Characterization, Microbial Community Structure, and Pathogen Occurrence in Two Typical Eel Farms
Abstract
1. Introduction
2. Materials and Methods
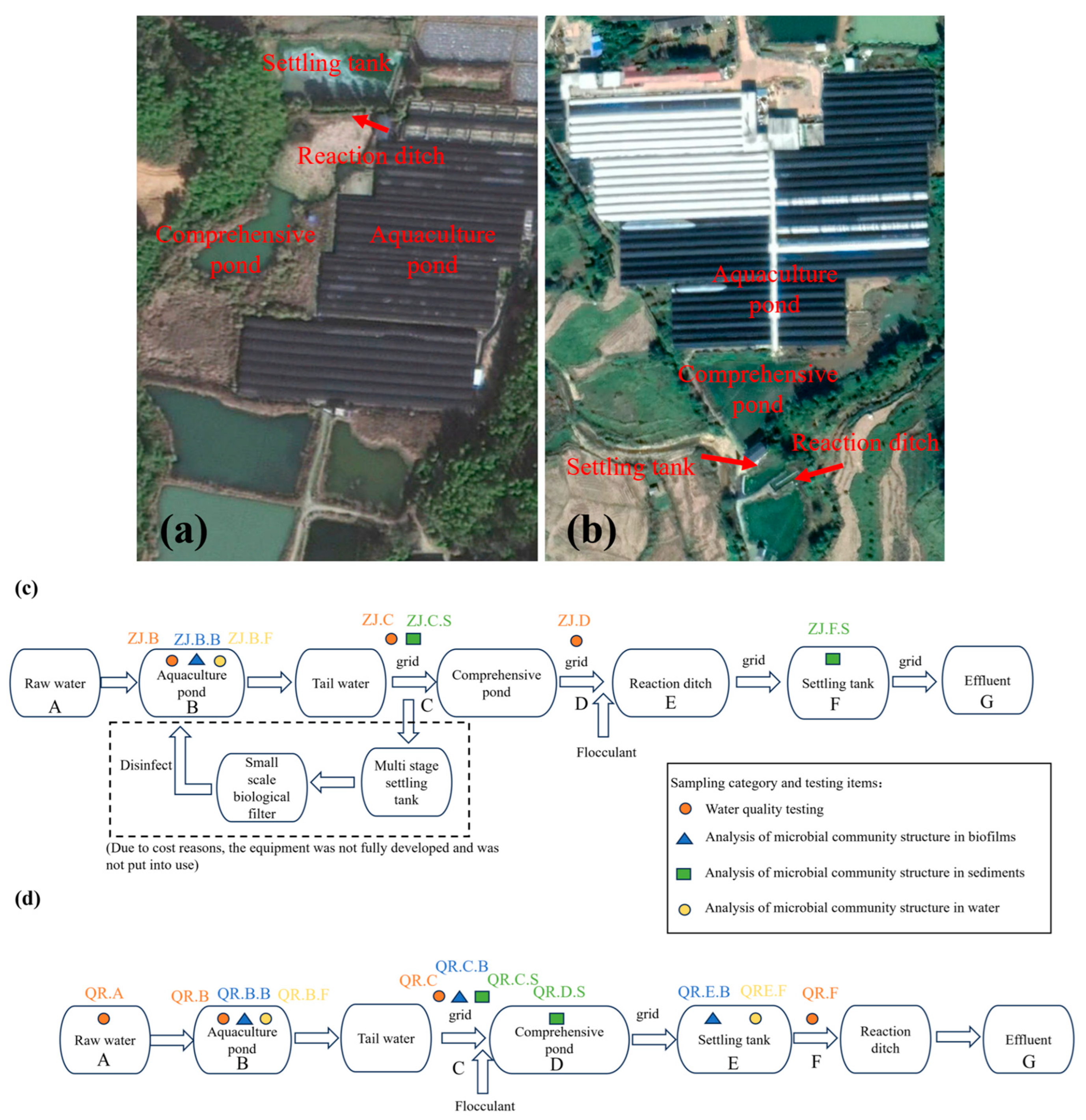
2.1. Overview of the Study Area and Sampling Locations
2.2. Water Quality Analysis
2.3. DNA Extraction
2.4. Microbial Community Structure Analysis
2.5. Data Analysis
3. Results
3.1. Water Quality in the Treatment Processes
3.2. Analysis of Microbial Community Composition and Diversity
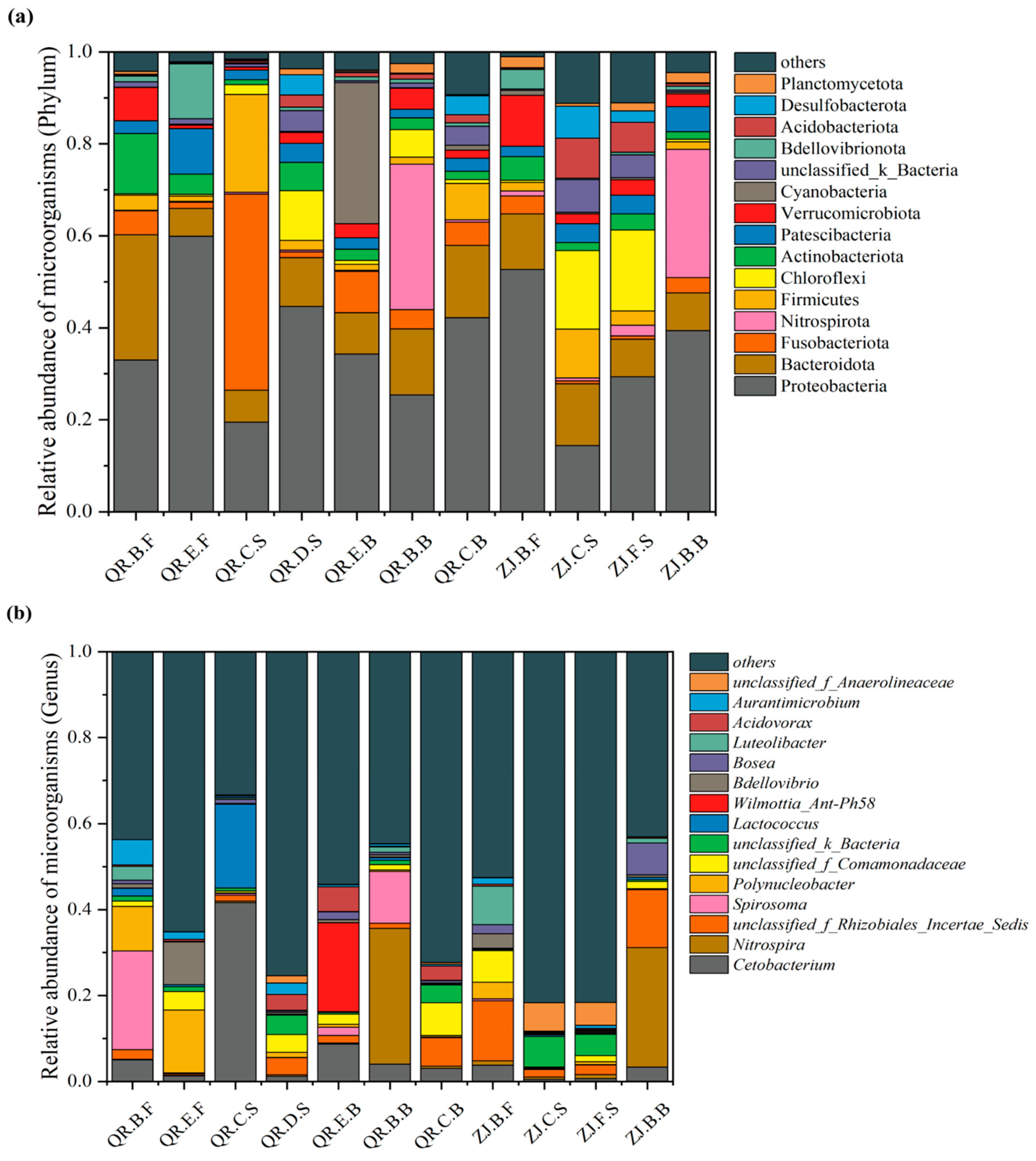
3.2.1. Analysis of Microorganisms at the Phylum and Genus Levels
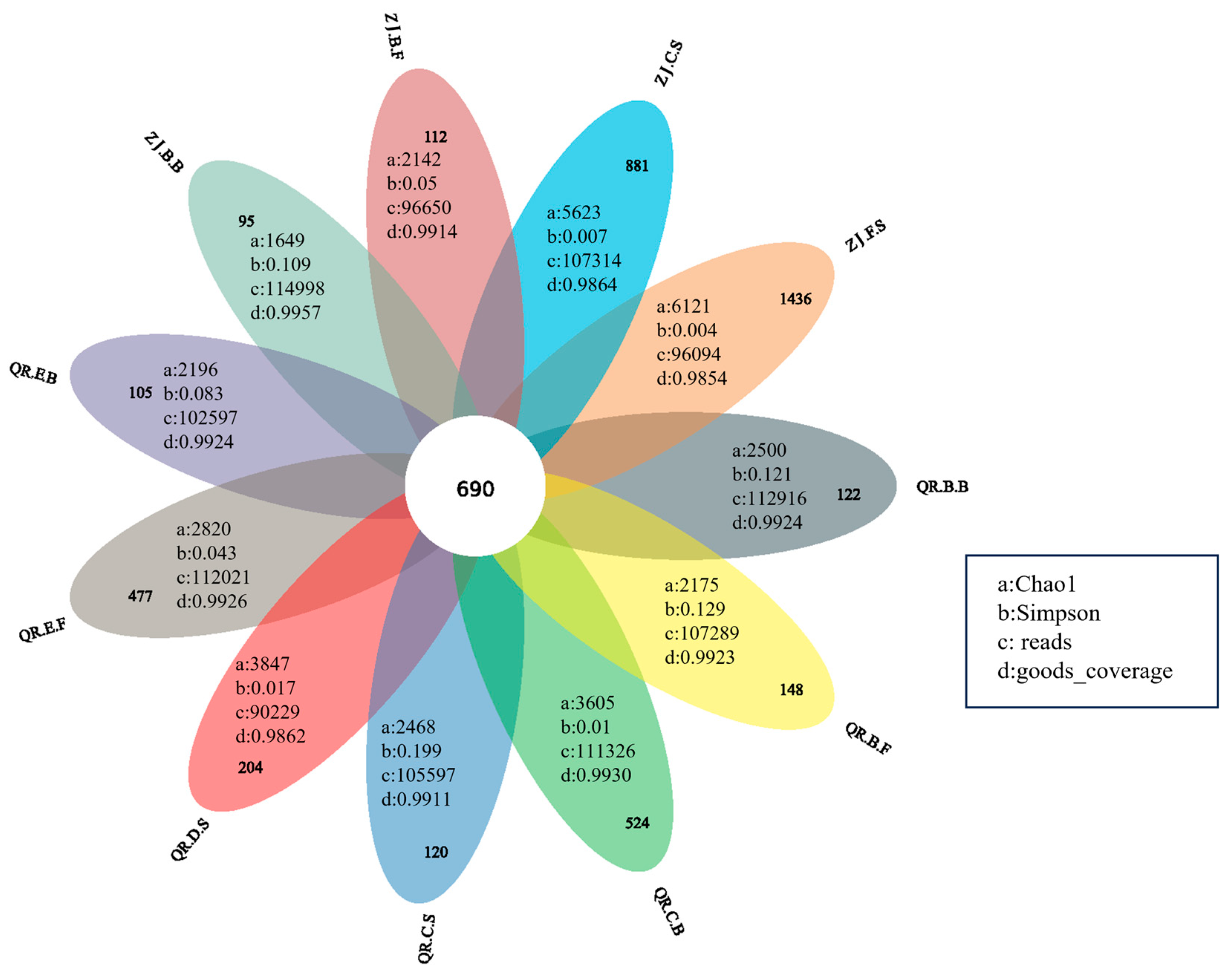
3.2.2. Analysis of Microbial Community Diversity and Similarity
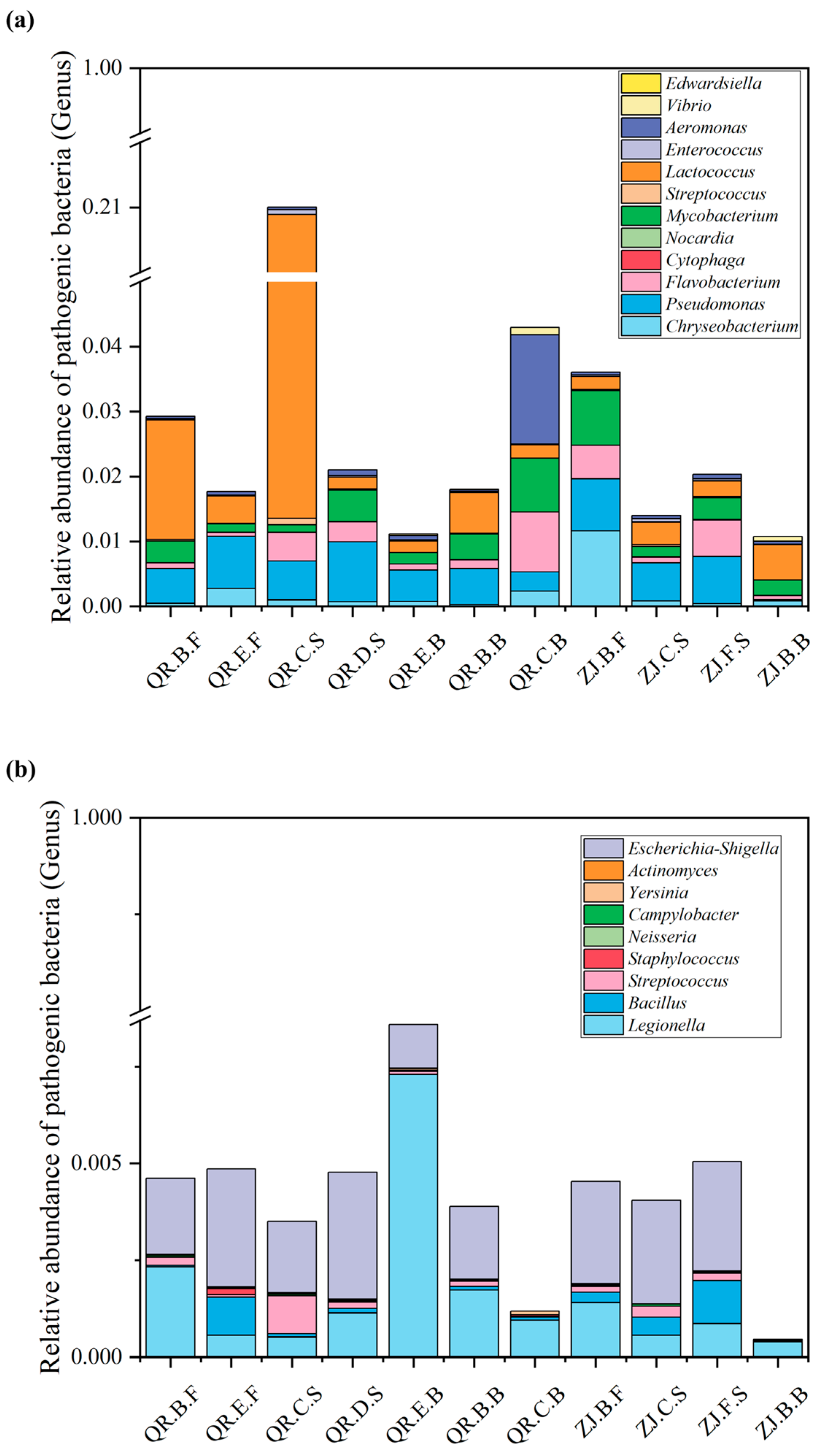
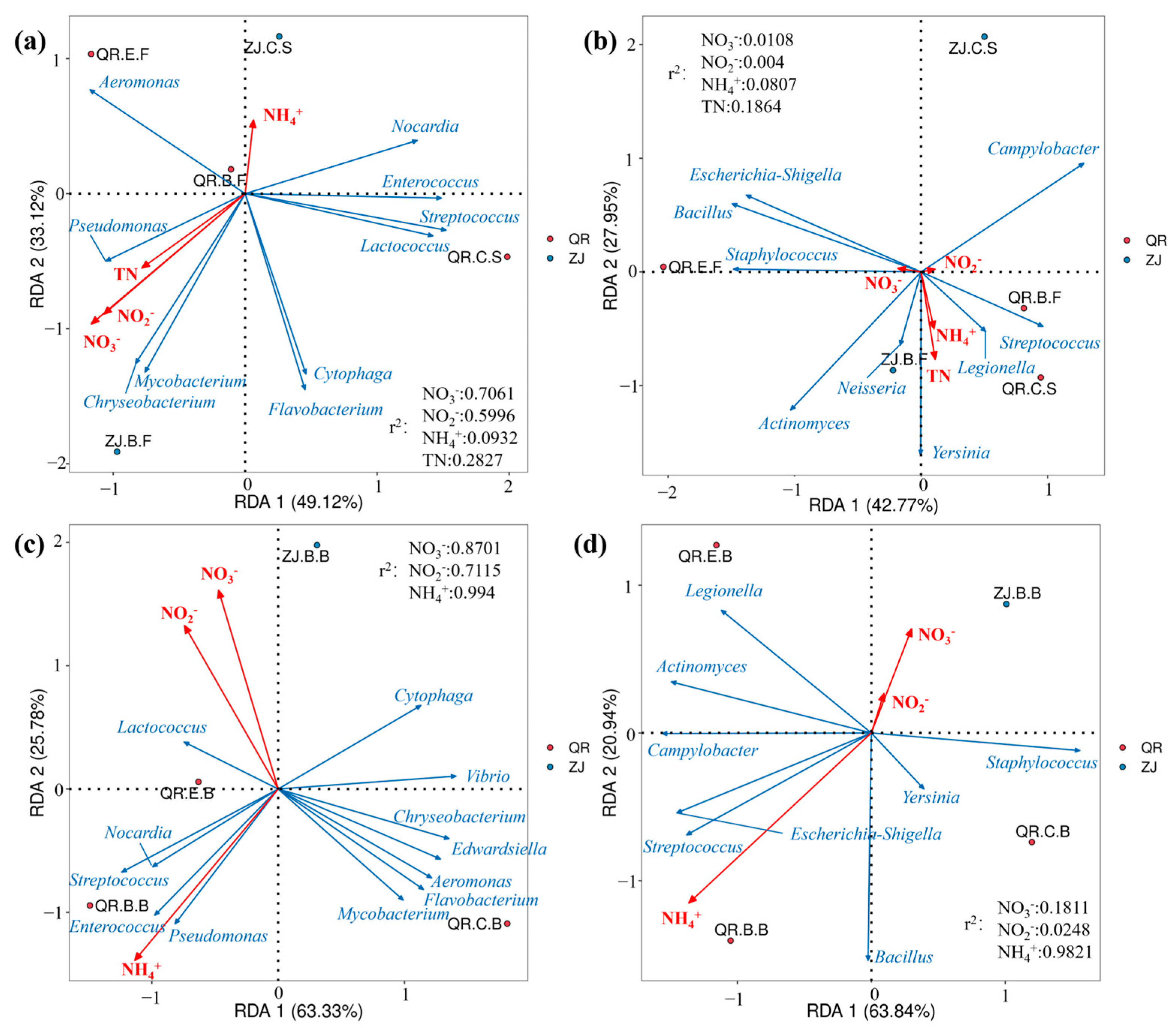
3.2.3. Abundance of Pathogenic Microorganisms
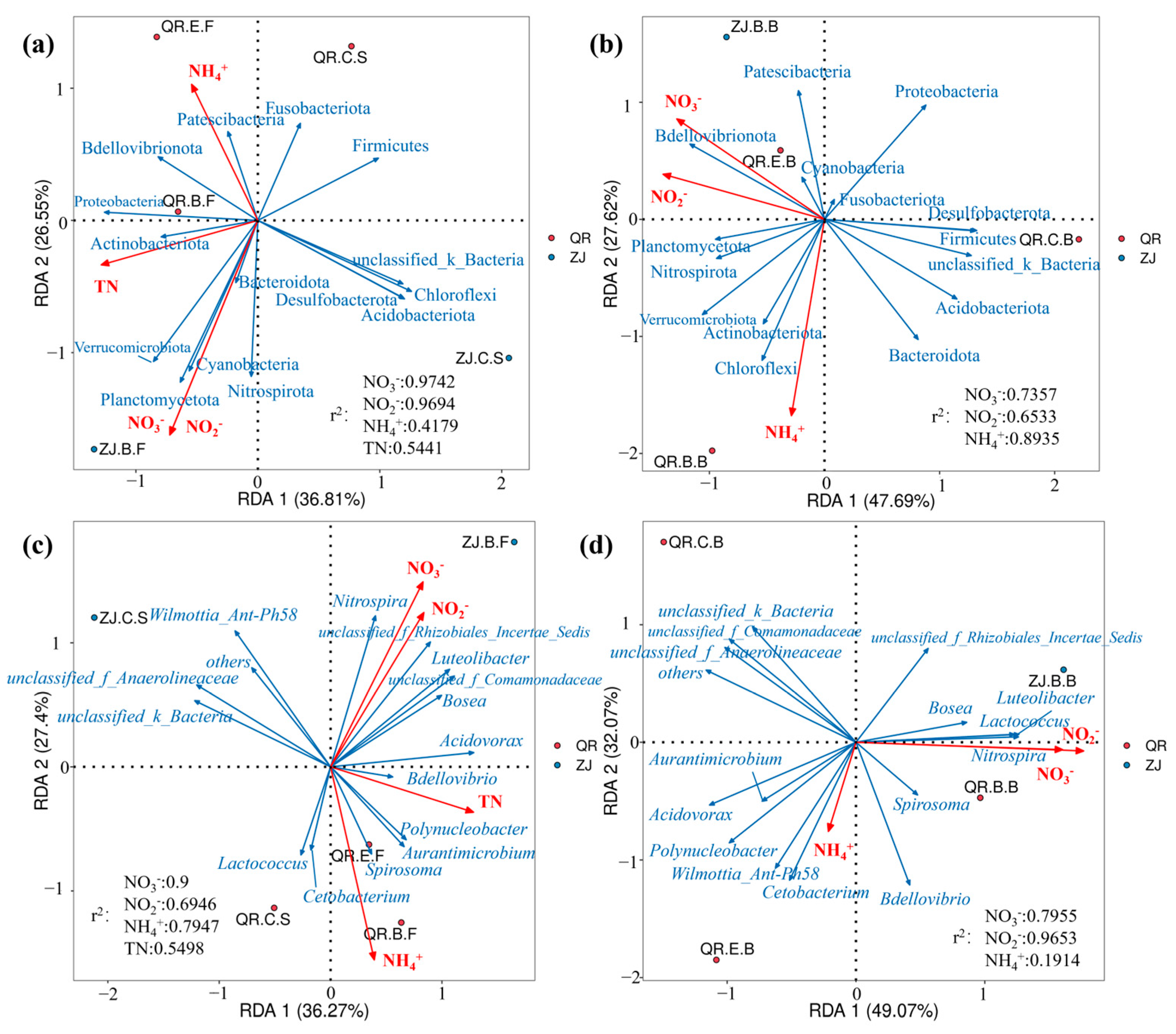
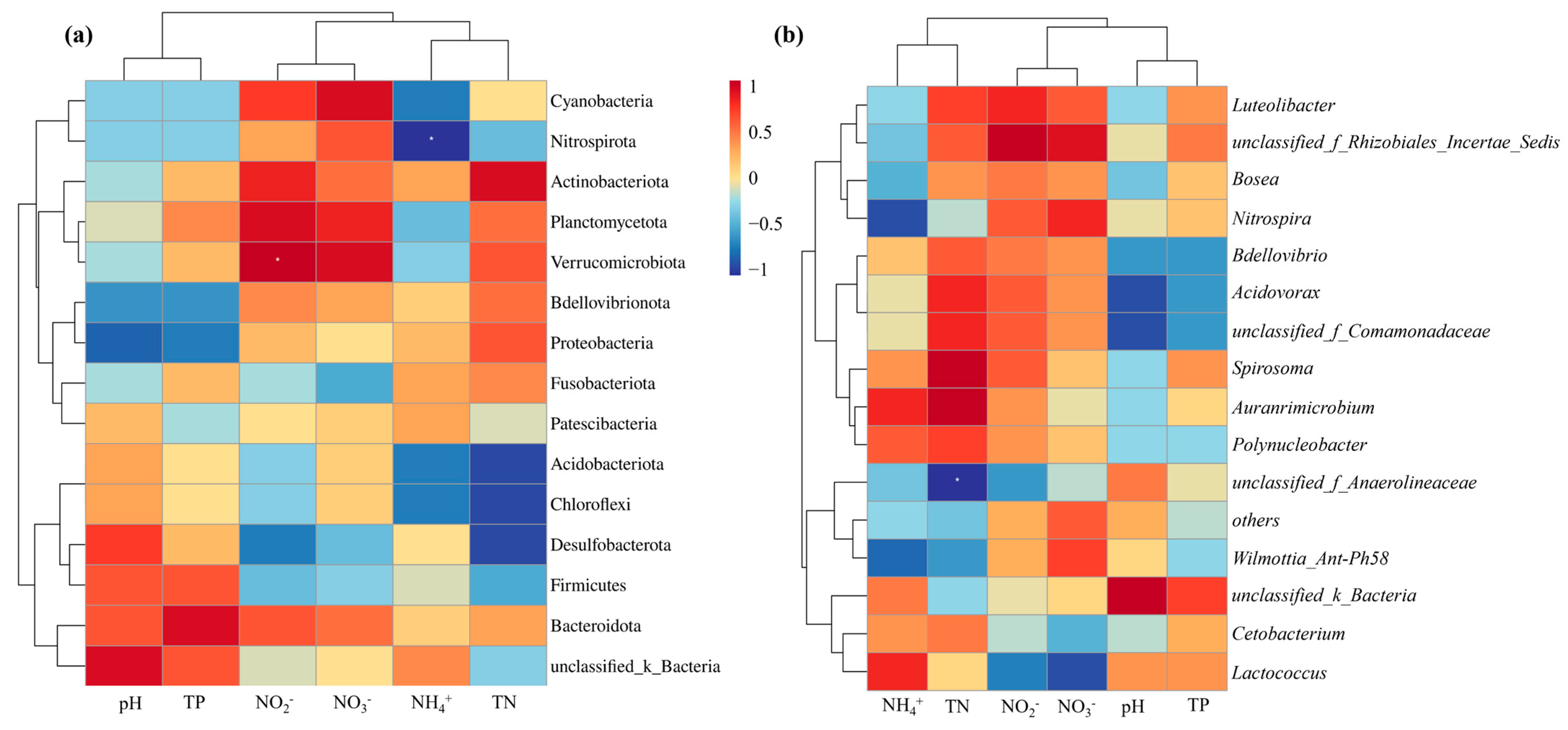
3.3. Correlation Analysis with Environmental Factors
4. Discussion
4.1. Shortcomings of the Water Treatment Processes
4.2. Pathogenic Microorganisms
4.3. Correlation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2022. [Google Scholar]
- Li, H.; Cui, Z.; Cui, H.; Bai, Y.; Yin, Z.; Qu, K. Hazardous substances and their removal in recirculating aquaculture systems: A review. Aquaculture 2023, 569, 739399. [Google Scholar] [CrossRef]
- Zhang, Y.; Bleeker, A.; Liu, J. Nutrient discharge from China’s aquaculture industry and associated environmental impacts. Environ. Res. Lett. 2015, 10, 045002. [Google Scholar] [CrossRef]
- Bai, D.; Li, X.; Liu, Z.; Wan, L.; Song, C.; Zhou, Y.; Cao, X. Nitrogen and phosphorus turnover and coupling in ponds with different aquaculture species. Aquaculture 2023, 563, 738997. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, M.; Zamani, A.; Liu, B. A review on the use of chitosan and chitosan derivatives as the bio-adsorbents for the water treatment: Removal of nitrogen-containing pollutants. Carbohydr. Polym. 2021, 273, 118625. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Xiao, Y.; Li, X.; Zhou, L.; Wang, Y.; Du, T.; Ma, X.; Li, J. Investigating the effect of nitrate on juvenile turbot (Scophthalmus maximus) growth performance, health status, and endocrine function in marine recirculation aquaculture systems. Ecotoxicol. Environ. Saf. 2021, 208, 111617. [Google Scholar] [CrossRef]
- Meinelt, T.; Kroupova, H.; Stüber, A.; Rennert, B.; Wienke, A.; Steinberg, C.E.W. Can dissolved aquatic humic substances reduce the toxicity of ammonia and nitrite in recirculating aquaculture systems? Aquaculture 2010, 306, 378–383. [Google Scholar] [CrossRef]
- Kakade, A.; Salama, E.-S.; Han, H.; Zheng, Y.; Kulshrestha, S.; Jalalah, M.; Harraz, F.A.; Alsareii, S.A.; Li, X. World eutrophic pollution of lake and river: Biotreatment potential and future perspectives. Environ. Technol. Innov. 2021, 23, 101604. [Google Scholar] [CrossRef]
- Bej, S.; Swain, S.; Bishoyi, A.K.; Mandhata, C.P.; Sahoo, C.R.; Padhy, R.N. Wastewater-associated infections: A public health concern. Water Air Soil Pollut. 2023, 234, 444. [Google Scholar] [CrossRef]
- Tang, S.; Gong, J.; Li, J.; Song, B.; Cao, W.; Zhao, J. Nitrogen and phosphorus in water-sediment system of eutrophic lake amended with biochar-supported Effective Microorganisms: Temporal variation and remediation. J. Environ. Manag. 2025, 380, 124732. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Simon, M.; Joshi, H. A review on green technologies for the rejuvenation of polluted surface water bodies: Field-scale feasibility, challenges, and future perspectives. J. Environ. Chem. Eng. 2021, 9, 105763. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, X.; Senbati, Y.; Song, K.; He, X. Nitrogen removal by heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas sp. DM02: Removal performance, mechanism and immobilized application for real aquaculture wastewater treatment. Bioresour. Technol. 2021, 322, 124555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, L.; Su, C.; Liu, L.; Dou, L. Simultaneous aerobic removal of phosphorus and nitrogen by a novel salt-tolerant phosphate-accumulating organism and the application potential in treatment of domestic sewage and aquaculture sewage. Sci. Total Environ. 2021, 758, 143580. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, T.; Zhang, H.; Guo, H.; Hu, R.; Lin, Z.; Li, Y.; Cheng, Y. Activation of indigenous denitrifying bacteria and enhanced nitrogen removal via artificial mixing in a drinking water reservoir: Insights into gene abundance, community structure, and co-existence model. Environ. Res. 2023, 236, 116830. [Google Scholar] [CrossRef]
- HJ 535-2009; Water Quality-Determination of Ammonia Nitrogen-Nessler’s Reagent Spectrophotometry. Sate Environmental Protection Administration: Beijing, China, 2009. (In Chinese)
- HJ/T 346-2007; Water Quality-Determination of Nitrate-Nitrogen-Ultraviolet Spectrophotometry. Sate Environmental Protection Administration: Beijing, China, 2007. (In Chinese)
- GB 7493-87; Water Quality-Determination of Nitrogen (Nitrite)-Spectrophotometric Method. Sate Environmental Protection Administration: Beijing, China, 1987. (In Chinese)
- HJ 636-2012; Water Quality-Determination of Total Nitrogen-Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method. Sate Environmental Protection Administration: Beijing, China, 2012. (In Chinese)
- GB 11893-89; Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method. Sate Environmental Protection Administration: Beijing, China, 1989. (In Chinese)
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- SC/T 9101-2007; Requirement for Water Discharge from Freshwater Aquaculture Pond. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2007. (In Chinese)
- Xu, P.X.; Han, L.L.; He, J.Z.; Luo, F.; Zhang, L.M. Research advance on molecular ecology of asymbiotic nitrogen fixation microbes. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2017, 28, 3440–3450. [Google Scholar]
- Yang, Y.; Fan, L.; Wang, X.; Chen, X.; Qiu, L.; Xu, H.; Li, D.; Song, C.; Meng, S.; Chen, J. In-situ pretreatment of aquaculture tail water by molasses addition and the responses of bacterioplankton communities. J. Water Process Eng. 2023, 56, 104526. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Change Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef]
- Huang, J.; Hu, B.; Qi, K.; Chen, W.; Pang, X.; Bao, W.; Tian, G. Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur. J. Soil Biol. 2016, 72, 35–41. [Google Scholar] [CrossRef]
- Tang, M.; Deng, Q.; Li, X.; Cao, X.; Zhang, Z.; Zhou, Y.; Sun, Q.; Song, C. The effect of natural materials used as sediment remediation on phosphorus and nitrogen control in a mesocosm. Environ. Sci. Eur. 2020, 32, 90. [Google Scholar] [CrossRef]
- Seiki, T.; Izawa, H.; Date, E. Benthic nutrient remineralization and oxygen consumption in the coastal area of Hiroshima Bay. Water Res. 1989, 23, 219–228. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, H.; Sun, X.; Zhu, Y.; Yang, L. Nitrification and denitrification by algae-attached and free-living microorganisms during a cyanobacterial bloom in Lake Taihu, a shallow Eutrophic Lake in China. Biogeochemistry 2016, 131, 135–146. [Google Scholar] [CrossRef]
- Cheng, H.; Li, W.; Zhang, C.; Dai, Z.; Dai, H.; Chen, H.; Wen, C.; Cheng, F.; Lu, X. Innovation of a biofilm system for odor mitigation and enhanced nitrogen removal in decentralized sewage treatment: Performance and microbial community analysis. J. Water Process Eng. 2025, 69, 106767. [Google Scholar] [CrossRef]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef]
- Rerkrungchariya, N.; Suyamud, B.; Phetrak, A.; Lohwacharin, J. Delineating multi-phase performance of ozone-enhanced ceramic filter–microfiltration system for treating polluted surface water. J. Water Process Eng. 2024, 67, 106272. [Google Scholar] [CrossRef]
- Yue, X.; Liang, J.; Lin, Y.; Xiao, X.; Chen, L.; Che, K.; Xiao, K. Acidobacteria as the dominant microorganism on the nitrogen-removal wastewater treatment with a low chemical oxygen demand/ammonium nitrogen ratio in biological contact oxidation reactor. J. Environ. Manag. 2025, 373, 123891. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Zhang, D.; Xing, C.; Liang, J.; Wang, C.; Yang, X.; Liu, Z.; Zhao, Z. Effects of nitrogen-driven eutrophication on the horizontal transfer of extracellular antibiotic resistance genes in water-sediment environments. Environ. Res. 2025, 274, 121317. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, M.; Zhao, S.; Peng, N.; Hu, R.; Hu, J.; Liang, Y. Algal growth enhances light-mediated limitation of bacterial nitrification in an aquaculture system. Water Air Soil Pollut. 2020, 231, 73. [Google Scholar] [CrossRef]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef]
- Daims, H.; Wagner, M. Nitrospira. Trends Microbiol. 2018, 26, 462–463. [Google Scholar] [CrossRef]
- Van Kessel, M.A.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.; Kartal, B.; Jetten, M.S.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Z.; Jiang, Y.; Ji, T.; Bai, H.; Zhao, H.; Yang, H. Addition of Bdellovibrio to aquaculture water can significantly alter the distribution of microbial community on the gills and enhance the survival rate of Carassius auratus gibelio. Aquaculture 2023, 576, 739820. [Google Scholar] [CrossRef]
- Geng, X.; Ma, Z.; Chen, E.; Wang, X.; Wang, B.; Zheng, F.; Sun, J.; Sun, B.; Zhang, Y.; Li, Z. Interactions between pH and Lactobacillus drove esters’ metabolism during the fermentation of Laobaigan Baijiu. Food Biosci. 2025, 67, 106297. [Google Scholar] [CrossRef]
- Manchanayake, T.; Salleh, A.; Amal, M.N.A.; Yasin, I.S.M.; Zamri-Saad, M. Pathology and pathogenesis of Vibrio infection in fish: A review. Aquac. Rep. 2023, 28, 101459. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, X.-H. Edwardsiella tarda: An intriguing problem in aquaculture. Aquaculture 2014, 431, 129–135. [Google Scholar] [CrossRef]
- Rahmawaty, A.; Chen, M.Y.; Byadgi, O.V.; Wang, P.C.; Chen, S.C. Phenotypic and genotypic analysis of Edwardsiella isolates from Taiwan indicates wide variation with a particular reference to Edwardsiella tarda and Edwardsiella anguillarum. J. Fish Dis. 2022, 45, 1659–1672. [Google Scholar] [CrossRef]
- Shao, S.; Lai, Q.; Liu, Q.; Wu, H.; Xiao, J.; Shao, Z.; Wang, Q.; Zhang, Y. Phylogenomics characterization of a highly virulent Edwardsiella strain ET080813T encoding two distinct T3SS and three T6SS gene clusters: Propose a novel species as Edwardsiella anguillarum sp. nov. Syst. Appl. Microbiol. 2015, 38, 36–47. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, L.; He, L.; Tang, Y.; Guo, S.; Zhai, S. Transcriptomic analysis using dual RNA sequencing revealed a Pathogen–Host interaction after Edwardsiella anguillarum infection in European eel (Anguilla anguilla). Fish Shellfish. Immunol. 2022, 120, 745–757. [Google Scholar] [CrossRef]
- Pirollo, T.; Perolo, A.; Mantegari, S.; Barbieri, I.; Scali, F.; Alborali, G.L.; Salogni, C. Mortality in farmed European eel (Anguilla anguilla) in Italy due to Streptococcus iniae. Acta Vet. Scand. 2023, 65, 5. [Google Scholar] [CrossRef]
- Zheng, C.-c.; Cai, X.-y.; Huang, M.-m.; Mkingule, I.; Sun, C.; Qian, S.-C.; Wu, Z.-j.; Han, B.-n.; Fei, H. Effect of biological additives on Japanese eel (Anguilla japonica) growth performance, digestive enzymes activity and immunology. Fish Shellfish. Immunol. 2019, 84, 704–710. [Google Scholar] [CrossRef]
- Egger, R.C.; Rosa, J.C.C.; Resende, L.F.L.; de Pádua, S.B.; de Oliveira Barbosa, F.; Zerbini, M.T.; Tavares, G.C.; Figueiredo, H.C.P. Emerging fish pathogens Lactococcus petauri and L. garvieae in Nile tilapia (Oreochromis niloticus) farmed in Brazil. Aquaculture 2023, 565, 739093. [Google Scholar] [CrossRef]
- Yajima, D.; Fujita, H.; Hayashi, I.; Shima, G.; Suzuki, K.; Toju, H. Core species and interactions prominent in fish-associated microbiome dynamics. Microbiome 2023, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wu, M.; Sonne, C.; Lam, S.S.; Kwong, R.W.M.; Jiang, Y.; Zhao, X.; Sun, X.; Zhang, X.; Li, C.; et al. The hidden risk of microplastic-associated pathogens in aquatic environments. Eco-Environ. Health 2023, 2, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Lee, Y.K.; Kwon, J.-H.; Hur, J. Microplastic biofilms in water treatment systems: Fate and risks of pathogenic bacteria, antibiotic-resistant bacteria, and antibiotic resistance genes. Sci. Total Environ. 2023, 892, 164523. [Google Scholar] [CrossRef]
- Feng, B.; Shen, H.; Yang, F.; Yan, J.; Yang, S.; Gan, N.; Shi, H.; Yu, S.; Wang, L. Efficient classification of Escherichia coli and Shigella using FT-IR spectroscopy and multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121369. [Google Scholar] [CrossRef]
- Nakanishi, T.; Hirose, M.; Asada, Y.; Itoh, S. Legionella community dynamics in a drinking water distribution system: Impact of residual chlorine depletion. Sci. Total Environ. 2024, 956, 177302. [Google Scholar] [CrossRef]
- Qi, Y.; Zhong, Y.; Luo, L.; He, J.; Feng, B.; Zhang, X.; Xia, Y.; Ren, H. Feasibility analysis of reclaimed water reuse based on water quality data and microbial community structure study. Sci. Total Environ. 2024, 951, 174781. [Google Scholar] [CrossRef]
- Vitorino, I.; Santos, J.D.N.; Godinho, O.; Vicente, F.; Vasconcelos, V.; Lage, O.M. Novel and conventional isolation techniques to obtain planctomycetes from marine environments. Microorganisms 2021, 9, 2078. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Yamada, T.; Sekiguchi, Y.; Imachi, H.; Kamagata, Y.; Ohashi, A.; Harada, H. Diversity, localization, and physiological properties of filamentous microbes belonging to Chloroflexi subphylum I in mesophilic and thermophilic methanogenic sludge granules. Appl. Environ. Microbiol. 2005, 71, 7493–7503. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, S.; Wan, Q.; Xu, M.; Chen, M.; Guo, S. Pathogenicity of Edwardsiella anguillarum to American eels (Anguilla rostrata) and RNA-seq analysis of host immune response to the E. anguillarum infection. Fish Shellfish. Immunol. 2023, 141, 109042. [Google Scholar] [CrossRef] [PubMed]
- Mallik, S.K.; Pathak, R.; Shahi, N.; Kala, K.; Chandra, S.; Das, P.; Singh, B.; Singh, M.; Giri, A.K.; Tandel, R.S. Pathological analysis and antimicrobial susceptibility of Chryseobacterium balustinum RTFCP 298 isolated from diseased rainbow trout, Oncorhynchus mykiss. Sci. Rep. 2023, 13, 13268. [Google Scholar] [CrossRef] [PubMed]
- Laltlanmawia, C.; Ghosh, L.; Saha, R.K.; Parhi, J.; Pal, P.; Dhar, B.; Saha, H. Isolation, identification and pathogenicity study of emerging multi-drug resistant fish pathogen Acinetobacter pittii from diseased rohu (Labeo rohita) in India. Aquac. Rep. 2023, 31, 101629. [Google Scholar] [CrossRef]
- Tawfeek, W.S.; Kassab, A.S.; Okasha, L.A.; Abdelsalam, M.; Sherif, A.H. The phenotypic and genetic characteristics of Pseudomonas anguilliseptica strains associated with mortalities in farmed sea bream and sea bass. Aquac. Int. 2024, 32, 3973–3992. [Google Scholar] [CrossRef]
- Juárez-Cortés, M.Z.; Vázquez, L.E.C.; Díaz, S.F.M.; Cardona Félix, C.S. Streptococcus iniae in aquaculture: A review of pathogenesis, virulence, and antibiotic resistance. Int. J. Vet. Sci. Med. 2024, 12, 25–38. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Tan, R.; Gao, Y. Identification, characterization and complete genome analysis of a Vibrio anguillarum isolated from Sebastes schlegelii. Microb. Pathog. 2024, 190, 106611. [Google Scholar] [CrossRef]
- Ortega, C.; Irgang, R.; Valladares-Carranza, B.; Collarte, C.; Avendaño-Herrera, R. First Identification and Characterization of Lactococcus garvieae Isolated from Rainbow Trout (Oncorhynchus mykiss) Cultured in Mexico. Animals 2020, 10, 1609. [Google Scholar] [CrossRef]
- Sombe, J.A.; Mzula, A.; Mwega, E.; Shirima, G.M.; Wambura, P.N. Optimization and Field Trial of an Aeromonas hydrophila Vaccine Candidate for Motile Aeromonads Septicemia in Farmed Nile Tilapia in Tanzania. Aquac. Res. 2024, 2024, 8891296. [Google Scholar] [CrossRef]
- Dayana Senthamarai, M.; Rajan, M.R.; Bharathi, P.V. Current risks of microbial infections in fish and their prevention methods: A review. Microb. Pathog. 2023, 185, 106400. [Google Scholar] [CrossRef]
- Rana, M.L.; Firdous, Z.; Ferdous, F.B.; Ullah, M.A.; Siddique, M.P.; Rahman, M.T. Antimicrobial resistance, biofilm formation, and virulence determinants in Enterococcus faecalis isolated from cultured and wild fish. Antibiotics 2023, 12, 1375. [Google Scholar] [CrossRef]
- Knupp, C.; Soto, E.; Loch, T.P. Varying Flavobacterium psychrophilum shedding dynamics in three bacterial coldwater disease-susceptible salmonid (Family Salmonidae) species. Microbiol. Spectr. 2024, 12, e03601–e03623. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, F.; Chen, B.; Deng, Y.; Chen, D.; Geng, Y.; Ouyang, P.; Huang, X. Isolation, identification and histopathological observation of Francisella noatunensis subsp. orientalis from Nile tilapia (Oreochromis niloticus). Aquaculture 2025, 595, 741532. [Google Scholar]
- Acosta, F.; Vega, B.; Monzón-Atienza, L.; Superio, J.; Torrecillas, S.; Gómez-Mercader, A.; Castro, P.; Montero, D.; Galindo-Villegas, J. Phylogenetic reconstruction, histopathological characterization, and virulence determination of a novel fish pathogen, Nocardia brasiliensis. Aquaculture 2024, 581, 740458. [Google Scholar] [CrossRef]
- Agriandini, M.; Sukenda, S.; Widanarni, W.; Lusiastuti, A.M. Fate and tissue distribution of Mycobacterium fortuitum through immersion challenge as a model of natural infection in Osphronemus goramy. Aquac. Int. 2021, 29, 1979–1989. [Google Scholar] [CrossRef]
| Sample | Nitrogen and Phosphorus Pollutants (mg/L) | pH | ||||
|---|---|---|---|---|---|---|
| NH4+ | NO3− | NO2− | TN | TP | ||
| ZJ.B | 0.06 ± 0.02 | 5.81 ± 0.20 | 1.930 ± 0.01 | 11.6 ± 0.18 | 0.98 ± 0.07 | 6.32 ± 0.05 |
| ZJ.C | 0.60 ± 0.03 | 3.81 ± 0.11 | 1.220 ± 0.01 | 6.16 ± 0.57 | 1.54 ± 0.02 | 7.39 ± 0.35 |
| ZJ.D | 0.67 ± 0.02 | 3.39 ± 0.23 | 1.154 ± 0.00 | 5.43 ± 0.48 | 1.29 ± 0.02 | 7.1 ± 0.01 |
| QR.A | 0.02 ± 0.01 | 0.25 ± 0.01 | 0.002 ± 0.00 | 0.33 ± 0.05 | 0.07 ± 0.00 | 6.86 ± 0.04 |
| QR.B | 9.81 ± 0.07 | 3.50 ± 0.11 | 1.388 ± 0.01 | 15.63 ± 1.15 | 2.00 ± 0.01 | 7.26 ± 0.02 |
| QR.C | 3.98 ± 0.12 | 1.89 ± 0.29 | 0.439 ± 0.00 | 6.74 ± 0.11 | 1.27 ± 0.03 | 6.84 ± 0.01 |
| QR.F | 5.36 ± 0.07 | 2.93 ± 0.07 | 0.762 ± 0.00 | 9.03 ± 0.69 | 0.04 ± 0.00 | 6.7 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, J.-Y.; Lin, H.-R.; Sun, X.-H.; Hu, G.-R.; Yu, R.-L.; Li, J.-Q. Characterization, Microbial Community Structure, and Pathogen Occurrence in Two Typical Eel Farms. Microorganisms 2025, 13, 1624. https://doi.org/10.3390/microorganisms13071624
Lai J-Y, Lin H-R, Sun X-H, Hu G-R, Yu R-L, Li J-Q. Characterization, Microbial Community Structure, and Pathogen Occurrence in Two Typical Eel Farms. Microorganisms. 2025; 13(7):1624. https://doi.org/10.3390/microorganisms13071624
Chicago/Turabian StyleLai, Jing-Ying, Hui-Rong Lin, Xiao-Hui Sun, Gong-Ren Hu, Rui-Lian Yu, and Jia-Qi Li. 2025. "Characterization, Microbial Community Structure, and Pathogen Occurrence in Two Typical Eel Farms" Microorganisms 13, no. 7: 1624. https://doi.org/10.3390/microorganisms13071624
APA StyleLai, J.-Y., Lin, H.-R., Sun, X.-H., Hu, G.-R., Yu, R.-L., & Li, J.-Q. (2025). Characterization, Microbial Community Structure, and Pathogen Occurrence in Two Typical Eel Farms. Microorganisms, 13(7), 1624. https://doi.org/10.3390/microorganisms13071624






