Metagenome Analysis Reveals Changes in Gut Microbial Antibiotic Resistance Genes and Virulence Factors in Reintroduced Giant Pandas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Source
2.2. DNA Extraction
2.3. Metagenomic Sequencing
2.4. Data Analysis
3. Results
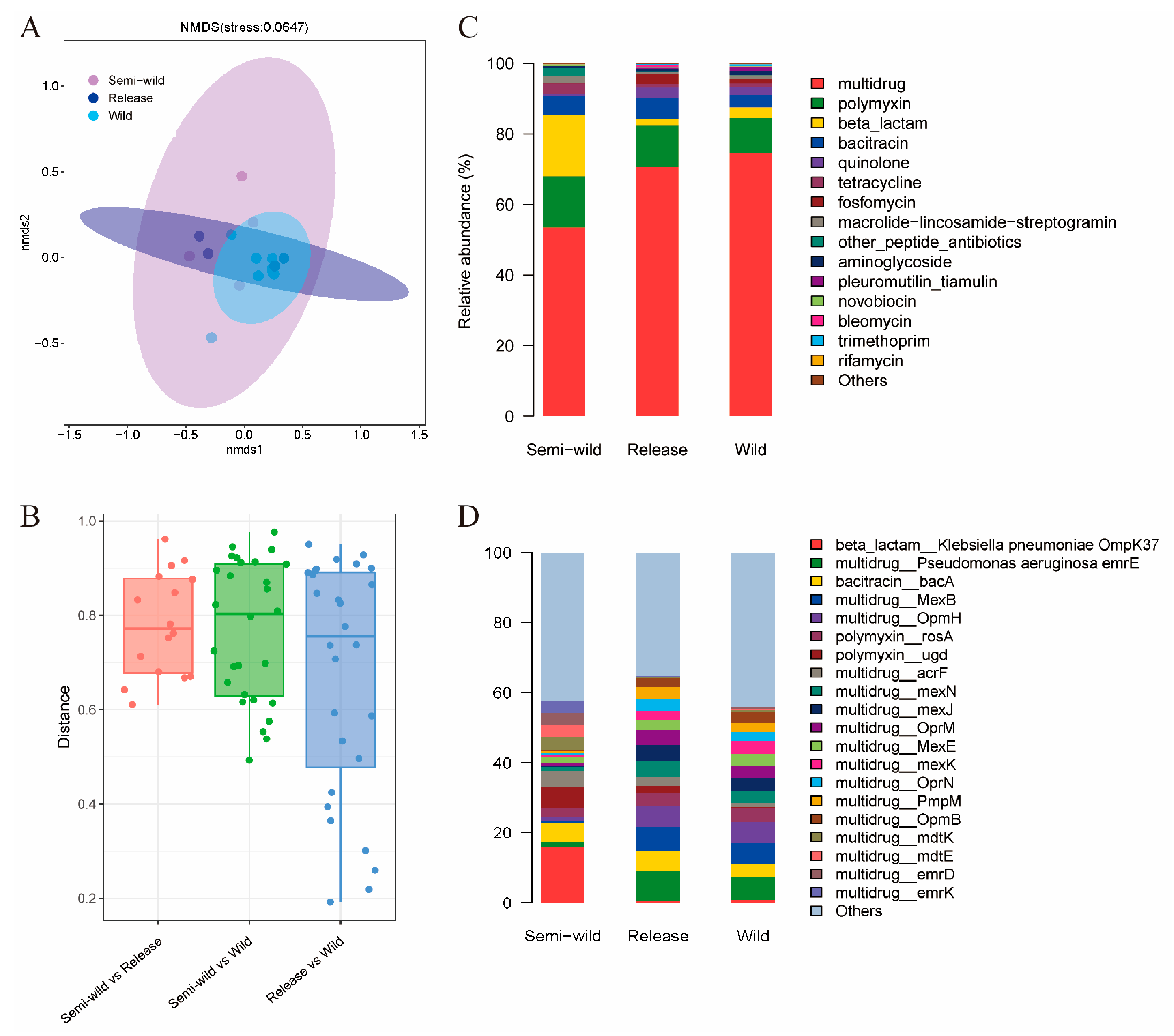
3.1. The Distributions of ARG in Released Giant Pandas Exhibited Similarity to Wild Giant Pandas
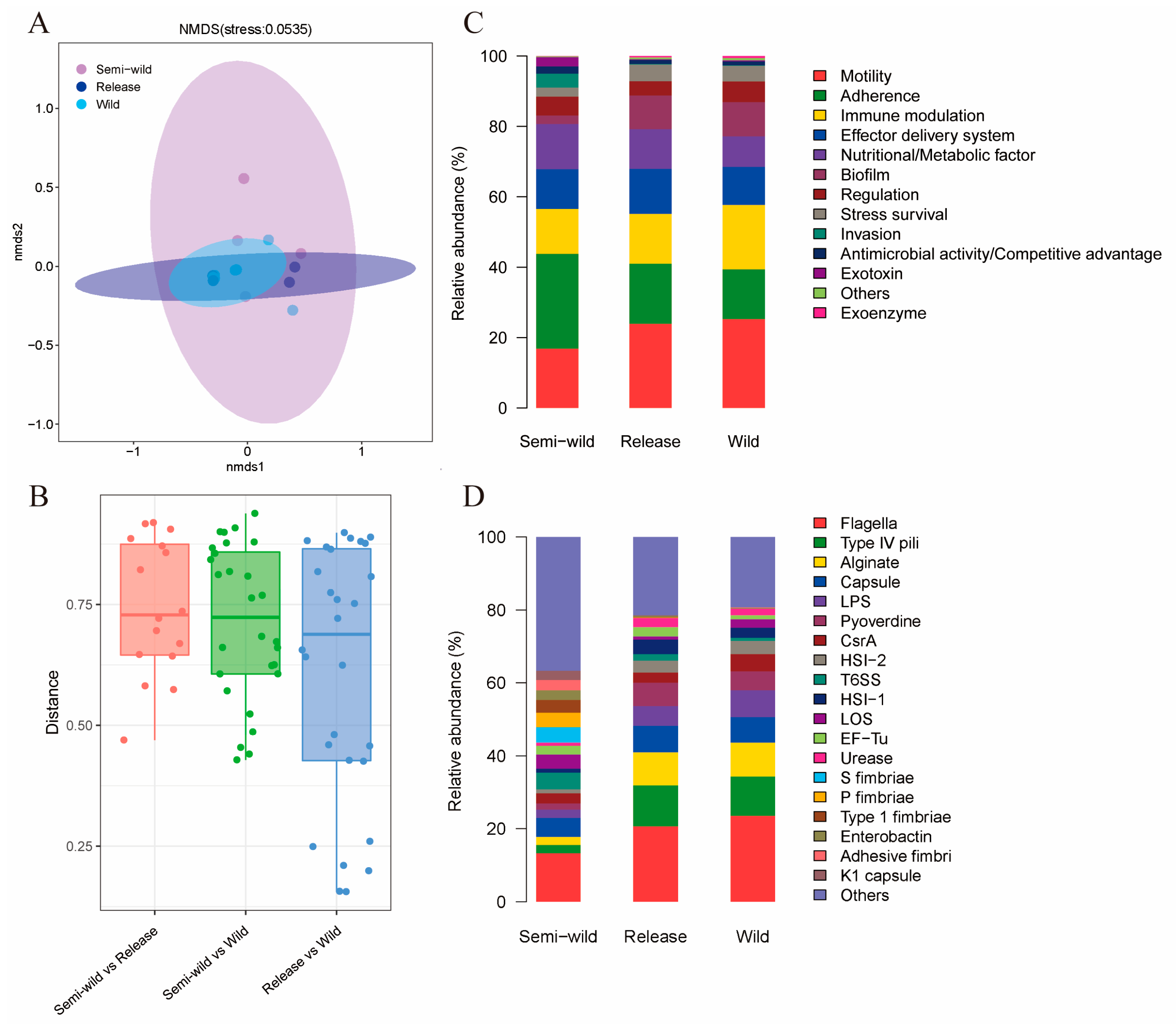
3.2. The Distributions of VF in Released Giant Pandas Exhibited Similarity to Wild Giant Pandas
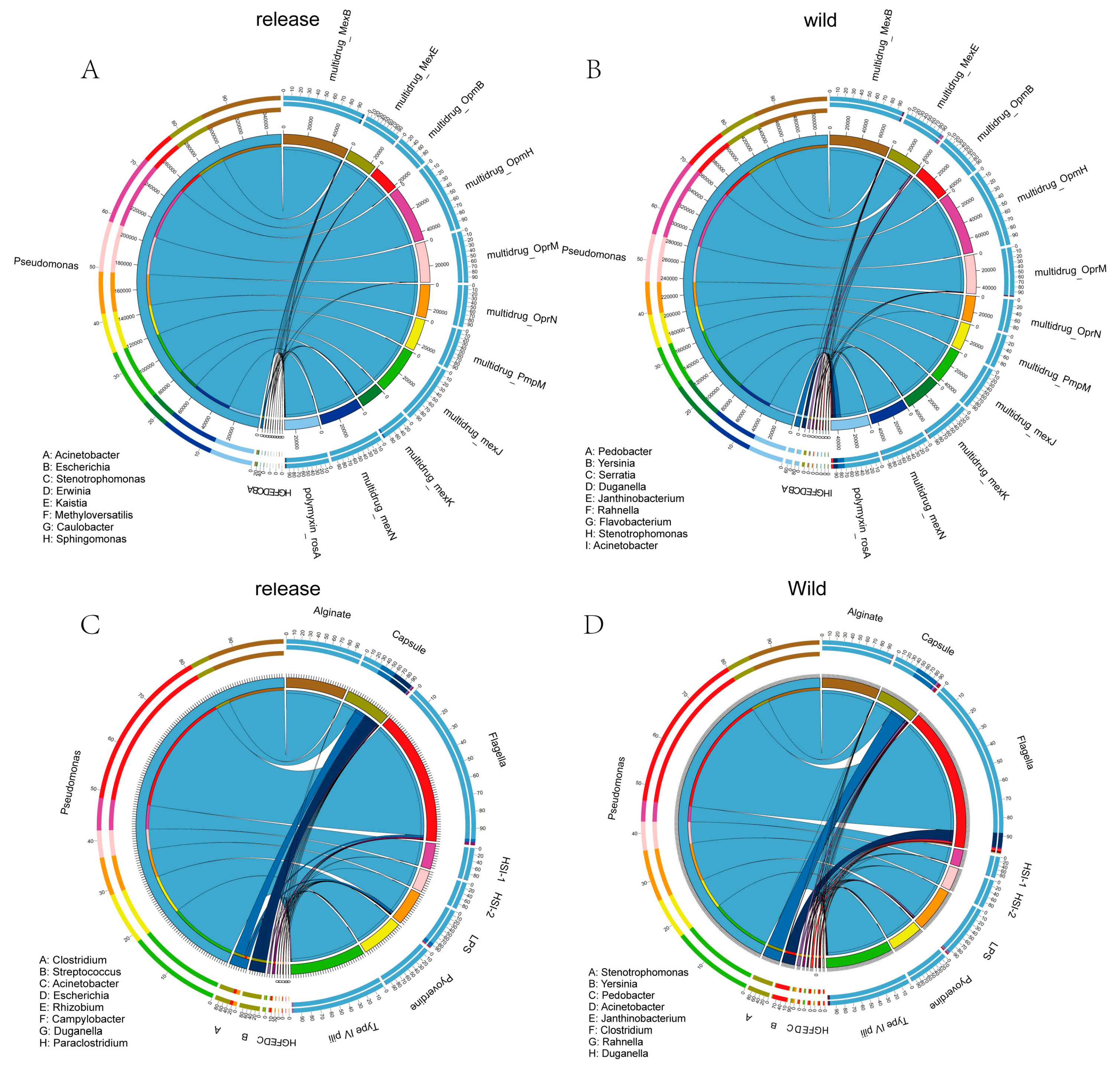
3.3. ARGs and VFs with Increased Abundance in Released and Wild Giant Pandas Were Mainly Found in Pseudomonas
4. Discussion
4.1. Characterization of Intestinal Microbial Resistance and Virulence Factors at Different Stages of Giant Pandas: Potential Risks for Wild Release
4.2. Geography and Diet May Be Key Drivers of the Distribution Patterns of ARG and VF in Giant Pandas’ Different Stages
4.3. Pseudomonas May Serve as a Critical Reservoir of Antibiotic Resistance and Virulence Factors in Giant Pandas: Implications for Public Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, F.; Wu, Q.; Hu, Y.; Huang, G.; Nie, Y.; Yan, L. Conservation metagenomics: A new branch of conservation biology. Sci. China Life Sci. 2019, 62, 168–178. [Google Scholar] [CrossRef]
- Wei, F.; Swaisgood, R.; Hu, Y.; Nie, Y.; Yan, L.; Zhang, Z.; Qi, D.; Zhu, L. Progress in the ecology and conservation of giant pandas. Conserv. Biol. 2015, 29, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, Y.; Wei, W.; Zhang, Z.; Zhou, H. The distribution variation of pathogens and virulence factors in different geographical populations of giant pandas. Front. Microbiol. 2023, 14, 1264786. [Google Scholar] [CrossRef]
- Yan, X.; Su, X.; Ren, Z.; Fan, X.; Li, Y.; Yue, C.; Yang, M.; Deng, H.; Deng, Y.; Xu, Z.; et al. High Prevalence of Antimicrobial Resistance and Integron Gene Cassettes in Multi-Drug-Resistant Klebsiella pneumoniae Isolates from Captive Giant Pandas (Ailuropoda melanoleuca). Front. Microbiol. 2021, 12, 801292. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Jiang, S.; Luo, L.; Zhou, Z.; Wang, L.; Huang, X.; Liu, H.; Zhang, S.; Luo, Y.; Ren, Z.; et al. Antibiotic-Resistant Escherichia coli Strains Isolated from Captive Giant Pandas: A Reservoir of Antibiotic Resistance Genes and Virulence-Associated Genes. Vet. Sci. 2022, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Huang, X.Y.; Li, Z.M.; Zhou, Z.Y.; Zhong, Z.J.; Peng, G.N. Virulence gene detection and antimicrobial resistance analysis of Enterococcus faecium in captive giant pandas (Ailuropoda melanoleuca) in China. Acta Vet. Scand. 2023, 65, 4. [Google Scholar] [CrossRef]
- One Health Commission. What Is One Health? 2018. Available online: https://www.onehealthcommission.org/en/why_one_health/what_is_one_health/ (accessed on 3 January 2017).
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Tunyong, W.; Arsheewa, W.; Santajit, S.; Kong-Ngoen, T.; Pumirat, P.; Sookrung, N.; Chaicumpa, W.; Indrawattana, N. Antibiotic Resistance Genes Among Carbapenem-resistant Enterobacterales (CRE) Isolates of Prapokklao Hospital, Chanthaburi Province, Thailand. Infect. Drug Resist. 2021, 14, 3485–3494. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- White, A.; Hughes, J.M. Critical Importance of a One Health Approach to Antimicrobial Resistance. Ecohealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- van Geelen, L.; Meier, D.; Rehberg, N.; Kalscheuer, R. (Some) current concepts in antibacterial drug discovery. Appl. Microbiol. Biotechnol. 2018, 102, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Metelkina, O.; Konstantinović, J.; Klein, A.; Shafiei, R.; Fares, M.; Alhayek, A.; Yahiaoui, S.; Elgaher, W.A.M.; Haupenthal, J.; Titz, A.; et al. Dual inhibitors of Pseudomonas aeruginosa virulence factors LecA and LasB. Chem. Sci. 2024, 15, 13333–13342. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, Y.; Lang, S.; Fondevila, M.F.; Schöler, D.; Harberts, A.; Cabré, N.; Chen, S.; Shao, Y.; Vervier, K.; et al. Targeted inhibition of pathobiont virulence factor mitigates alcohol-associated liver disease. Cell Host Microbe 2025, 33, 957–972.e956. [Google Scholar] [CrossRef]
- Mendes, G.; Santos, M.L.; Ramalho, J.F.; Duarte, A.; Caneiras, C. Virulence factors in carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1325077. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Xu, L.; Hu, T.; Chen, H.; Qi, D.; Gu, X.; Yang, X.; Yang, Z.; Zhu, L. The "wildness" of the giant panda gut microbiome and its relevance to effective translocation. Glob. Ecol. Conserv. 2019, 18, e00644. [Google Scholar] [CrossRef]
- Huang, G.; Qi, D.; Yang, Z.; Hou, R.; Shi, W.; Zhao, F.; Li, Z.; Yan, L.; Wei, F. Gut microbiome as a key monitoring indicator for reintroductions of captive animals. Conserv. Biol. 2024, 38, e14173. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, X.; Nie, Y.; Huang, F.; Huang, Y.; Dai, Q.; Hu, Y.; Yang, Y.; Zhou, X.; Zhang, H.; et al. Reintroduction of the giant panda into the wild: A good start suggests a bright future. Biol. Conserv. 2018, 217, 181–186. [Google Scholar] [CrossRef]
- Tang, J.; Wang, C.; Zhang, H.; Zhao, J.; Guo, W.; Mishra, S.; Kong, F.; Zeng, B.; Ning, R.; Li, D.; et al. Gut microbiota in reintroduction of giant panda. Ecol. Evol. 2020, 10, 1012–1028. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Sun, S.W.; Liang, B.; Jiang, B.W.; Feng, N.; Liu, J.; Ji, X. Prevalence of antimicrobial resistance and virulence genes in Klebsiella pneumoniae and Congenetic Raoultella Isolates from captive giant pandas. PLoS ONE 2023, 18, e0283738. [Google Scholar] [CrossRef]
- Guo, W.; Mishra, S.; Wang, C.; Zhang, H.; Ning, R.; Kong, F.; Zeng, B.; Zhao, J.; Li, Y. Comparative Study of Gut Microbiota in Wild and Captive Giant Pandas (Ailuropoda melanoleuca). Genes 2019, 10, 827. [Google Scholar] [CrossRef]
- Hu, T.; Dai, Q.; Chen, H.; Zhang, Z.; Dai, Q.; Gu, X.; Yang, X.; Yang, Z.; Zhu, L. Geographic pattern of antibiotic resistance genes in the metagenomes of the giant panda. Microb. Biotechnol. 2021, 14, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Lu, L.; Zhang, Z.; Qi, D.; Zhang, M.; O’Connor, P.; Wei, F.; Zhu, Y.G. Insights into the roles of fungi and protist in the giant panda gut microbiome and antibiotic resistome. Environ. Int. 2021, 155, 106703. [Google Scholar] [CrossRef]
- Yang, S.; Deng, W.; Li, G.; Jin, L.; Huang, Y.; He, Y.; Wu, D.; Li, D.; Zhang, A.; Liu, C.; et al. Reference gene catalog and metagenome-assembled genomes from the gut microbiome reveal the microbial composition, antibiotic resistome, and adaptability of a lignocellulose diet in the giant panda. Environ. Res. 2024, 245, 118090. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, Z.; Yao, R.; Xu, L.; Chen, H.; Gu, X.; Wu, T.; Yang, X. Potential Mechanism of Detoxification of Cyanide Compounds by Gut Microbiomes of Bamboo-Eating Pandas. mSphere 2018, 3, e00229-18. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.M.; Leung, C.M.; Ting, H.F.; Sadakane, K.; Yamashita, H.; Lam, T.W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Hyatt, D.; LoCascio, P.F.; Hauser, L.J.; Uberbacher, E.C. Gene and translation initiation site prediction in metagenomic sequences. Bioinformatics 2012, 28, 2223–2230. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Jiang, X.T.; Chai, B.; Li, L.; Yang, Y.; Cole, J.R.; Tiedje, J.M.; Zhang, T. ARGs-OAP v2.0 with an expanded SARG database and Hidden Markov Models for enhancement characterization and quantification of antibiotic resistance genes in environmental metagenomes. Bioinformatics 2018, 34, 2263–2270. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Beals, E.W. Bray-Curtis Ordination: An Effective Strategy for Analysis of Multivariate Ecological Data. In Advances in Ecological Research; MacFadyen, A., Ford, E.D., Eds.; Academic Press: Cambridge, MA, USA, 1984; Volume 14, pp. 1–55. [Google Scholar]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Han, Y.; Huang, Y.; Li, D.; Chai, J.; Deng, L.; Wei, M.; Wu, K.; Zhao, H.; Yang, G.; et al. A comprehensive analysis of antibiotic resistance genes in the giant panda gut. Imeta 2024, 3, e171. [Google Scholar] [CrossRef]
- Huang, G.; Qu, Q.; Wang, M.; Huang, M.; Zhou, W.; Wei, F. Global landscape of gut microbiome diversity and antibiotic resistomes across vertebrates. Sci. Total Environ. 2022, 838, 156178. [Google Scholar] [CrossRef]
- Gao, R.; Hu, Y.; Li, Z.; Sun, J.; Wang, Q.; Lin, J.; Ye, H.; Liu, F.; Srinivas, S.; Li, D.; et al. Dissemination and Mechanism for the MCR-1 Colistin Resistance. PLoS Pathog. 2016, 12, e1005957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, F.; Jia, L.; Yan, H.; Gao, L.; Tian, Y.; Su, X.; Zhang, X.; Lv, C.; Ma, Z.; et al. Edwardsiella piscicida infection reshapes the intestinal microbiome and metabolome of big-belly seahorses: Mechanistic insights of synergistic actions of virulence factors. Front. Immunol. 2023, 14, 1135588. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-Resistant Klebsiella pneumoniae: Virulence Factors, Molecular Epidemiology and Latest Updates in Treatment Options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef]
- Ozer, E.; Yaniv, K.; Chetrit, E.; Boyarski, A.; Meijler, M.M.; Berkovich, R.; Kushmaro, A.; Alfonta, L. An inside look at a biofilm: Pseudomonas aeruginosa flagella biotracking. Sci. Adv. 2021, 7, eabg8581. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Wang, L.; Huang, G.; Hou, R.; Qi, D.; Wu, Q.; Nie, Y.; Zuo, Z.; Ma, R.; Zhou, W.; Ma, Y.; et al. Multi-omics reveals the positive leverage of plant secondary metabolites on the gut microbiota in a non-model mammal. Microbiome 2021, 9, 192. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.; Wan, D.; Wang, X.; Zhang, X.; Zhu, Y.; Guo, J. Pollution characteristics of heavy metals, antibiotic and antibiotic resistance genes in the crested ibis and their habitat across different lifestyle and geography. Environ. Res. 2024, 261, 119701. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.G.; Zhang, J.D.; Lu, L.; Wang, Y.F.; Zhu, D. Captivity increased the abundance of high-risk antibiotic resistance genes in the giant panda gut microbiome. Environ. Res. 2024, 263, 120220. [Google Scholar] [CrossRef]
- Jin, L.; Xie, J.; He, T.; Wu, D.; Li, X. Airborne transmission as an integral environmental dimension of antimicrobial resistance through the “One Health” lens. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4172–4193. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Huang, C.; Han, W.; Gu, P.; Jing, R.; Yang, Q. Antibiotic resistance genes and virulence factors in the plastisphere in wastewater treatment plant effluent: Health risk quantification and driving mechanism interpretation. Water Res. 2025, 271, 122896. [Google Scholar] [CrossRef]
- Yi, G.; Jin, M.K.; Cai, T.G.; Xu, R.; Gou, X.W.; Yang, N.; Feng, Y.L.; Zhang, S.W.; Qi, X.J.; Zhu, Y.G.; et al. Antibiotics and Pesticides Enhancing the Transfer of Resistomes among Soil-Bayberry-Fruit Fly Food Chain in the Orchard Ecosystem. Environ. Sci. Technol. 2024, 58, 18167–18176. [Google Scholar] [CrossRef]
- Kim, Y.; Leung, M.H.Y.; Kwok, W.; Fournié, G.; Li, J.; Lee, P.K.H.; Pfeiffer, D.U. Antibiotic resistance gene sharing networks and the effect of dietary nutritional content on the canine and feline gut resistome. Anim. Microbiome 2020, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Kang, L.; Zhang, Z.; Zhang, D.; Li, P.; Zhang, Q.; Ma, X.; Wang, J.; Hou, Y.; et al. Diversity, functions, and antibiotic resistance genes of bacteria and fungi are examined in the bamboo plant phyllosphere that serve as food for the giant pandas. Int. Microbiol. 2025, 28, 751–763. [Google Scholar] [CrossRef]
- Tan, R.; Jin, M.; Shao, Y.; Yin, J.; Li, H.; Chen, T.; Shi, D.; Zhou, S.; Li, J.; Yang, D. High-sugar, high-fat, and high-protein diets promote antibiotic resistance gene spreading in the mouse intestinal microbiota. Gut Microbes 2022, 14, 2022442. [Google Scholar] [CrossRef]
- Fu, Y.; Jia, F.; Su, J.; Xu, X.; Zhang, Y.; Li, X.; Jiang, X.; Schäffer, A.; Virta, M.; Tiedje, J.M.; et al. Co-occurrence patterns of gut microbiome, antibiotic resistome and the perturbation of dietary uptake in captive giant pandas. J. Hazard. Mater. 2024, 471, 134252. [Google Scholar] [CrossRef]
- Preston, G.M. Plant perceptions of plant growth-promoting Pseudomonas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Hayes, D., Jr.; Wozniak, D.J. Cystic Fibrosis and Pseudomonas aeruginosa: The Host-Microbe Interface. Clin. Microbiol. Rev. 2019, 32, e00138-18. [Google Scholar] [CrossRef] [PubMed]
- Paz-Zarza, V.M.; Mangwani-Mordani, S.; Martínez-Maldonado, A.; Álvarez-Hernández, D.; Solano-Gálvez, S.G.; Vázquez-López, R. Pseudomonas aeruginosa: Pathogenicity and antimicrobial resistance in urinary tract infection. Rev. Chilena Infectol. 2019, 36, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Rekha, N.D.; Gopal, S. Pseudomonas aeruginosa biofilm: Treatment strategies to combat infection. Arch. Microbiol. 2025, 207, 141. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Tenover, F.C.; Nicolau, D.P.; Gill, C.M. Carbapenemase-producing Pseudomonas aeruginosa -an emerging challenge. Emerg. Microbes Infect. 2022, 11, 811–814. [Google Scholar] [CrossRef]
- Obritsch, M.D.; Fish, D.N.; MacLaren, R.; Jung, R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: Epidemiology and treatment options. Pharmacotherapy 2005, 25, 1353–1364. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Lei, T.; Zhang, X.; Yao, J.; He, J.; Liu, H.; Cai, H.; Ji, J.; Zhu, Y.; et al. Genomic epidemiology and ceftazidime-avibactam high-level resistance mechanisms of Pseudomonas aeruginosa in China from 2010 to 2022. Emerg. Microbes Infect. 2024, 13, 2324068. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Gao, C.; Cui, X.; Yang, B.; He, K.; Huang, Q.; Yang, X.; Wen, K.; Xie, J.; Yang, Z.; et al. Metagenome Analysis Reveals Changes in Gut Microbial Antibiotic Resistance Genes and Virulence Factors in Reintroduced Giant Pandas. Microorganisms 2025, 13, 1616. https://doi.org/10.3390/microorganisms13071616
Feng W, Gao C, Cui X, Yang B, He K, Huang Q, Yang X, Wen K, Xie J, Yang Z, et al. Metagenome Analysis Reveals Changes in Gut Microbial Antibiotic Resistance Genes and Virulence Factors in Reintroduced Giant Pandas. Microorganisms. 2025; 13(7):1616. https://doi.org/10.3390/microorganisms13071616
Chicago/Turabian StyleFeng, Wanju, Chenyi Gao, Xinyuan Cui, Bing Yang, Ke He, Qiuyu Huang, Xinru Yang, Kaizhi Wen, Jiadong Xie, Zhisong Yang, and et al. 2025. "Metagenome Analysis Reveals Changes in Gut Microbial Antibiotic Resistance Genes and Virulence Factors in Reintroduced Giant Pandas" Microorganisms 13, no. 7: 1616. https://doi.org/10.3390/microorganisms13071616
APA StyleFeng, W., Gao, C., Cui, X., Yang, B., He, K., Huang, Q., Yang, X., Wen, K., Xie, J., Yang, Z., & Zhu, L. (2025). Metagenome Analysis Reveals Changes in Gut Microbial Antibiotic Resistance Genes and Virulence Factors in Reintroduced Giant Pandas. Microorganisms, 13(7), 1616. https://doi.org/10.3390/microorganisms13071616





