicrobial regimens improves DFO eradication rates. An ongoing clinical trial is investigating outcomes of adjunctive rifampicin versus placebo. | Rifampicin can be hepatotoxic; may also introduce drug interactions with concomitant use of medications metabolized by CYP3A4. |
| Antimicrobial peptides | preclinical | AMPs target bacterial membranes, causing depolarization and disruption of the proton-motive force, which impairs efflux pump function and enhances susceptibility to antibiotics. | Data on AMPs in DFO are limited to preclinical studies; in vivo models have demonstrated the ability to resensitize antibiotic-resistant bacteria by disrupting bacterial efflux mechanisms. | Evidence is limited to preclinical studies without human data; foreseeable challenges include stability, delivery, and potential cytotoxicity. |
| Topical and local therapy | ||||
| Topical oxygen therapy | IIb, IV (therapeutic) | Topical oxygen promotes phagocytosis, increases reactive oxygen species, and stimulates angiogenesis, supporting wound healing. | Case series report complete recovery in patients with diabetic foot abscesses treated with topical oxygen and drainage, with no recurrences overlong-term follow-up. A prospective study found higher healing rates in diabetic foot ulcers treated with topical oxygen compared to silver-based dressings. | Evidence focuses on abscess and ulcer healing, with limited utility for directly treating underlying DFO. Topical oxygen therapy on an additional medium for sustained delivery. |
| Medicated wound dressing | IIb, IV (therapeutic) | Silver-impregnated dressing provides sustained antimicrobial activity within the wound bed while facilitating drainage. | Case series using PWSR with drainage in diabetic foot abscesses reported complete recovery within 2–9 months and no recurrence during follow-up. | Wound dressings exhibit limited utility in treating underlying DFO, silver presents concern as potential topical irritant. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, C.; Ralph, J.E.; Lim, J.; Cathey, J.M.; O'Neill, C.N.; Anastasio, A.T. Novel Treatments for Diabetic Foot Osteomyelitis: A Narrative Review. Microorganisms 2025, 13, 1639. https://doi.org/10.3390/microorganisms13071639
Jing C, Ralph JE, Lim J, Cathey JM, O'Neill CN, Anastasio AT. Novel Treatments for Diabetic Foot Osteomyelitis: A Narrative Review. Microorganisms. 2025; 13(7):1639. https://doi.org/10.3390/microorganisms13071639
Chicago/Turabian StyleJing, Crystal, Julia E. Ralph, Jamie Lim, Jackson M. Cathey, Conor N. O'Neill, and Albert T. Anastasio. 2025. "Novel Treatments for Diabetic Foot Osteomyelitis: A Narrative Review" Microorganisms 13, no. 7: 1639. https://doi.org/10.3390/microorganisms13071639
APA StyleJing, C., Ralph, J. E., Lim, J., Cathey, J. M., O'Neill, C. N., & Anastasio, A. T. (2025). Novel Treatments for Diabetic Foot Osteomyelitis: A Narrative Review. Microorganisms, 13(7), 1639. https://doi.org/10.3390/microorganisms13071639