Comparative Analysis of Soil Microbial Community Structures in Rhizosphere of Two Texture-Differentiated Lotus Root Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site Description and Experimental Designs
2.2. Soil Samples Collection
2.3. Test Methods
2.4. Statistical Analyses
3. Results
3.1. Biological Characteristics of Different Varieties of Lotus Roots
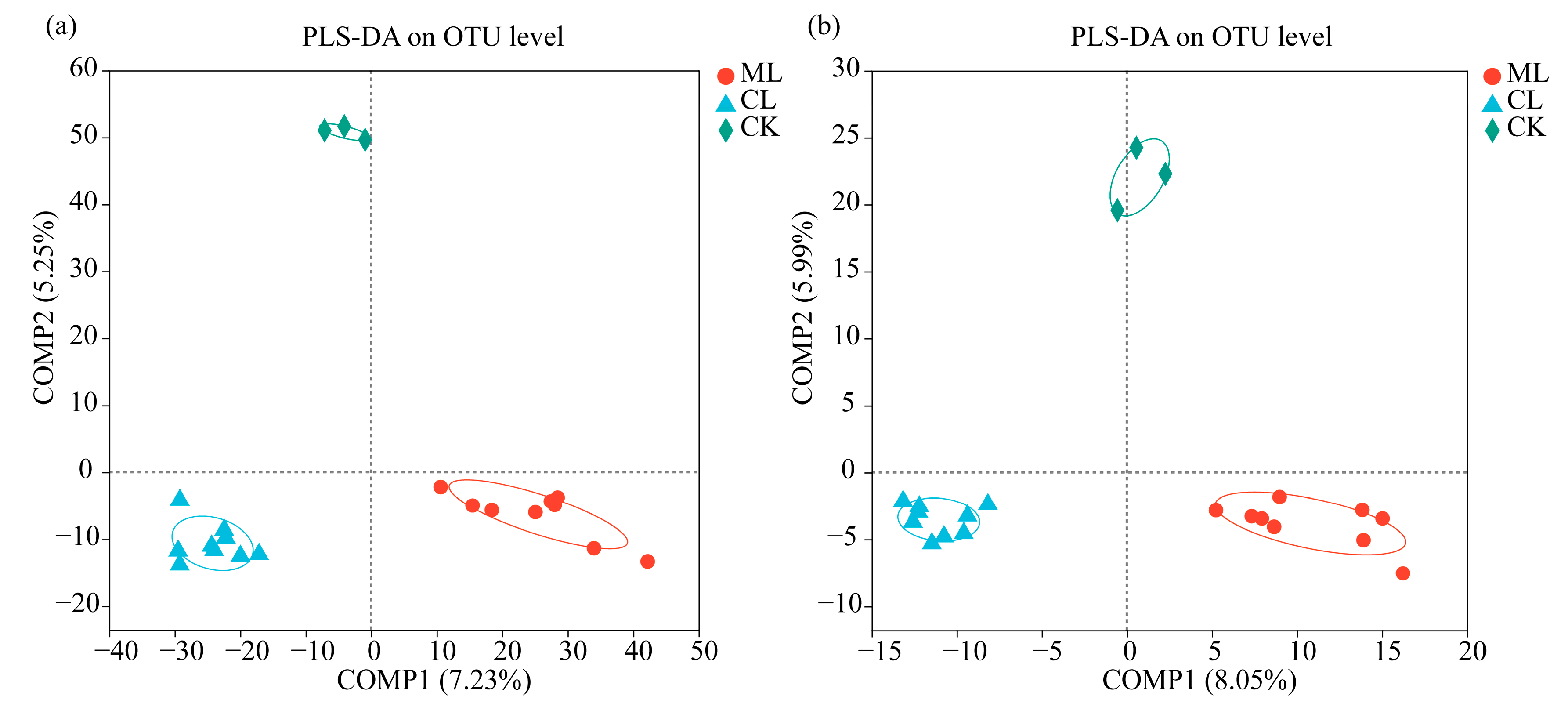
3.2. Soil Microbial Diversity in Rhizospheres in Mealy and Crunchy Lotus Root Varieties
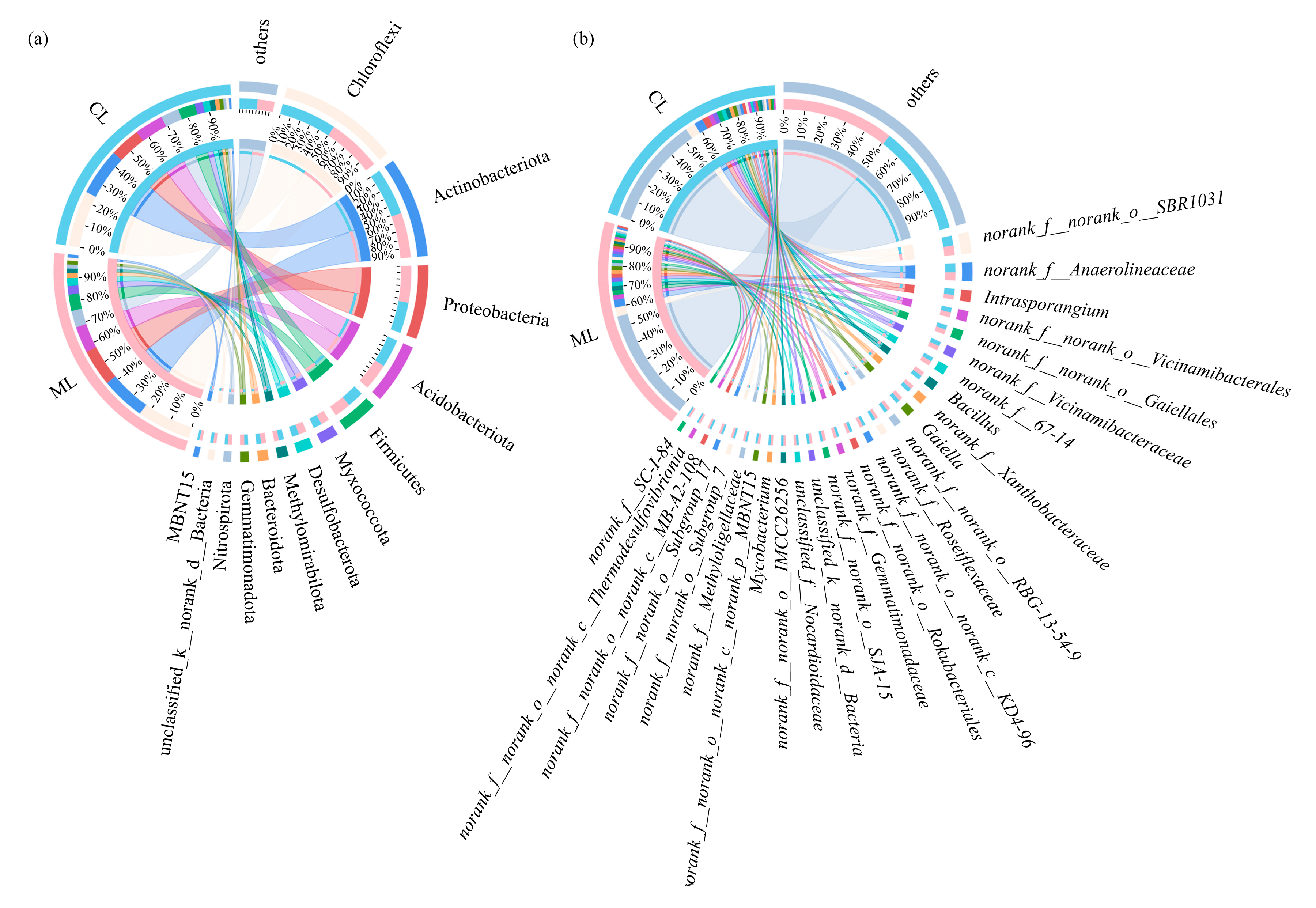
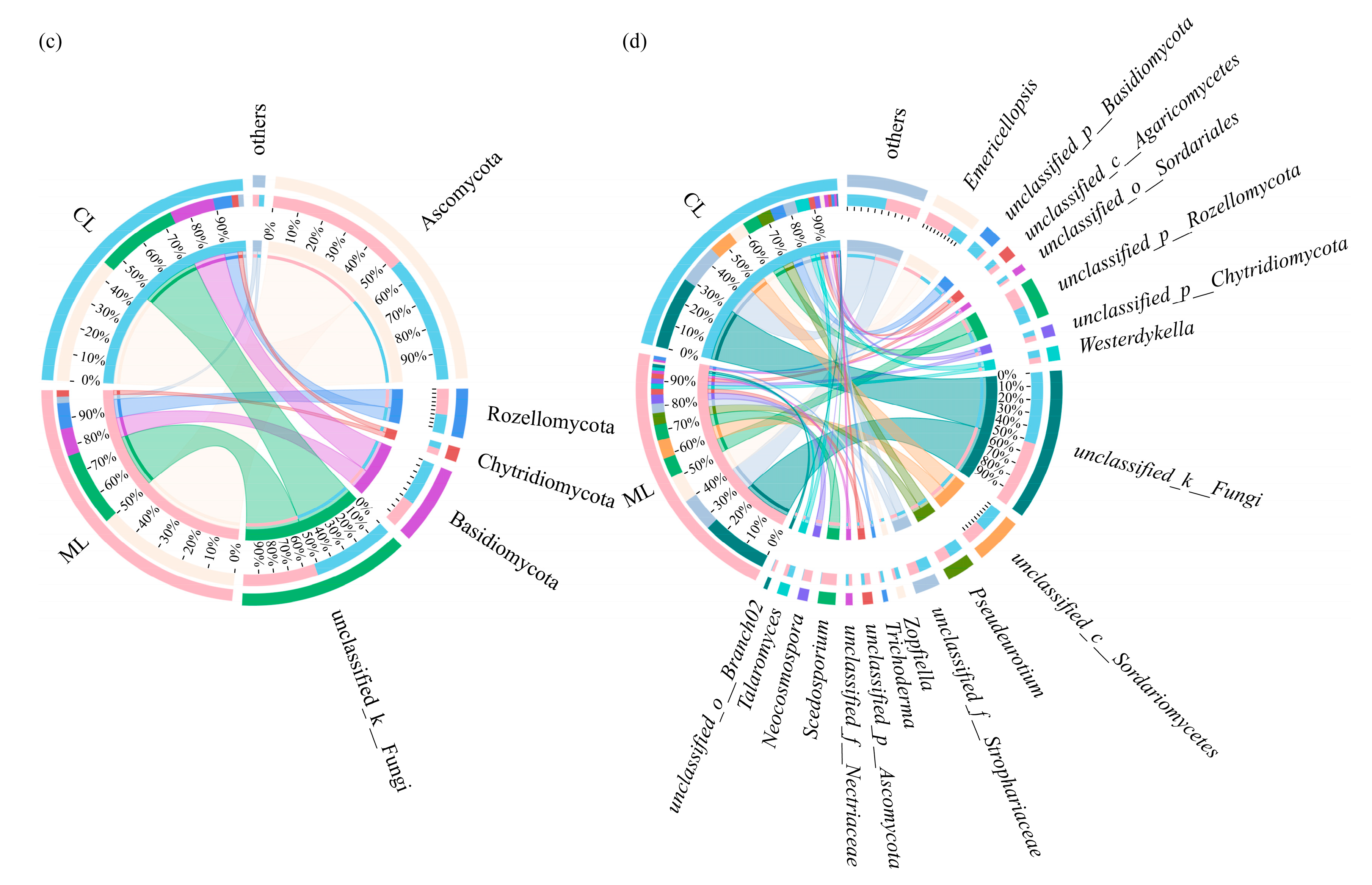
3.3. Soil Microbial Compositions of Rhizospheres in Mealy and Crunchy Lotus Root Varieties
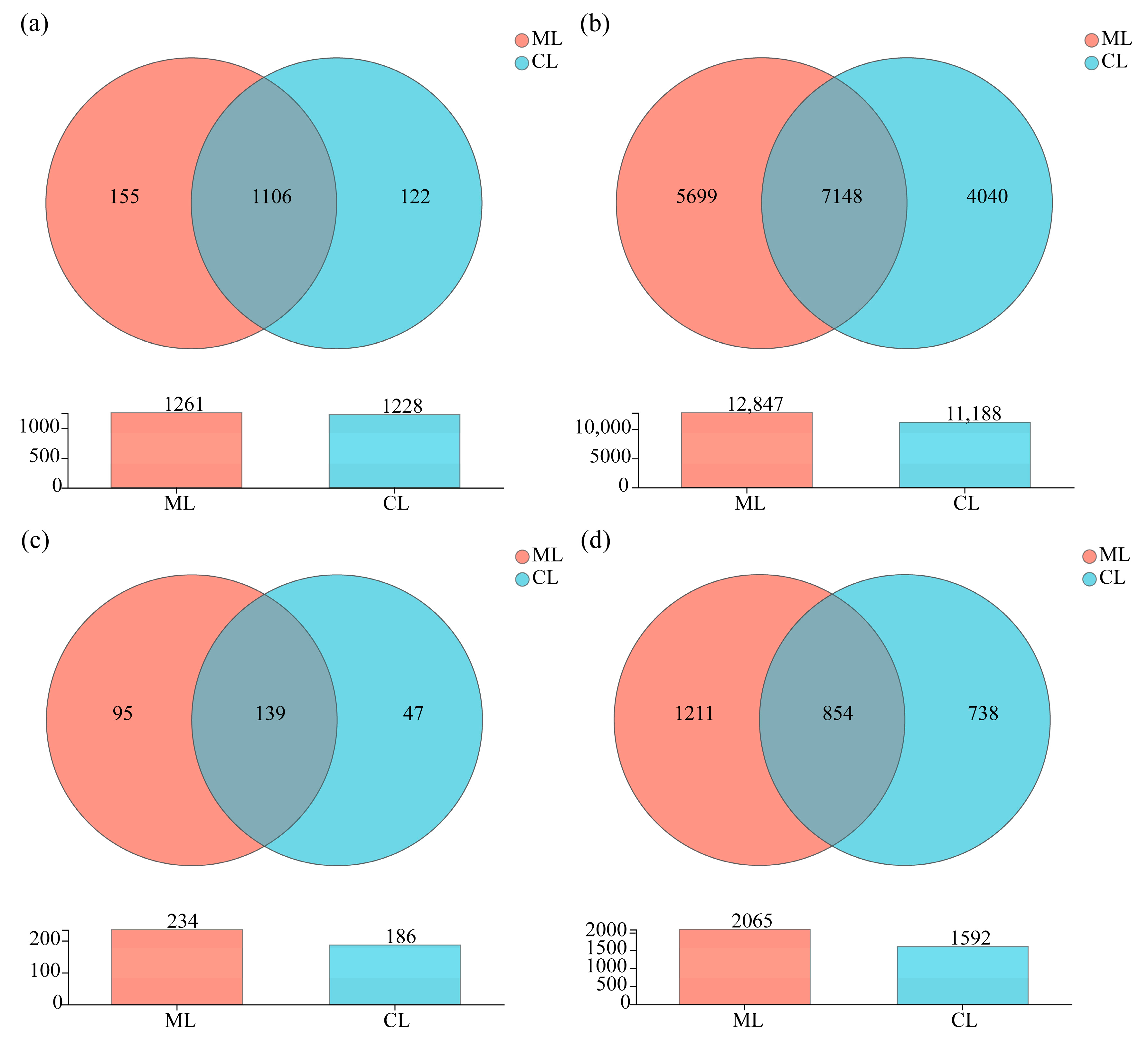
3.4. Venn Diagram Analysis of Soil Microorganisms in Rhizosphere of Mealy and Crunchy Lotus Root Varieties
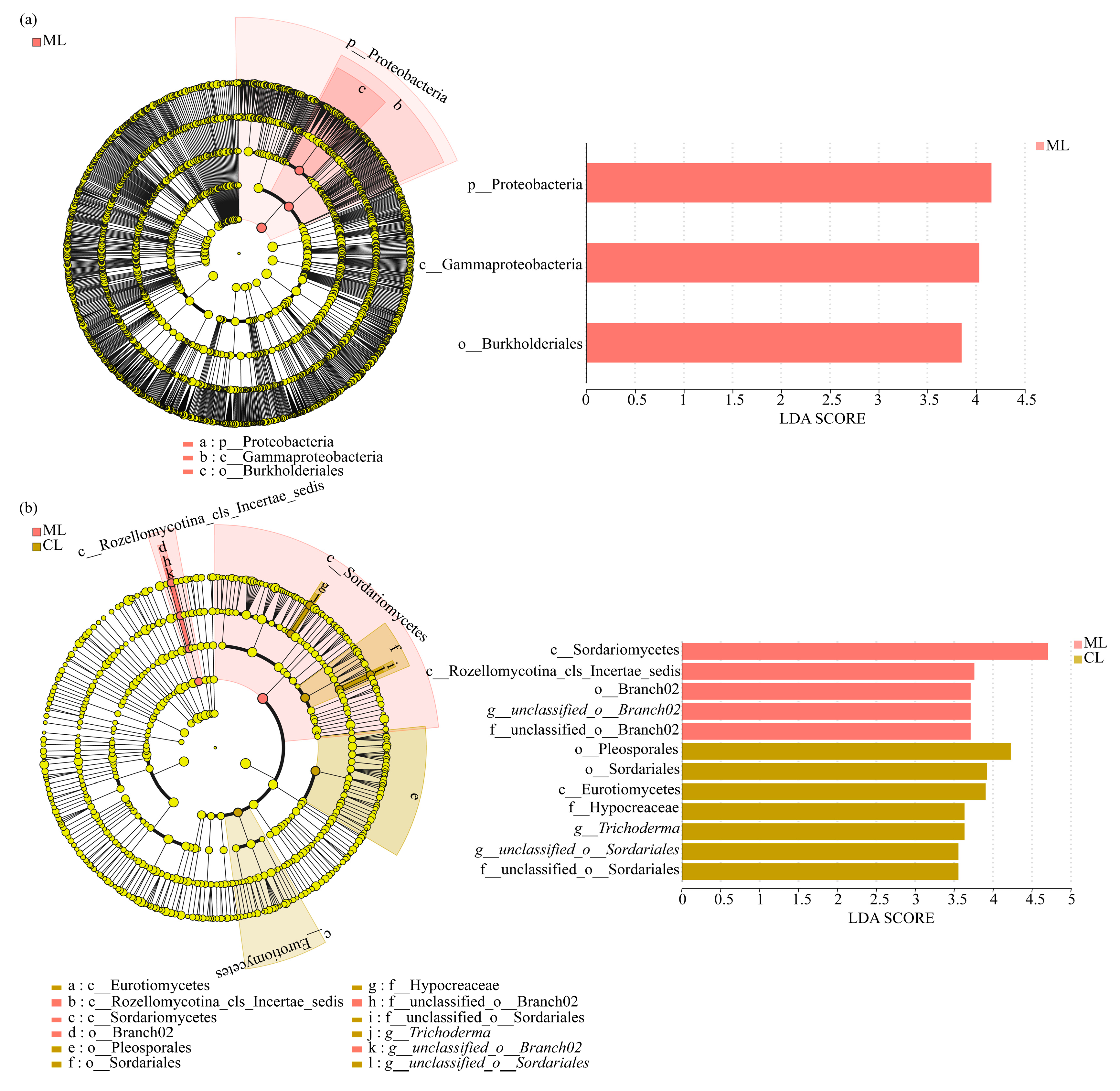
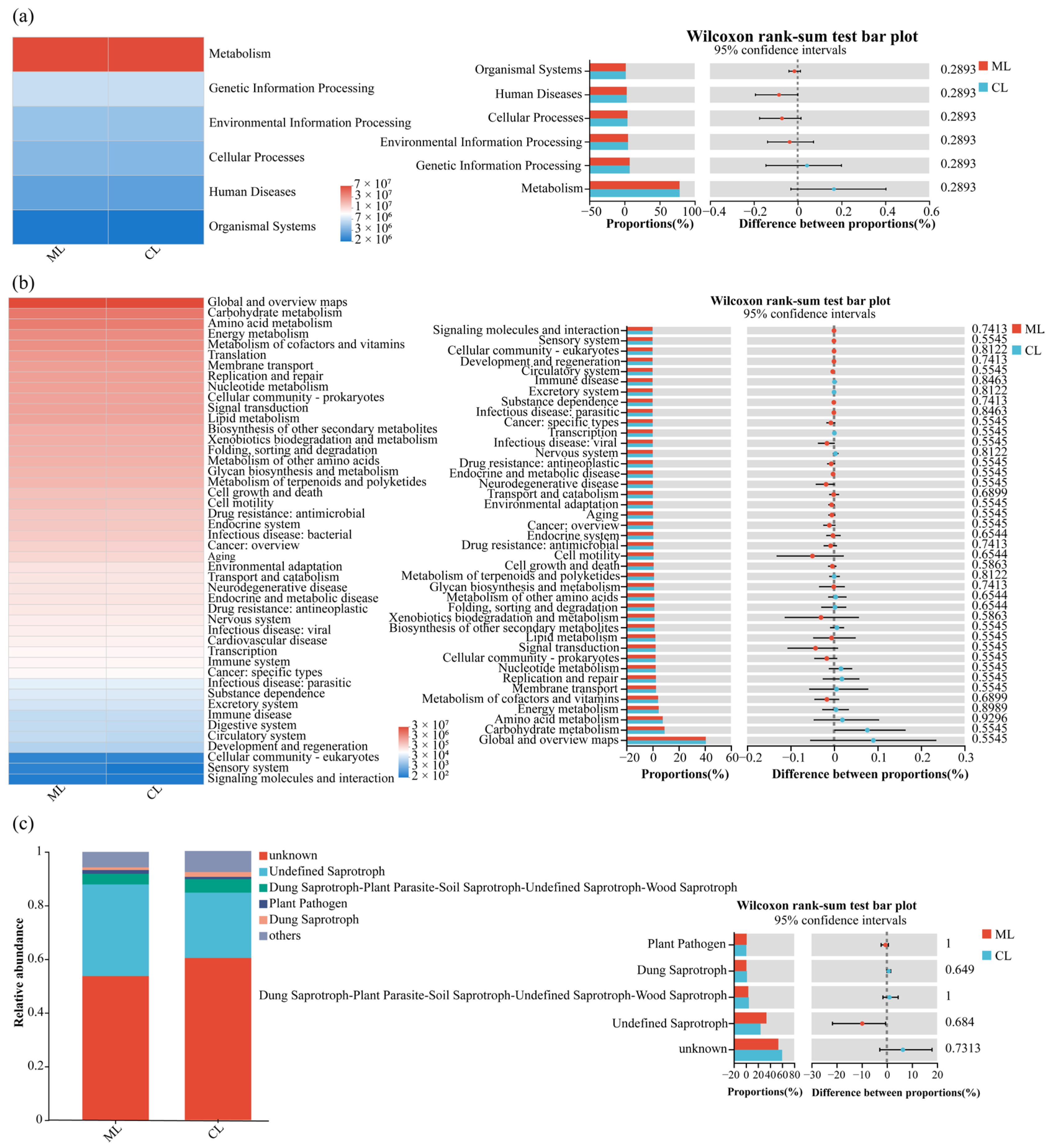
3.5. Functional Predictions of Soil Microbial Communities in Rhizospheres of Mealy and Crunchy Lotus Root Varieties
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, J.; Dong, W.; Cheng, T.; Zhou, S. Nelumbonaceae: Systematic position and species diversification revealed by the complete chloroplast genome. J. Syst. Evol. 2012, 50, 477–487. [Google Scholar] [CrossRef]
- Thanushree, M.P.; Sudha, M.L.; Crassina, K. Lotus (Nelumbo nucifera) rhizome powder as a novel ingredient in bread sticks: Rheological characteristics and nutrient composition. J. Food Meas. Charact. 2017, 11, 1795–1803. [Google Scholar] [CrossRef]
- Xu, S.Y.; Shoemaker, C.F. Gelatinization properties of Chinese water chestnut starch and lotus root starch. J. Food Sci. 1986, 51, 445–449. [Google Scholar] [CrossRef]
- Xu, Y. Perspectives on the 21st century development of functional foods: Bridging Chinese medicated diet and functional foods. Int. J. Food Sci. Technol. 2001, 36, 229–242. [Google Scholar] [CrossRef]
- Guo, H.B. Cultivation of lotus (Nelumbo nucifera Gaertn. ssp nucifera) and its utilization in China. Genet. Resour. Crop Evol. 2009, 56, 323–330. [Google Scholar] [CrossRef]
- Yang, D. Antioxidant Activities of Lotus Rhizome. Ph.D. Thesis, Dissertation of Zhejiang University, Zhejiang, China, 2007. [Google Scholar]
- Mukerjee, P.K.; Das, K.; Balasubramanian, R.; Saha, K.; Pal, M.; Saha, B.P. Anti-Diarrhoel evaluation of Nelumbo nucifera rhizome extract. Indian. J. Pharmacol. 1995, 27, 262–264. [Google Scholar]
- Mukherjee, P.K.; Balasubramanian, R.; Saha, K.; Saha, B.P.; Pal, M. Antibacterial efficiency of Nelumbo nucifera. Indian Drugs 1995, 32, 274–276. [Google Scholar]
- Eon-Hwan, S.; Jae-Geun, K. Effects of Nelumbo nucifera on the regional cerebral blood flow and blood pressure in rats. J. East Asian Soc. Diet. Life 2005, 15, 49–56. [Google Scholar]
- Lee, J.; Park, S.; Lee, M. Effect of lotus root (Nelumbo nucifera G) on lipid metabolism in rats with diet-induced hypercholesterolemia. Food Sci. Preserv. 2006, 13, 634–642. [Google Scholar]
- Mukherjee, P.K.; Pal, S.K.; Saha, K.; Saha, B.P. Hypoglycaemic activity of Nelumbo nucifera Gaertn. (Fam. Nymphaeaceae) rhizome (methanolic extract) in streptozotocin-induced diabetic rats. Phytother. Res. 1995, 9, 522–524. [Google Scholar] [CrossRef]
- Du, H.; Zhao, X.; You, J.; Park, J.; Kim, S.; Chang, K. Antioxidant and hepatic protective effects of lotus root hot water extract with taurine supplementation in rats fed a high fat diet. J. Biomed. Sci. 2010, 17, S39. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Pal, M.; Saha, K.; Saha, B.P.; Das, J. Diuretic activity of extract of the rhizomes of Nelumbo nucifera Gaertn. (Fam. Nymphaeaceae). Phytother. Res. 1996, 10, 424–425. [Google Scholar] [CrossRef]
- Chiang, P.; Luo, Y. Effects of pressurized cooking on the relationship between the chemical compositions and texture changes of lotus root (Nelumbo nucifera Gaertn.). Food Chem. 2007, 105, 480–484. [Google Scholar] [CrossRef]
- Northcote, D.H. The cell walls of higher plants: Their composition, structure and growth. Biol. Rev. 1958, 33, 53–102. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Yi, Y.; Hou, W.; Wang, L.; Ai, Y.; Min, T. Varying concentrations of ethephon induce antioxidant defences and cell wall degradation to regulate storage quality of fresh-cut lotus root. Food Biosci. 2024, 61, 104975. [Google Scholar] [CrossRef]
- Cai, Z.; Jiang, Y.; Wang, F.; Liu, J.; Kan, J.; Zhang, M.; Qi, X.; Li, L.; Zhao, S.; Qian, C. Study on Quality and Starch Characteristics of Powdery and Crispy Lotus Roots. Foods 2024, 13, 3335. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, L.; Yin, J.; Yan, S.; Liu, K.; Zhang, F.; Xu, B.; Li, L. Structure and physicochemical properties of starches in lotus (Nelumbo nucifera Gaertn) rhizome. Food Sci. Nutr. 2013, 1, 273–283. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef]
- Rochefort, A.; Briand, M.; Marais, C.; Wagner, M.; Laperche, A.; Vallee, P.; Barret, M.; Sarniguet, A. Influence of Environment and Host Plant Genotype on the Structure and Diversity of the Brassica napus Seed Microbiota. Phytobiomes J. 2019, 3, 326–336. [Google Scholar] [CrossRef]
- Ge, Y.; Xu, S.; Xu, Y. Review on influencing factors of rhizosphere microbiome assemblage. Acta Agric. Zhejiangensis 2019, 31, 2120–2130. [Google Scholar] [CrossRef]
- Cregger, M.A.; Veach, A.M.; Yang, Z.K.; Crouch, M.J.; Vilgalys, R.; Tuskan, G.A.; Schadt, C.W. The Populus holobiont: Dissecting the effects of plant niches and genotype on the microbiome. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Igwe, A.N.; Vannette, R.L. Bacterial communities differ between plant species and soil type, and differentially influence seedling establishment on serpentine soils. Plant Soil 2019, 441, 423–437. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Ferrari, E.; Tanunchai, B.; Wahdan, S.F.M.; Sadubsarn, D.; Farneti, B.; Checcucci, A.; Buscot, F.; et al. Taxonomical and functional composition of strawberry microbiome is genotype-dependent. J. Adv. Res. 2022, 42, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Chanclud, E.; Morel, J. Plant hormones: A fungal point of view. Mol. Plant Pathol. 2016, 17, 1289–1297. [Google Scholar] [CrossRef]
- Frankenberger, W.T., Jr.; Brunner, W. Method of detection of auxin-indole-3-acetic acid in soils by high performance liquid chromatography. Soil Sci. Soc. Am. J. 1983, 47, 237–241. [Google Scholar] [CrossRef]
- Nieto, K.F.; Frankenberger, W.T., Jr. Biosynthesis of cytokinins in soil. Soil Sci. Soc. Am. J. 1989, 53, 735–740. [Google Scholar] [CrossRef]
- Smith, A.M.; Cook, R.J. Implications of ethylene production by bacteria for biological balance of soil. Nature 1974, 252, 703–705. [Google Scholar] [CrossRef]
- Tudzynski, B.; Sharon, A. Biosynthesis, Biological Role and Application of Fungal Phytohormones. In Industrial Applications; Osiewacz, H.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 183–211. ISBN 978-3-662-10378-4. [Google Scholar]
- Chaves, F.C.; Gianfagna, T.J. Necrotrophic phase of Monihophthora perniciosa causes salicylic acid accumulation in infected stems of cacao. Physiol. Mol. Plant Pathol. 2006, 69, 104–108. [Google Scholar] [CrossRef]
- Arshad, M.; Frankenberger, W.T. Plant Growth-Regulating Substances in the Rhizosphere: Microbial Production and Functions. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 1997; Volume 62, pp. 45–151. ISBN 0065-2113. [Google Scholar]
- Zhou, P.; Jin, Q.; Qian, P.; Wang, Y.; Wang, X.; Jiang, H.; Yao, D.; Liu, X.; Liu, F.; Li, J.; et al. Genetic resources of lotus (Nelumbo) and their improvement. Ornam. Plant Res. 2022, 2, 5. [Google Scholar] [CrossRef]
- Zhao, S.; Deng, K.; Zhu, Y.; Jiang, T.; Wu, P.; Feng, K.; Li, L. Optimization of slow-release fertilizer application improves lotus rhizome quality by affecting the physicochemical properties of starch. J. Integr. Agric. 2023, 22, 1045–1057. [Google Scholar] [CrossRef]
- Dong, W.; Liu, X.; Yi, Y.; Wang, L.; Hou, W.; Ai, Y.; Wang, H.; Min, T. Evaluation of Pre-Harvest Nutrient Composition and Functional Active Substances in Various Lotus Roots. Foods 2024, 13, 2297. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Huang, X.; Zhong, Z.; Huang, F.; Li, S.; Wang, L.; Min, T.; Wang, H. Structural and biological properties of polysaccharides from lotus root. Int. J. Biol. Macromol. 2019, 130, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Salo-väänänen, P.P.; Koivistoinen, P.E. Determination of protein in foods: Comparison of net protein and crude protein (N× 6.25) values. Food Chem. 1996, 57, 27–31. [Google Scholar] [CrossRef]
- Zhu, F.; Sun, H.; Wang, J.; Zheng, X.; Wang, T.; Diao, Y.; Hu, Z. Differential expression involved in starch synthesis pathway genes reveal various starch characteristics of seed and rhizome in lotus (Nelumbo nucifera). J. Food Sci. 2022, 87, 4250–4263. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, F.; Yang, H.; LI, L.; Gong, B.; Wang, L. Research advances on cell wall disassembly in fruit ripening and softening. Food Sci. Technol. 2010, 35, 62–66. [Google Scholar] [CrossRef]
- Kan, J.; Liu, T.; Jin, C.; Xie, H. Degradation of Cell Wall Polysaccharides and Related Enzyme Activities during Non-melting-fleshPeach Fruit Softening. Food Sci. 2011, 32, 268–274. [Google Scholar]
- Owino, W.O.; Nakano, R.; Kubo, Y.; Inaba, A. Coordinated expression patterns of genes encoding cell wall modifying enzymes during ripening in distinct anatomical tissue regions of the fig (Ficus carica L.) fruit. Postharvest Biol. Technol. 2004, 32, 253–261. [Google Scholar] [CrossRef]
- Yu, S. Study on Extraction and Characteristics of Lotus Root. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2017. [Google Scholar]
- Haichar, F.E.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, S.; Luo, X. Role of generalists, specialists and opportunists in Nitrospira community assembly in soil aggregates from nearby cropland and grassland areas in a campus. Appl. Soil Ecol. 2024, 198, 105377. [Google Scholar] [CrossRef]
- Zhao, Z.; Luo, J.; Jin, B.; Zhang, J.; Li, B.; Ma, B.; An, X.; Zhang, S.; Shan, B. Analysis of Bacterial Communities in Partial Nitritation and Conventional Nitrification Systems for Nitrogen Removal. Sci. Rep. 2018, 8, 12930. [Google Scholar] [CrossRef]
- Xu, C.M.; Zhou, C.N.; Zheng, G.S.; Wang, D.Y.; Zhao, F.; Zhu, X.D.; Zhang, X.F. Effects of Nitrogen Fertilizer Application and Densities on Non-structural Carbohydrates Accumulation, Distribution and Quality of Super Late Rice Tianyouhuazhan. J. Agric. Sci. Technol. 2010, 12, 86–91. [Google Scholar] [CrossRef]
- Lapébie, P.; Lombard, V.; Drula, E.; Terrapon, N.; Henrissat, B. Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat. Commun. 2019, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, B.; Bhatt, K.; Lal, S.; Srinivasan, R.; Bhatt, V. Production of a novel cellulase by Bacillus amyloliquefaciens OKB3 isolated from soil: Purification and characterization. Int. J. Biol. Macromol. 2024, 282, 137454. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.N.; Mongkolthanaruk, W.; Riddech, N. Enhancing soil amendment for salt stress using pretreated rice straw and cellulolytic fungi. Sci. Rep. 2024, 14, 13903. [Google Scholar] [CrossRef]
- Goncalves, M.F.M.; Hilario, S.; van de Peer, Y.; Esteves, A.C.; Alves, A. Genomic and Metabolomic Analyses of the Marine Fungus Emericellopsis cladophorae: Insights into Saltwater Adaptability Mechanisms and Its Biosynthetic Potential. J. Fungi 2022, 8, 31. [Google Scholar] [CrossRef]

| Sequence Type | Primer Name | Primer Sequence | Length Sequencing | Sequencing Platform |
|---|---|---|---|---|
| Bacterial 16S rRNA | 338F | 5′-ACTCCTACGGGAGGCAGCAG-3′ | 416 bp | Miseq PE300 |
| 806R | 5′-GGACTACHVGGGTWTCTAAT-3′ | |||
| Fungal ITS | ITS1F | 5′-CTTGGTCATTTAGAGGAAGTAA-3′ | 251 bp | MiSeq PE300 |
| ITS2R | 5′-GCTGCGTTCTTCATCGATGC-3′ |
| Samples | Shannon Index | Simpson Index | Ace Index | Chao 1 Index | Coverage | |
|---|---|---|---|---|---|---|
| Bacteria | ML | 7.17 ± 0.07a | 0.0024 ± 0.0003a | 5847.07 ± 325.66a | 5506.40 ± 290.86a | 0.96 |
| CL | 7.04 ± 0.26a | 0.0041 ± 0.0051a | 5587.18 ± 286.22a | 5294.94 ± 247.26a | 0.96 | |
| CK | 7.13 ± 0.01a | 0.0024 ± 0.0002a | 5895.46 ± 81.78a | 5551.98 ± 47.85a | 0.96 | |
| Fungi | ML | 4.33 ± 0.71a | 0.069 ± 0.0948a | 556.56 ± 114.02a | 559.30 ± 113.97a | 0.99 |
| CL | 4.47 ± 0.31a | 0.0356 ± 0.0186a | 385.24 ± 89.06b | 387.66 ± 89.26b | 0.99 | |
| CK | 4.43 ± 0.35a | 0.0471 ± 0.0386a | 417.13 ± 80.85ab | 419.51 ± 80.97ab | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liang, Q.; Gao, M.; Ou, Y.; Hu, Y.; Jiang, W.; Jiang, H.; Yang, S. Comparative Analysis of Soil Microbial Community Structures in Rhizosphere of Two Texture-Differentiated Lotus Root Varieties. Microorganisms 2025, 13, 1637. https://doi.org/10.3390/microorganisms13071637
Li X, Liang Q, Gao M, Ou Y, Hu Y, Jiang W, Jiang H, Yang S. Comparative Analysis of Soil Microbial Community Structures in Rhizosphere of Two Texture-Differentiated Lotus Root Varieties. Microorganisms. 2025; 13(7):1637. https://doi.org/10.3390/microorganisms13071637
Chicago/Turabian StyleLi, Xinni, Qiyue Liang, Meiping Gao, Yangxiu Ou, Yifeng Hu, Wen Jiang, Huiping Jiang, and Shangdong Yang. 2025. "Comparative Analysis of Soil Microbial Community Structures in Rhizosphere of Two Texture-Differentiated Lotus Root Varieties" Microorganisms 13, no. 7: 1637. https://doi.org/10.3390/microorganisms13071637
APA StyleLi, X., Liang, Q., Gao, M., Ou, Y., Hu, Y., Jiang, W., Jiang, H., & Yang, S. (2025). Comparative Analysis of Soil Microbial Community Structures in Rhizosphere of Two Texture-Differentiated Lotus Root Varieties. Microorganisms, 13(7), 1637. https://doi.org/10.3390/microorganisms13071637






