Enhancing Soil Health and Corn Productivity with a Co-Fermented Microbial Inoculant (CFMI-8): A Field-Based Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition and Preparation of CFMI-8
2.2. Study Location and Conditions
2.3. Experimental Design
2.4. Sample Collection
2.5. Soil Health Analysis
2.6. Soil Chemical Composition and Leaf Tissue Analysis
2.7. Statistical Analysis
2.8. Statistical Correlation of Soil Health Metrics ad Environmental Variables
2.9. Machine Learning Classification Using Random Forests
3. Results
3.1. Soil Health Metrics
3.2. Agronomic Performance
3.3. Micronutrients
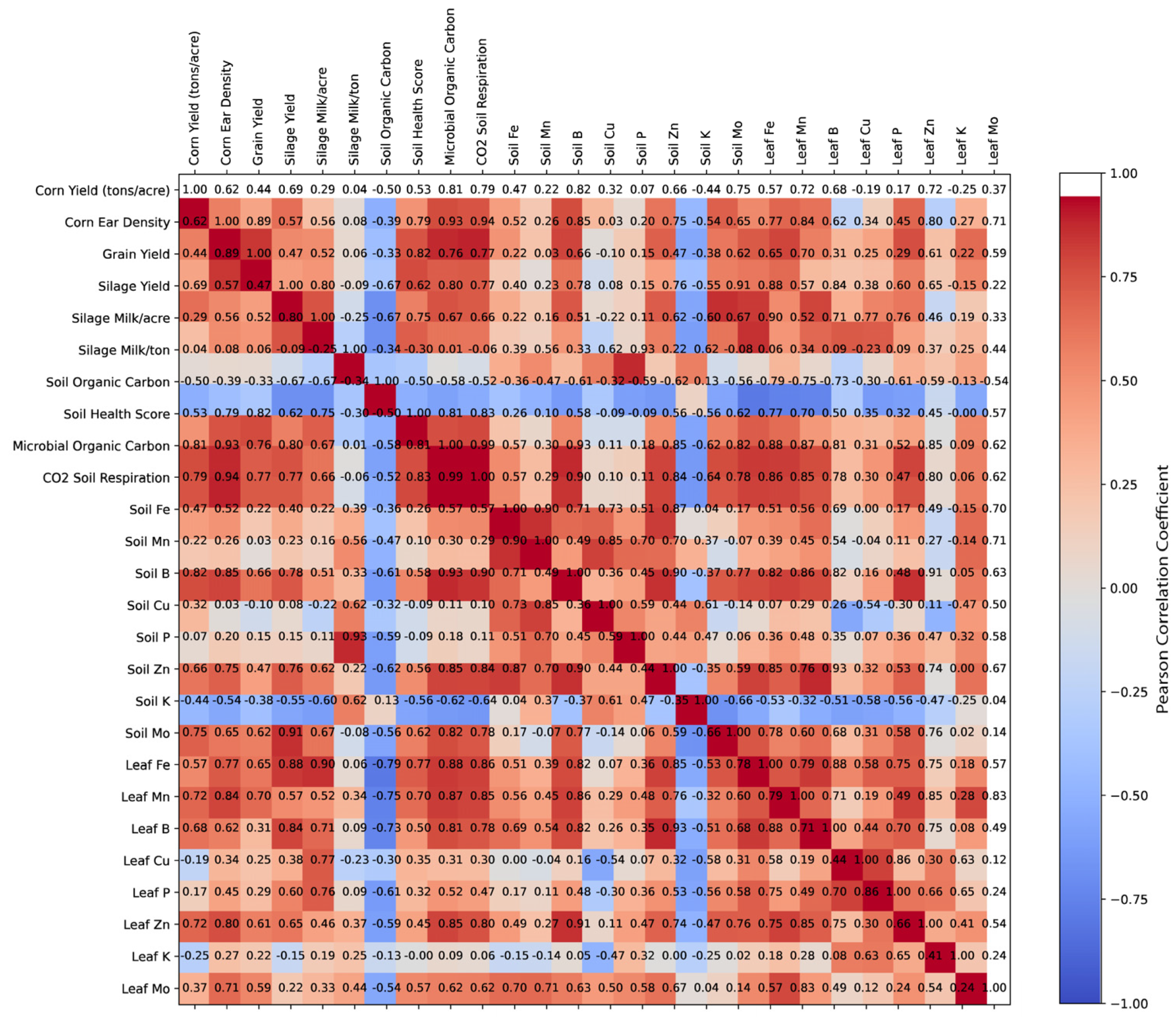
3.4. Spearman Correlation Analysis of Agronomic, Soil, and Microbial Parameters
3.5. Impact of Environmental Factors on Soil Health and Productivity
3.6. Predictive Modeling with Random Forest
3.6.1. Determinants of Corn Yield
3.6.2. Determinants of Micronutrient Uptake
4. Discussion
4.1. Soil Health Parameters
4.2. Agronomic Results
4.3. Micronutrients Analysis
4.4. Soil Health, Microbial Activity, and Crop Productivity
4.5. Soil Health and Fertility
4.6. Random Forest Analysis and Predictive Metrics
4.7. Key Biogeochemical Predictors of Corn Productivity
4.8. Biological and Agronomic Drivers of Micronutrient Uptake
4.9. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing soil health and plant growth through microbial fertilizers: Mechanisms, benefits, and sustainable agricultural practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Al-Shammary, A.A.G.; Al-Shihmani, L.S.S.; Fernández-Gálvez, J.; Caballero-Calvo, A. Optimizing sustainable agriculture: A comprehensive review of agronomic practices and their impacts on soil attributes. J. Environ. Manag. 2024, 364, 121487. [Google Scholar] [CrossRef]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; de Araujo Pereira, A.P.; Araujo, A.S.F.; Vaishnav, A.; Karpouzas, D.G.; Singh, B.K. Soil microbial diversity plays an important role in resisting and restoring degraded ecosystems. Plant Soil 2024, 500, 325–349. [Google Scholar] [CrossRef]
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, abe0725. [Google Scholar] [CrossRef]
- Islam, W.; Zeng, F.; Alotaibi, M.O.; Khan, K.A. Unlocking the potential of soil microbes for sustainable desertification management. Earth-Sci. Rev. 2024, 252, 104738. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Jia, Z.; Zhai, L.; Zhang, B.; Grüters, U.; Ma, S.; Qian, J.; Liu, X.; Zhang, J. Meta-analysis reveals the effects of microbial inoculants on the biomass and diversity of soil microbial communities. Nat. Ecol. Evol. 2024, 8, 1270–1284. [Google Scholar]
- Upadhayay, V.K.; de los Santos Villalobos, S.; Aravindharajan, S.; Kukreti, B.; Chitara, M.K.; Jaggi, V.; Sharma, A.; Singh, A.V. Microbial Advancement in Agriculture. In Microbial Inoculants: Applications for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2024; pp. 95–125. [Google Scholar]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v. 3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef]
- Arkin, A.P.; Cottingham, R.W.; Henry, C.S.; Harris, N.L.; Stevens, R.L.; Maslov, S.; Dehal, P.; Ware, D.; Perez, F.; Canon, S. KBase: The United States department of energy systems biology knowledgebase. Nat. Biotechnol. 2018, 36, 566–569. [Google Scholar] [CrossRef]
- Samantaray, A.; Chattaraj, S.; Mitra, D.; Ganguly, A.; Kumar, R.; Gaur, A.; Mohapatra, P.K.D.; de los Santos-Villalobos, S.; Rani, A.; Thatoi, H. Advances in microbial based bio-inoculum for amelioration of soil health and sustainable crop production. Curr. Res. Microb. Sci. 2024, 7, 100251. [Google Scholar] [CrossRef]
- Kapoor, D.; Sharma, P.; Sharma, M.M.M.; Yadav, S.; Husen, A. Exploring soil microbiota and their role in plant growth, stress tolerance, disease control and nutrient immobilizer. Biocatal. Agric. Biotechnol. 2024, 61, 103358. [Google Scholar] [CrossRef]
- Visca, A.; Di Gregorio, L.; Clagnan, E.; Bevivino, A. Sustainable strategies: Nature-based solutions to tackle antibiotic resistance gene proliferation and improve agricultural productivity and soil quality. Environ. Res. 2024, 248, 118395. [Google Scholar] [CrossRef]
- de Jesus Cano, R.; Daniels, J.M.; Carlin, M.; Huber, D.M. Microbial Approach to Sustainable Cotton Agriculture: The Role of PaleoPower® in Soil Health and Glyphosate Mitigation. Preprints 2025. [Google Scholar] [CrossRef]
- Khan, M.T.; Aleinikovienė, J.; Butkevičienė, L.-M. Innovative organic fertilizers and cover crops: Perspectives for sustainable agriculture in the era of climate change and organic agriculture. Agronomy 2024, 14, 2871. [Google Scholar] [CrossRef]
- Allen, B.H.; Gupta, N.; Edirisinghe, J.N.; Faria, J.P.; Henry, C.S. Application of the metabolic modeling pipeline in KBase to categorize reactions, predict essential genes, and predict pathways in an isolate genome. Microb. Syst. Biol. Methods Protoc. 2022, 2349, 291–320. [Google Scholar]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Haney, R.L.; Haney, E.B.; Smith, D.R.; Harmel, R.D.; White, M.J. The soil health tool—Theory and initial broad-scale application. Appl. Soil Ecol. 2018, 125, 162–168. [Google Scholar] [CrossRef]
- Undersander, D.; Mertens, D.; Thiex, N. Forage Analyses; National Forage Testing Association: Omaha, NE, USA, 1993; Volume 10301. [Google Scholar]
- Shenk, J.; Westerhaus, M. The application of near infrared reflectance spectroscopy (NIRS) to forage analysis. In Forage Quality, Evaluation, and Utilization; American Society of Agronomy: Madison, WI, USA, 1994; pp. 406–449. [Google Scholar]
- Jones Jr, J.B.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. Soil Test. Plant. Anal. 1990, 3, 389–427. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Flynn, M.R. Analysis of censored exposure data by constrained maximization of the Shapiro–Wilk W statistic. Ann. Occup. Hyg. 2010, 54, 263–271. [Google Scholar]
- Pearson, E.S. The test of significance for the correlation coefficient. J. Am. Stat. Assoc. 1931, 26, 128–134. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Han, H.; Guo, X.; Yu, H. Variable selection using mean decrease accuracy and mean decrease gini based on random forest. In Proceedings of the 2016 7th Ieee International Conference on Software Engineering and Service Science (ICSESS), Beijing, China, 26–28 August 2016; pp. 219–224. [Google Scholar]
- Tatachar, A.V. Comparative assessment of regression models based on model evaluation metrics. Int. Res. J. Eng. Technol. (IRJET) 2021, 8, 853–860. [Google Scholar]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant graphics for data analysis. Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Wickens, T.D.; Keppel, G. Design and Analysis: A Researcher’s Handbook; Pearson Prentice-Hall: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- José, U. Python Programming for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Weng, Z.; Lehmann, J.; Van Zwieten, L.; Joseph, S.; Archanjo, B.S.; Cowie, B.; Thomsen, L.; Tobin, M.J.; Vongsvivut, J.; Klein, A. Probing the nature of soil organic matter. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4072–4093. [Google Scholar] [CrossRef]
- Carvalho, M.L.; Maciel, V.F.; Bordonal, R.d.O.; Carvalho, J.L.N.; Ferreira, T.O.; Cerri, C.E.P.; Cherubin, M.R. Stabilization of organic matter in soils: Drivers, mechanisms, and analytical tools—A literature review. Rev. Bras. Ciência Solo 2023, 47, e0230130. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s mineral nutrition of higher plants. Plants 2012, 89, 315–330. [Google Scholar]
- Drinkwater, L.E.; Snapp, S.S. Advancing the science and practice of ecological nutrient management for smallholder farmers. Front. Sustain. Food Syst. 2022, 6, 921216. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 13. [Google Scholar]
- Yadav, B.K.; Sidhu, A.S. Dynamics of potassium and their bioavailability for plant nutrition. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 187–201. [Google Scholar]
- Manning, D.A. Mineral sources of potassium for plant nutrition. A review. Agron. Sustain. Dev. 2010, 30, 281–294. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Lazcano, C.; Domínguez, J. The use of vermicompost in sustainable agriculture: Impact on plant growth and soil fertility. Soil Nutr. 2011, 10, 187. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Snapp, S. Nutrients in agroecosystems: Rethinking the management paradigm. Adv. Agron. 2007, 92, 163–186. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Ali, Q.; Ali, S.; El-Esawi, M.A.; Rizwan, M.; Azeem, M.; Hussain, A.I.; Perveen, R.; El-Sheikh, M.A.; Alyemeni, M.N.; Wijaya, L. Foliar spray of Fe-Asp confers better drought tolerance in sunflower as compared with FeSO4: Yield traits, osmotic adjustment, and antioxidative defense mechanisms. Biomolecules 2020, 10, 1217. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Kang, Y.; Shi, M.; Yang, X.; Li, H.; Yu, H.; Wang, Y.; Qin, S. Application of different foliar iron fertilizers for improving the photosynthesis and tuber quality of potato (Solanum tuberosum L.) and enhancing iron biofortification. Chem. Biol. Technol. Agric. 2022, 9, 79. [Google Scholar] [CrossRef]
- Huber, D.; Wilhelm, N. The role of manganese in resistance to plant diseases. In Manganese in Soils and Plants: Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ Held at the Waite Agricultural Research Institute, the University of Adelaide, Glen Osmond, South Australia, August 22–26, 1988 as an Australian Bicentennial Event; Springer: Dordrecht, The Netherlands, 1988; pp. 155–173. [Google Scholar]
- Schmidt, S.B.; Husted, S. The biochemical properties of manganese in plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Goldberg, S.; Gupta, U.C. 8 Boron. In Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015; p. 305. [Google Scholar]
- Rasheed, M.K. Role of boron in plant growth: A review. J. Agric. Res. 2009, 47, 329–338. [Google Scholar]
- Haque, M.A. Boron Impact on Maize Growth and Yield: A Review. Int. J. Plant Soil. Sci. 2024, 36, 353–363. [Google Scholar] [CrossRef]
- Rudani, K.; Vishal, P.; Kalavati, P. The importance of zinc in plant growth—A review. Int. Res. J. Nat. Appl. Sci. 2018, 5, 38–48. [Google Scholar]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef]
- Zimmer, W.; Mendel, R. Molybdenum metabolism in plants. Plant Biol. 1999, 1, 160–168. [Google Scholar] [CrossRef]
- Tokasheva, D.; Nurbekova, Z.A.; Akbassova, A.Z.; Omarov, R. Molybdoenzyme participation in plant biochemical processes. Eurasian J. Appl. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Ngaire Brady, J.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Tremblay, A.; Fatani, A.; Ford, A.L.; Piano, A.; Nagulesapillai, V.; Auger, J.; MacPherson, C.W.; Christman, M.C.; Tompkins, T.A.; Dahl, W.J. Safety and Effect of a Low- and High-Dose Multi-Strain Probiotic Supplement on Microbiota in a General Adult Population: A Randomized, Double-Blind, Placebo-Controlled Study. J. Diet. Suppl. 2021, 18, 227–247. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Lizcano-Toledo, R.; Reyes-Martín, M.P.; Celi, L.; Fernández-Ondoño, E. Phosphorus dynamics in the soil–plant–environment relationship in cropping systems: A review. Appl. Sci. 2021, 11, 11133. [Google Scholar] [CrossRef]
- Amadou, I.; Houben, D.; Faucon, M.-P. Unravelling the role of rhizosphere microbiome and root traits in organic phosphorus mobilization for sustainable phosphorus fertilization. A review. Agronomy 2021, 11, 2267. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Fageria, V. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Alloway, B.J. Micronutrients and crop production: An introduction. In Micronutrient Deficiencies in Global Crop Production; Springer: Dordrecht, The Netherlands, 2008; pp. 1–39. [Google Scholar]
- Rahman, R.; Sofi, J.A.; Javeed, I.; Malik, T.H.; Nisar, S. Role of micronutrients in crop production. Int. J. Curr. Microbiol. Appl. Sci. 2020, 8, 2265–2287. [Google Scholar]
- Yan, P.; Zhang, Q.; Shuai, X.; Pan, J.; Zhang, W.; Shi, J.; Wang, M.; Chen, X.; Cui, Z. Interaction between plant density and nitrogen management strategy in improving maize grain yield and nitrogen use efficiency on the North China Plain. J. Agric. Sci. 2016, 154, 978–988. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Fageria, N.; Baligar, V.; Li, Y. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Davidson, E.A.; Reis de Carvalho, C.J.; Vieira, I.C.; Figueiredo, R.d.O.; Moutinho, P.; Yoko Ishida, F.; Primo dos Santos, M.T.; Benito Guerrero, J.; Kalif, K.; Tuma Sabá, R. Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecol. Appl. 2004, 14, 150–163. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Cao, D.; Wu, C.; Wang, X.; Wei, L.; Guo, B.; Wang, S.; Ding, J.; Chen, H. Microbial carbon and phosphorus metabolism regulated by C: N: P stoichiometry stimulates organic carbon accumulation in agricultural soils. Soil Tillage Res. 2024, 242, 106152. [Google Scholar] [CrossRef]
- Nadeem, F.; Abbas, S.; Waseem, F.; Ali, N.; Mahmood, R.; Bibi, S.; Deng, L.; Wang, R.; Zhong, Y.; Li, X. Phosphorus (P) and Zinc (Zn) nutrition constraints: A perspective of linking soil application with plant regulations. Environ. Exp. Bot. 2024, 226, 105875. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Bozdar, B.; Tu, P. Micronutrients and their effects on horticultural crop quality, productivity and sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Yang, W.; Liu, J.; Wang, Z. Community metagenomics reveals the processes of nutrient cycling regulated by microbial functions in soils with P fertilizer input. Plant Soil 2024, 499, 139–154. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Furtak, K. Soil–Plant–Microbe interactions determine soil biological fertility by altering rhizospheric nutrient cycling and biocrust formation. Sustainability 2022, 15, 625. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, Z.; Liu, L.; Yang, W.; Wang, L.; Li, J.; Zhang, D. Soil respiration and organic carbon response to biochar and their influencing factors. Atmosphere 2022, 13, 2038. [Google Scholar] [CrossRef]
- San Román, A.X.; Srikanthan, N.; Hamid, A.A.; Muratore, T.J.; Knorr, M.A.; Frey, S.D.; Simpson, M.J. Long-term warming in a temperate forest accelerates soil organic matter decomposition despite increased plant-derived inputs. Biogeochemistry 2024, 167, 1159–1174. [Google Scholar] [CrossRef]
- Akhtar, M.; Gulab, M.; Ghazanfar, M. Assessing Soil Health and Fertility through Microbial Analysis and Nutrient Profiling Implications for Sustainable Agriculture. Innov. Res. Appl. Biol. Chem. Sci. 2023, 1, 29–42. [Google Scholar]
- Liptzin, D.; Norris, C.E.; Cappellazzi, S.B.; Mac Bean, G.; Cope, M.; Greub, K.L.; Rieke, E.L.; Tracy, P.W.; Aberle, E.; Ashworth, A. An evaluation of carbon indicators of soil health in long-term agricultural experiments. Soil Biol. Biochem. 2022, 172, 108708. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.; Parra-Saldívar, R. Soil carbon sequestration–An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Deng, J.; Fu, L.; Chen, H.; Li, C.; Xu, L.; Jiao, J.; Zhang, S.; Wang, J. Cover crop by irrigation and fertilization improves soil health and maize yield: Establishing a soil health index. Appl. Soil Ecol. 2023, 182, 104727. [Google Scholar] [CrossRef]
- Sainju, U.M.; Liptzin, D.; Dangi, S.M. Enzyme activities as soil health indicators in relation to soil characteristics and crop production. Agrosystems Geosci. Environ. 2022, 5, e20297. [Google Scholar] [CrossRef]
- Liang, J.; Chen, K.; Siqintana; Huo, T.; Zhang, Y.; Jing, J.; Feng, W. Towards improved modeling of SOC decomposition: Soil water potential beyond the wilting point. Glob. Change Biol. 2022, 28, 3665–3673. [Google Scholar] [CrossRef]
- Mauget, S.A.; Himanshu, S.K.; Goebel, T.S.; Ale, S.; Lascano, R.J.; Gitz III, D.C. Soil and soil organic carbon effects on simulated Southern High Plains dryland Cotton production. Soil Tillage Res. 2021, 212, 105040. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Drinkwater, L.; Letourneau, D.; Workneh, F.; Van Bruggen, A.; Shennan, C. Fundamental differences between conventional and organic tomato agroecosystems in California. Ecol. Appl. 1995, 5, 1098–1112. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–281. [Google Scholar]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef]
- Beattie, G.A.; Edlund, A.; Esiobu, N.; Gilbert, J.; Nicolaisen, M.H.; Jansson, J.K.; Jensen, P.; Keiluweit, M.; Lennon, J.T.; Martiny, J. Soil microbiome interventions for carbon sequestration and climate mitigation. mSystems 2024, 10, e01129-24. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
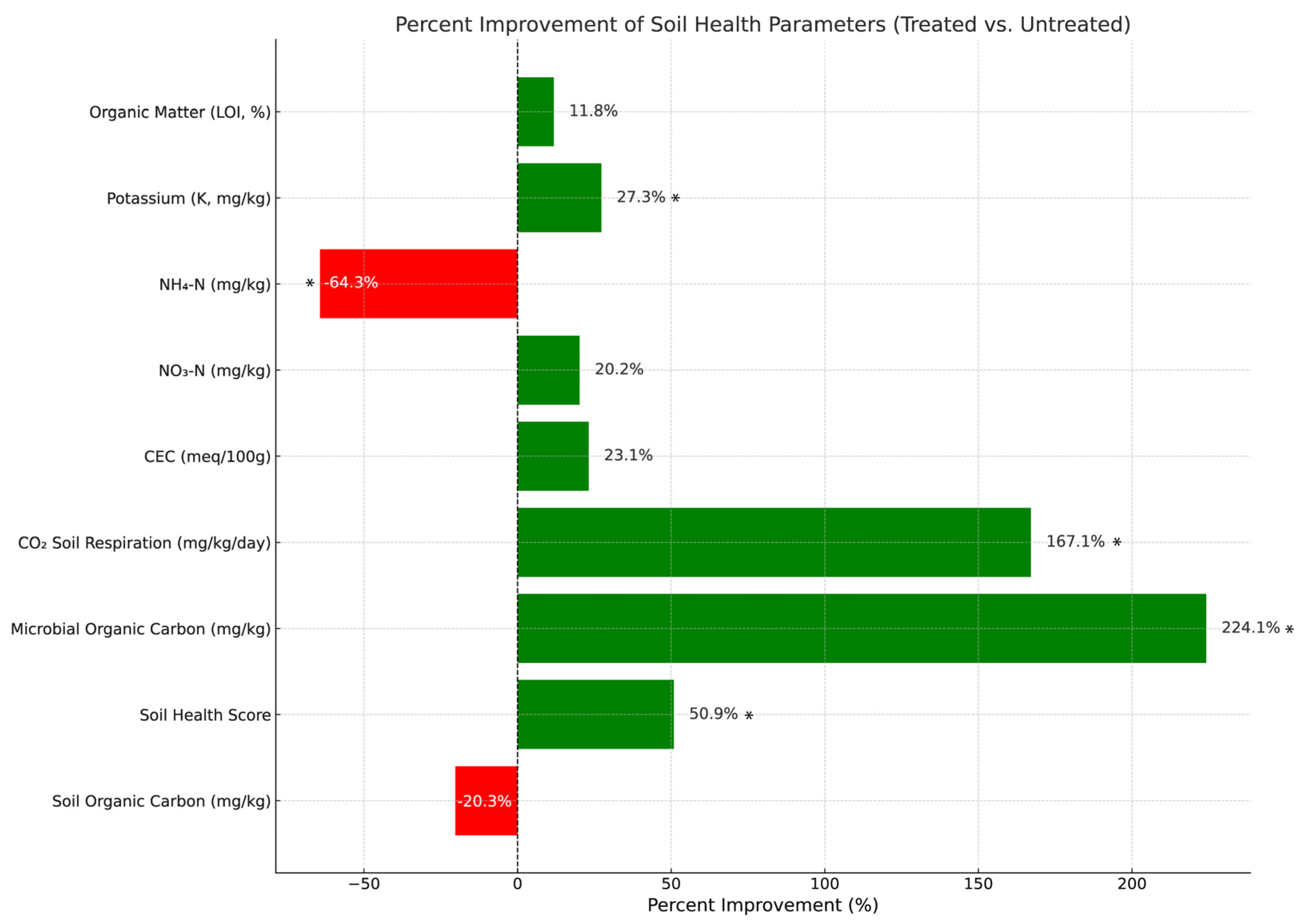



| Parameter | Treated (Mean ± SD) | Untreated (Mean ± SD) |
|---|---|---|
| Soil Organic Carbon (mg/kg) | 118.00 ± 5.62 | 148.00 ± 11.37 |
| Soil Health Score | 13.26 ± 2.33 | 8.79 ± 0.80 |
| Microbial Organic Carbon (mg/kg) | 80.48 ± 23.54 | 24.83 ± 5.18 |
| CO2 Soil Respiration (mg/kg/day) | 95.83 ± 30.34 | 35.88 ± 5.92 |
| Cation Exchange Capacity (meq/100 g) | 8.78 ± 0.22 | 7.13 ± 0.55 |
| NO3-N (mg/kg) | 38.15 ± 3.58 | 31.75 ± 4.43 |
| NH4-N (mg/kg) | 0.6 ± 0.08 | 1.68 ± 0.41 |
| Potassium (K, mg/kg) | 368.75 ± 11.14 | 289.75 ± 34.11 |
| Organic Matter (LOI, %) | 1.90 ± 0.07 | 1.70 ± 0.07 |
| Parameter | Treated (Mean ± SD) | Untreated (Mean ± SD) | % Change |
|---|---|---|---|
| Corn Yield (tons/acre) | 7.20 ± 1.79 | 5.60 ± 0.68 | +28.6% |
| Corn Ear Density | 38.50 ± 1.87 | 32.75 ± 2.05 | +17.6% |
| Grain Yield | 246.40 ± 6.68 | 235.57 ± 7.03 | +4.6% |
| Silage Yield (tons/acre) | 36.26 ± 1.18 | 33.08 ± 1.51 | +9.6% |
| Silage Milk/acre (lbs.) | 121,703.00 ± 6320.59 | 111,333.75 ± 3652.58 | +9.3% |
| Silage Milk/ton (lbs.) | 3426.00 ± 183.84 | 3332.25 ± 67.36 | +2.8% |
| Metric | Untreated | Treated | % Change | p-Value | ||
|---|---|---|---|---|---|---|
| Mean | Std Dev | Mean | Std Dev | |||
| Soil Fe | 31.500 | 7.188 | 65.750 | 28.745 | 108.730 | 0.094 |
| Soil Mn | 1.250 | 0.500 | 2.000 | 0.816 | 60.000 | 0.178 |
| Soil B | 0.300 | 0.000 | 0.450 | 0.058 | 50.000 | 0.014 |
| Soil Cu | 0.575 | 0.150 | 0.650 | 0.238 | 13.043 | 0.617 |
| Soil P | 56.750 | 4.500 | 67.500 | 12.583 | 18.943 | 0.188 |
| Soil Zn | 1.350 | 0.058 | 1.750 | 0.129 | 29.630 | 0.004 |
| Soil K | 130.000 | 25.245 | 106.250 | 36.087 | −18.269 | 0.327 |
| Soil Mo | 0.023 | 0.005 | 0.035 | 0.010 | 55.556 | 0.083 |
| Leaf Fe | 110.500 | 2.380 | 121.250 | 2.754 | 9.729 | 0.001 |
| Leaf Mn | 31.100 | 1.738 | 34.750 | 0.957 | 11.736 | 0.016 |
| Leaf B | 10.150 | 0.819 | 12.150 | 0.473 | 19.704 | 0.009 |
| Leaf Cu | 10.000 | 0.816 | 11.750 | 2.217 | 17.500 | 0.216 |
| Leaf P | 0.372 | 0.015 | 0.402 | 0.017 | 8.054 | 0.039 |
| Leaf Zn | 2.128 | 0.025 | 2.203 | 0.025 | 3.525 | 0.005 |
| Leaf K | 32.750 | 1.708 | 33.750 | 1.708 | 3.053 | 0.439 |
| Leaf Mo | 0.310 | 0.036 | 0.355 | 0.013 | 14.516 | 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, R.D.J.; Daniels, J.M.; Carlin, M.; Huber, D. Enhancing Soil Health and Corn Productivity with a Co-Fermented Microbial Inoculant (CFMI-8): A Field-Based Evaluation. Microorganisms 2025, 13, 1638. https://doi.org/10.3390/microorganisms13071638
Cano RDJ, Daniels JM, Carlin M, Huber D. Enhancing Soil Health and Corn Productivity with a Co-Fermented Microbial Inoculant (CFMI-8): A Field-Based Evaluation. Microorganisms. 2025; 13(7):1638. https://doi.org/10.3390/microorganisms13071638
Chicago/Turabian StyleCano, Raul De Jesus, Judith M. Daniels, Martha Carlin, and Don Huber. 2025. "Enhancing Soil Health and Corn Productivity with a Co-Fermented Microbial Inoculant (CFMI-8): A Field-Based Evaluation" Microorganisms 13, no. 7: 1638. https://doi.org/10.3390/microorganisms13071638
APA StyleCano, R. D. J., Daniels, J. M., Carlin, M., & Huber, D. (2025). Enhancing Soil Health and Corn Productivity with a Co-Fermented Microbial Inoculant (CFMI-8): A Field-Based Evaluation. Microorganisms, 13(7), 1638. https://doi.org/10.3390/microorganisms13071638






