Shifts in Fungal Communities and Potential Functions Under Masson Pine Forest-to-Tea Plantation Conversion in Subtropical China
Abstract
1. Introduction
- (1)
- The conversion of Masson pine forests to tea plantations would initially increase soil pH but decrease SOC and TN, resulting in a reduction in fungal abundance due to a reduction in litter production and alterations in litter quality.
- (2)
- Fertilization and increasing stand age would increase soil nutrients, potentially changing fungal abundance and community composition and improving fungal functional characteristics (such as inhibition of pathogens and enrichment of beneficial fungi).
2. Materials and Methods
2.1. Research Site
2.2. Experimental Design
2.3. Soil Sampling and Physicochemical Properties Analysis
2.4. Soil Microbial Analysis
2.4.1. Soil DNA Extraction and Illumina Sequencing
2.4.2. Quantitative Polymerase Chain Reaction
2.4.3. Sequence Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Environmental Factors
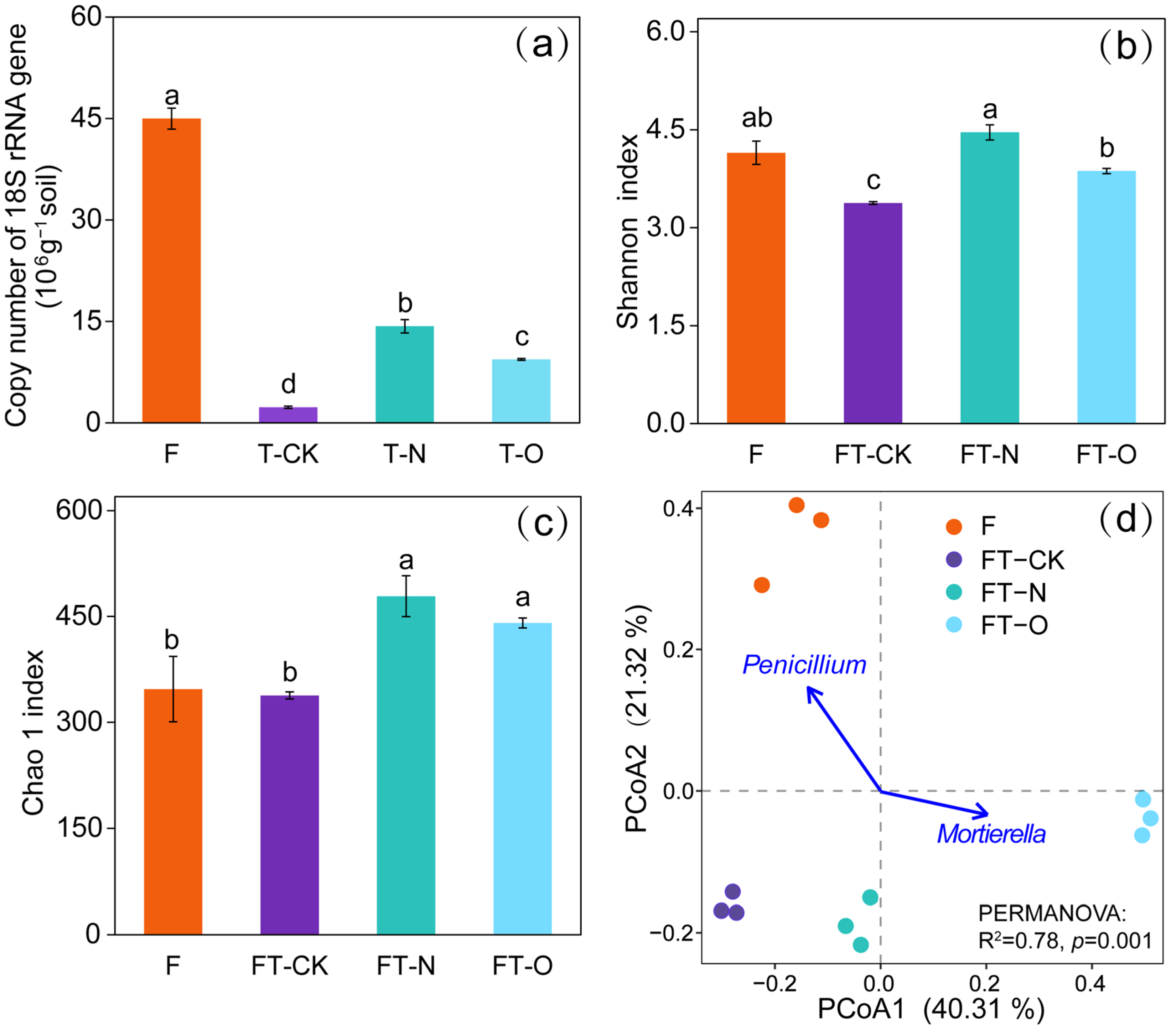
3.2. Soil Fungal Abundance
3.3. Soil Fungal Diversity and Community Composition
3.3.1. Soil Fungal Diversity
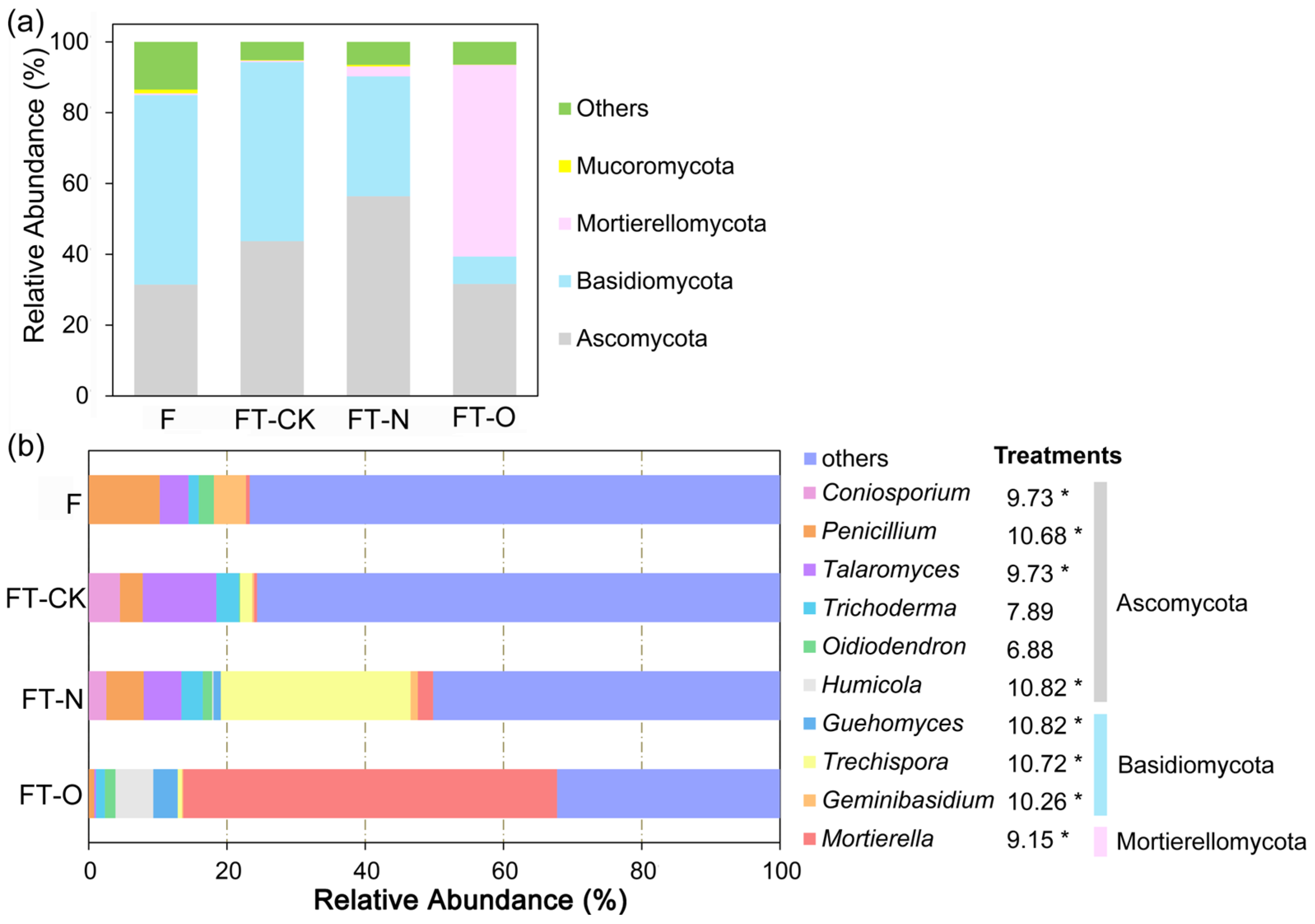
3.3.2. Soil Fungal Community Composition
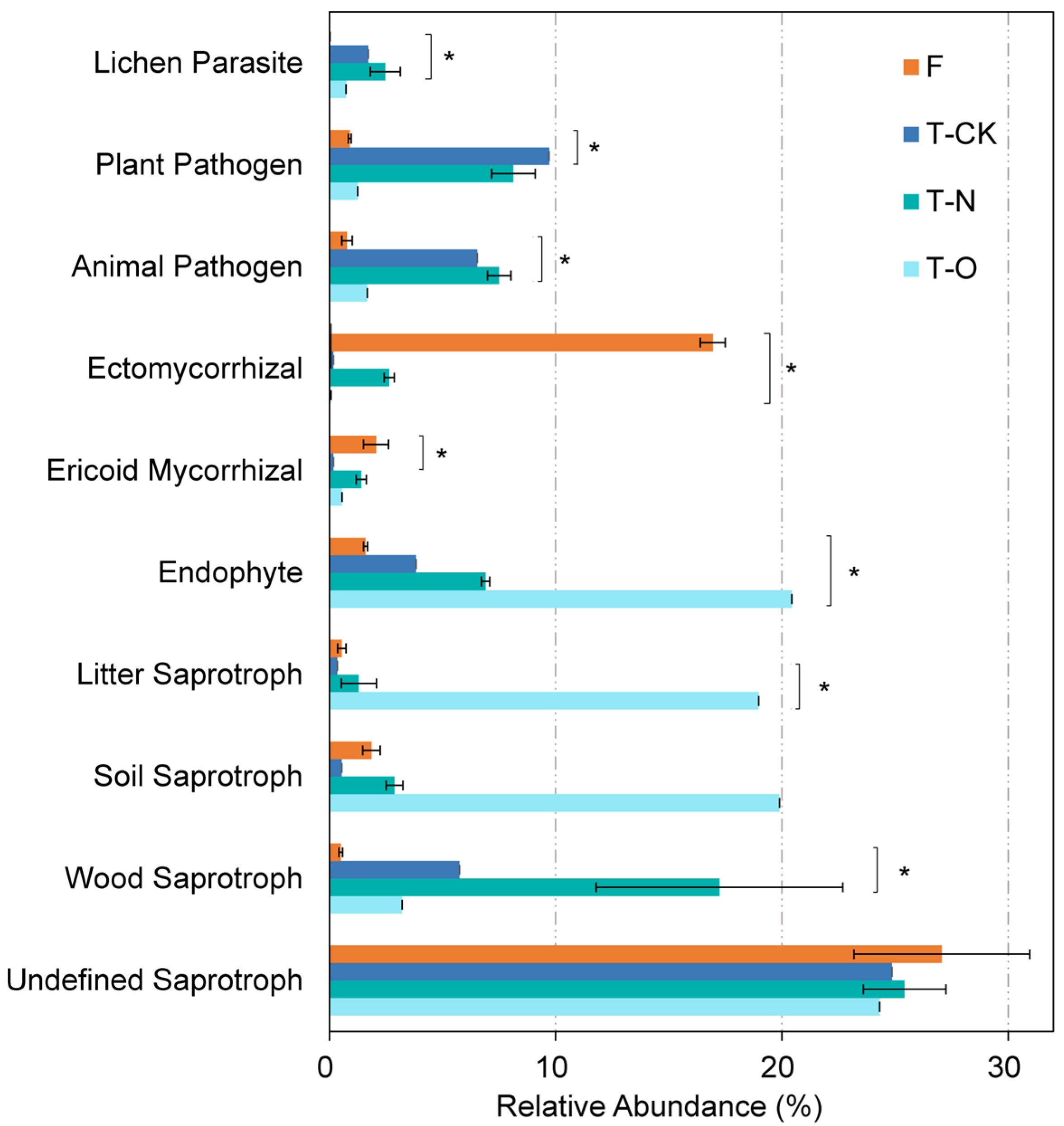
3.4. Soil Fungal Functional Profiles
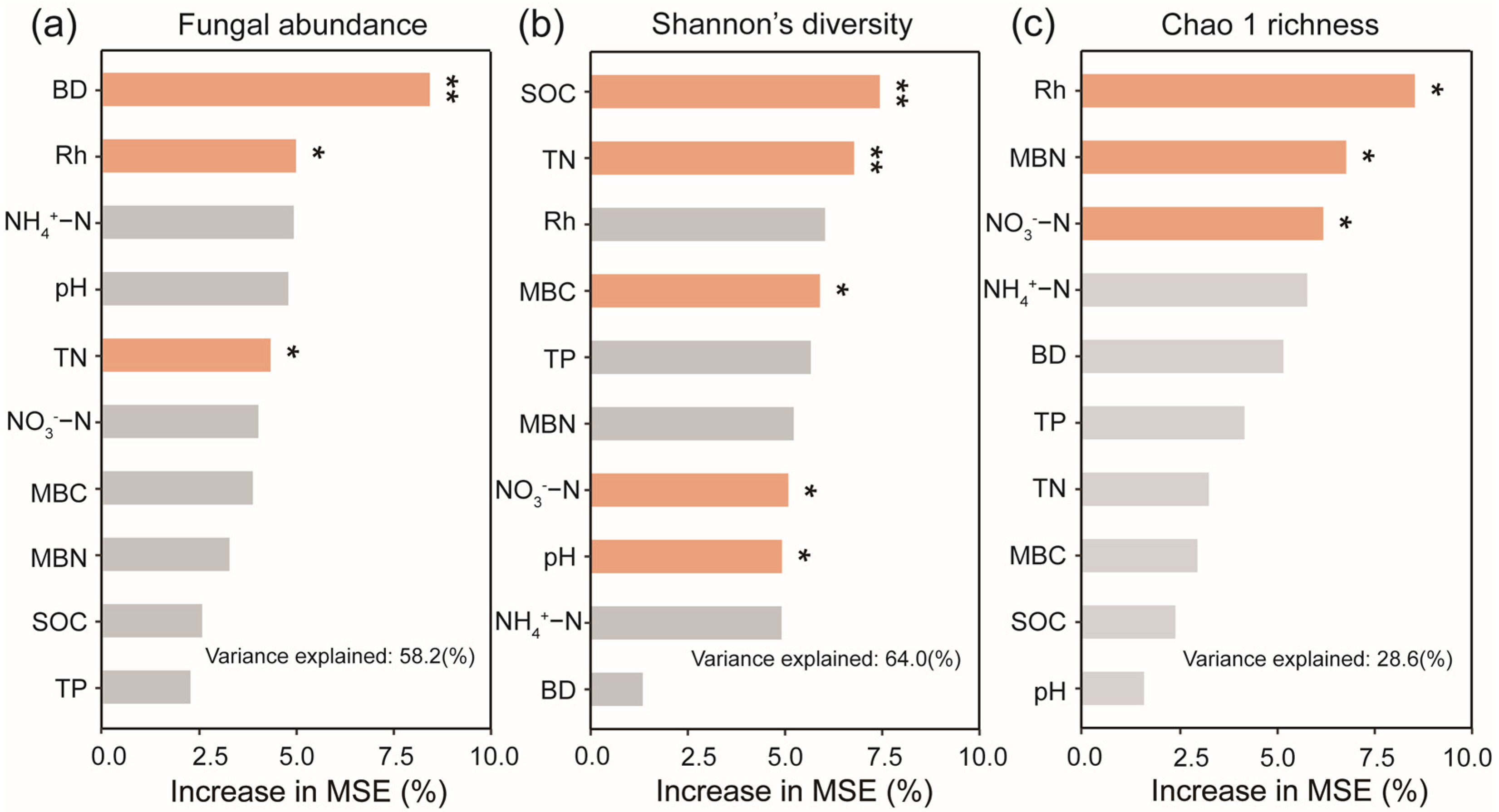
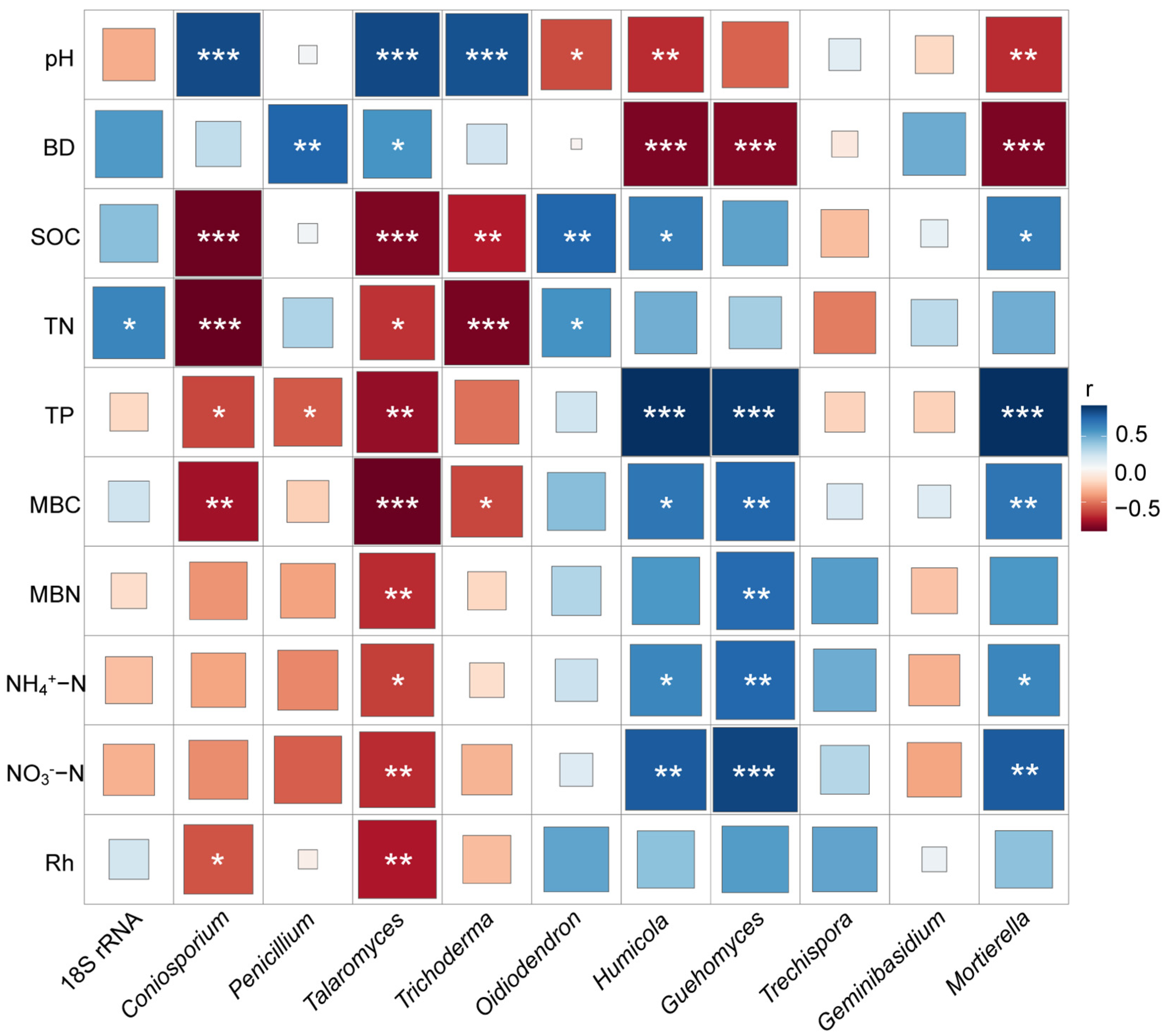
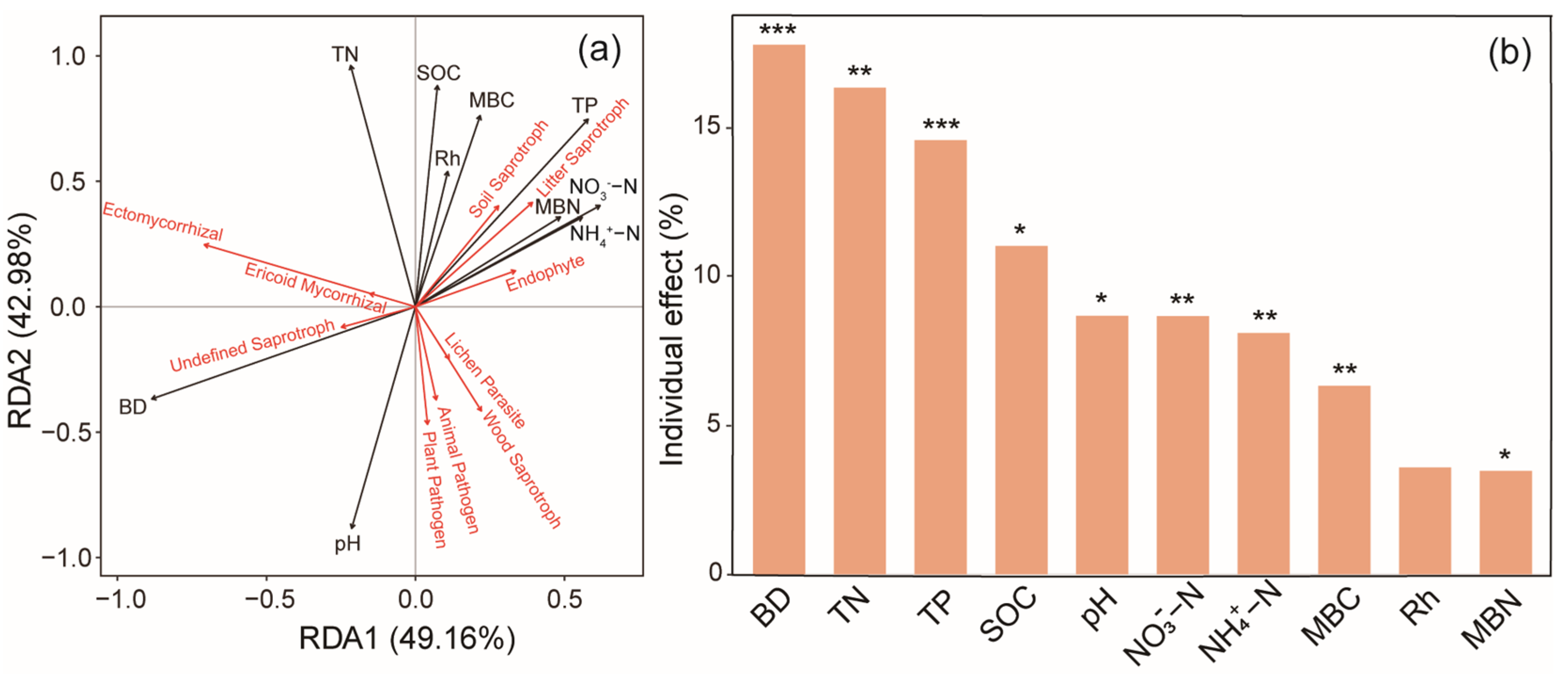
3.5. Relationships Among Fungal Abundance, Community Composition, Functional Groups, and Soil Characteristics
4. Discussion
4.1. Conversion from Masson Pine Forest to Tea Field Decreased Soil Fungal Abundance
4.2. Conversion from Masson Pine Forest to Tea Field-Induced Changes in Fungal Communities
4.3. Impacts of Conversion from Masson Pine Forest to Tea Field on Soil Fungal Functional Groups
4.4. The Present Research Applications and Future Research Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NBSC. China Statistical Yearbook, Annual Publication; National Bureau of Statistics of China: Beijing, China, 2024; Available online: https://www.stats.gov.cn/sj/ndsj/2024/indexeh.htm (accessed on 7 January 2025).
- Zheng, N.; Yu, Y.; Wang, J.; Chapman, S.J.; Yao, H.; Zhang, Y. The Conversion of Subtropical Forest to Tea Plantation Changes the Fungal Community and the Contribution of Fungi to N2O Production. Environ. Pollut. 2020, 265, 115106. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Wang, C.; Liu, X.; Wang, Y.; Shen, J.; Qin, J.; Wu, J. Dynamics and Underlying Mechanisms of N2O and NO Emissions in Response to a Transient Land-Use Conversion of Masson Pine Forest to Tea Field. Sci. Total Environ. 2019, 693, 133549. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Huang, B.H.; Huang, C.L.; Li, G.; Liao, P.C. The Aboveground Vegetation Type and Underground Soil Property Mediate the Divergence of Soil Microbiomes and the Biological Interactions. Microb. Ecol. 2018, 75, 434–446. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Panico, S.C.; Memoli, V.; Santorufo, L.; Zarrelli, A.; Barile, R.; Maisto, G. Differences in Soil Carbon and Nitrogen Pools between Afforested Pine Forests and Natural Shrublands in a Mediterranean Area. Appl. Soil Ecol. 2022, 170, 104262. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; An, S.; Sun, H.; Bhople, P.; Chen, Z. Soil Physicochemical and Microbial Characteristics of Contrasting Land-Use Types along Soil Depth Gradients. CATENA 2018, 162, 345–353. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.; Chen, L.; Wang, J.; Zhang, L. Soil pH Determines Fungal Diversity along an Elevation Gradient in Southwestern China. Sci. China Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef]
- Peng, S.; Liu, W.; Xu, G.; Pei, X.; Millerick, K.; Duan, B. A Meta-Analysis of Soil Microbial and Physicochemical Properties Following Native Forest Conversion. CATENA 2021, 204, 105447. [Google Scholar] [CrossRef]
- Yamashita, N.; Ohta, S.; Hardjono, A. Soil Changes Induced by Acacia mangium Plantation Establishment: Comparison with Secondary Forest and Imperata Cylindrica Grassland Soils in South Sumatra, Indonesia. Forest Ecol. Manag. 2008, 254, 362–370. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.; Li, Y.; Liu, X.; Wang, Y.; Qin, J.; Wu, J. Effects of Land-Use Conversion from Masson Pine Forests to Tea Plantations on Net Ecosystem Carbon and Greenhouse Gas Budgets. Agr. Ecosyst. Environ. 2021, 320, 107578. [Google Scholar] [CrossRef]
- Dai, X.; Yuan, Y.; Wang, H. Changes of Anaerobic to Aerobic Conditions but Not of Crop Type Induced Bulk Soil Microbial Community Variation in the Initial Conversion of Paddy Soils to Drained Soils. CATENA 2016, 147, 578–585. [Google Scholar] [CrossRef]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R.; et al. A Meta-Analysis of Global Fungal Distribution Reveals Climate-Driven Patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef]
- Hu, L.; Li, Q.; Yan, J.; Liu, C.; Zhong, J. Vegetation Restoration Facilitates Belowground Microbial Network Complexity and Recalcitrant Soil Organic Carbon Storage in Southwest China Karst Region. Sci. Total Environ. 2022, 820, 153137. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Y.; Li, J.; Li, X.; Ruan, H.; Bhople, P.; Keiblinger, K.; Mao, L.; Liu, D. Interaction among Soil Nutrients, Plant Diversity and Hypogeal Fungal Trophic Guild Modifies Root-Associated Fungal Diversity in Coniferous Forests of Chinese Southern Himalayas. Plant Soil 2022, 481, 395–408. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Yuan, Y.; Dai, X.; Xu, M.; Wang, H.; Fu, X.; Yang, F. Responses of Microbial Community Structure to Land-Use Conversion and Fertilization in Southern China. Eur. J. Soil Biol. 2015, 70, 1–6. [Google Scholar] [CrossRef]
- Navarro-Noya, Y.E.; Montoya-Ciriaco, N.; Muñoz-Arenas, L.C.; Hereira-Pacheco, S.; Estrada-Torres, A.; Dendooven, L. Conversion of a High-Altitude Temperate Forest for Agriculture Reduced Alpha and Beta Diversity of the Soil Fungal Communities as Revealed by a Metabarcoding Analysis. Front. Microbiol. 2021, 12, 667566. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chapman, S.J.; Yao, H.; Zheng, N.; Müller, C. Tea Plantation Affects Soil Nitrogen Transformations in Subtropical China. J. Soil. Sediment. 2021, 21, 441–451. [Google Scholar] [CrossRef]
- Yang, X.; Ni, K.; Shi, Y.; Yi, X.; Zhang, Q.; Fang, L.; Ma, L.; Ruan, J. Effects of Long-Term Nitrogen Application on Soil Acidification and Solution Chemistry of a Tea Plantation in China. Agr. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Lin, F.; Jiang, C.; Zeng, X.; Feng, D.; Huang, F.; Tang, J. Land Use Change Alters Carbon and Nitrogen Dynamics Mediated by Fungal Functional Guilds within Soil Aggregates. Sci. Total Environ. 2023, 902, 166080. [Google Scholar] [CrossRef]
- Palta, Ş.; Tokel, E.; Baş, E.; Souza, T. Effects of Forest-to-Agriculture Conversion on Soil Arbuscular Mycorrhizal Fungi in the Western Black Sea Region. Soil Till. Res. 2025, 252, 106581. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular No. 939; U.S. Government printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Wu, J.S.; Jorgensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass-C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Hoshino, Y.T.; Morimoto, S. Comparison of 18S rDNA Primers for Estimating Fungal Diversity in Agricultural Soils Using Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis. Soil Sci. Plant Nutr. 2008, 54, 701–710. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Han, W.; Kemmitt, S.J.; Brookes, P.C. Soil Microbial Biomass and Activity in Chinese Tea Gardens of Varying Stand Age and Productivity. Soil Biol. Biochem. 2007, 39, 1468–1478. [Google Scholar] [CrossRef]
- Panico, S.C.; Memoli, V.; Esposito, F.; Maisto, G.; De Marco, A. Plant Cover and Management Practices as Drivers of Soil Quality. Appl. Soil Ecol. 2018, 129, 34–42. [Google Scholar] [CrossRef]
- Pereira, C.M.R.; López-García, Á.; Maia, L.C.; Frøslev, T.G.; Kjøller, R.; Rosendahl, S. Arbuscular Mycorrhizal Fungal Communities of Pristine Rainforests and Adjacent Sugarcane Fields Recruit from Different Species Pools. Soil Biol. Biochem. 2022, 167, 108585. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Frey, B.; Yang, L.; Li, M.-H.; Ni, H. Land Use Change Effects on Diversity of Soil Bacterial, Acidobacterial and Fungal Communities in Wetlands of the Sanjiang Plain, Northeastern China. Sci. Rep. 2019, 9, 18535. [Google Scholar] [CrossRef]
- Fan, L.; Han, W. Soil Respiration after Forest Conversion to Tea Gardens: A Chronosequence Study. CATENA 2020, 190, 104532. [Google Scholar] [CrossRef]
- Wei, Z.; Shan, J.; Well, R.; Yan, X.; Senbayram, M. Land Use Conversion and Soil Moisture Affect the Magnitude and Pattern of Soil-Borne N2, NO, and N2O Emissions. Geoderma 2022, 407, 115568. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.M.; Guhr, A.; Borer, E.T.; Seabloom, E.W.; Spohn, M. Effects of Nitrogen and Phosphorus Addition on Microbial Community Composition and Element Cycling in a Grassland Soil. Soil Biol. Biochem. 2020, 151, 108041. [Google Scholar] [CrossRef]
- Di Marco, M.; Harwood, T.D.; Hoskins, A.J.; Ware, C.; Hill, S.L.L.; Ferrier, S. Projecting Impacts of Global Climate and Land-use Scenarios on Plant Biodiversity Using Compositional-turnover Modelling. Glob. Change Biol. 2019, 25, 2763–2778. [Google Scholar] [CrossRef]
- Ren, C.; Liu, W.; Zhao, F.; Zhong, Z.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Soil Bacterial and Fungal Diversity and Compositions Respond Differently to Forest Development. CATENA 2019, 181, 104071. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, J.; Meng, T.; Zhang, Y.; Yang, J.; Müller, C.; Cai, Z. Tea Plantation Destroys Soil Retention of NO3− and Increases N2O Emissions in Subtropical China. Soil Biol. Biochem. 2014, 73, 106–114. [Google Scholar] [CrossRef]
- Yan, B.; Sun, L.; Li, J.; Liang, C.; Wei, F.; Xue, S.; Wang, G. Change in Composition and Potential Functional Genes of Soil Bacterial and Fungal Communities with Secondary Succession in Quercus Liaotungensis Forests of the Loess Plateau, Western China. Geoderma 2020, 364, 114199. [Google Scholar] [CrossRef]
- Huang, C.; Wu, X.; Liu, X.; Fang, Y.; Liu, L.; Wu, C. Functional Fungal Communities Dominate Wood Decomposition and Are Modified by Wood Traits in a Subtropical Forest. Sci. Total Environ. 2022, 806, 151377. [Google Scholar] [CrossRef]
- Zhang, Y.; Shangguan, Z. Long-Term N Addition Accelerated Organic Carbon Mineralization in Aggregates by Shifting Microbial Community Composition. Agr. Ecosyst. Environ. 2023, 342, 108249. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Shen, J.; Yuan, Q.; Fan, F.; Wei, W.; Li, Y.; Wu, J. Biochar Alters Soil Microbial Communities and Potential Functions 3–4 Years after Amendment in a Double Rice Cropping System. Agr. Ecosyst. Environ. 2021, 311, 107291. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bol, R. Sources and Mechanisms of Priming Effect Induced in Two Grassland Soils Amended with Slurry and Sugar. Soil Biol. Biochem. 2006, 38, 747–758. [Google Scholar] [CrossRef]
- Hoysted, G.A.; Kowal, J.; Jacob, A.; Rimington, W.R.; Duckett, J.G.; Pressel, S.; Orchard, S.; Ryan, M.H.; Field, K.J.; Bidartondo, M.I. A Mycorrhizal Revolution. Curr. Opin. Plant Biol. 2018, 44, 1–6. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Ji, L.; Shi, Y.; Yi, X.; Yang, Q.; Ni, K.; Ruan, J. Long-Term Nitrogen Fertilization Indirectly Affects Soil Fungi Community Structure by Changing Soil and Pruned Litter in a Subtropical Tea (Camellia sinensis L.) Plantation in China. Plant Soil 2019, 444, 409–426. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, B.X.; Xu, C.Y.; Raza, M.; Wang, Q.; Wang, Q.Z.; Fu, Y.N.; Hu, J.Y.; Imoulan, A.; Hussain, M.; et al. Intercropping Walnut and Tea: Effects on Soil Nutrients, Enzyme Activity, and Microbial Communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef]
- Torres-Cruz, T.J.; Hesse, C.; Kuske, C.R.; Porras-Alfaro, A. Presence and Distribution of Heavy Metal Tolerant Fungi in Surface Soils of a Temperate Pine Forest. Appl. Soil Ecol. 2018, 131, 66–74. [Google Scholar] [CrossRef]
- Hei, J.; Wang, S.; He, X. Effects of Exogenous Organic Acids on the Growth, Edaphic Factors, Soil Extracellular Enzymes, and Microbiomes Predict Continuous Cropping Obstacles of Panax notoginseng from the Forest Understorey. Plant Soil 2023, 503, 105–122. [Google Scholar] [CrossRef]
- Osorio, N.W. Effectiveness of phosphate solubilizing microorganisms in increasing plant phosphate uptake and growth in tropical soils. In Bacteria in Agrobiology: Plant Nutrient Management Volume III; Masheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 65–80. [Google Scholar]
- Ning, C.; Egerton-Warburton, L.M.; Mueller, G.M.; Xiang, W.; Yan, W.; Liu, S. Shifts in Ectomycorrhizal Fungal Community Composition during the Early Establishment of Native and Exotic Pine Seedlings. Appl. Soil Ecol. 2021, 157, 103722. [Google Scholar] [CrossRef]
- Liu, T.; Wu, X.; Li, H.; Ning, C.; Li, Y.; Zhang, X.; He, J.; Filimonenko, E.; Chen, S.; Chen, X.; et al. Soil Quality and r—K Fungal Communities in Plantations after Conversion from Subtropical Forest. CATENA 2022, 219, 106584. [Google Scholar] [CrossRef]
- Hannula, S.E.; Di Lonardo, D.P.; Christensen, B.T.; Crotty, F.V.; Elsen, A.; Van Erp, P.J.; Hansen, E.M.; Rubæk, G.H.; Tits, M.; Toth, Z.; et al. Inconsistent Effects of Agricultural Practices on Soil Fungal Communities across 12 European Long-term Experiments. Eur. J. Soil Sci. 2021, 72, 1902–1923. [Google Scholar] [CrossRef]
- Zhang, J.H.; Ge, X.; Pan, D.D.; Qiao, P.L.; Wu, F.Z.; Zhou, X.G. Effects of Vanillin on Cucumber (Cucumis sativus L.) Seedling Rhizosphere Fungal Community Composition. Allelopathy J. 2018, 44, 169–180. [Google Scholar] [CrossRef]
- Su, Y.; Yu, M.; Xi, H.; Lv, J.; Ma, Z.; Kou, C.; Shen, A. Soil Microbial Community Shifts with Long-Term of Different Straw Return in Wheat-Corn Rotation System. Sci. Rep. 2020, 10, 6360. [Google Scholar] [CrossRef]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal Community Composition in Soils Subjected to Long-term Chemical Fertilization Is Most Influenced by the Type of Organic Matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Li, F.; Zhang, S.; Wang, Y.; Li, Y.; Li, P.; Chen, L.; Jie, X.; Hu, D.; Feng, B.; Yue, K.; et al. Rare Fungus, Mortierella Capitata, Promotes Crop Growth by Stimulating Primary Metabolisms Related Genes and Reshaping Rhizosphere Bacterial Community. Soil Bio. Biochem. 2020, 151, 108017. [Google Scholar] [CrossRef]
- Cao, Y.; Thomashow, L.S.; Luo, Y.; Hu, H.; Deng, X.; Liu, H.; Shen, Z.; Li, R.; Shen, Q. Resistance to Bacterial Wilt Caused by Ralstonia Solanacearum Depends on the Nutrient Condition in Soil and Applied Fertilizers: A Meta-Analysis. Agr. Ecosyst. Environ. 2022, 329, 107874. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White Jr, J.F.; Arnold, A.E.; Redman, R.S. Fungal Endophytes: Diversity and Functional Roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Tack, A.J.M.; Wasserman, B.; Liu, J.; Berg, G.; Norelli, J.; Droby, S.; Wisniewski, M. Evidence for Host–Microbiome Co-evolution in Apple. New Phytol. 2022, 234, 2088–2100. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Hyde, K.D.; McKenzie, E.H.C.; Saxena, R.K.; Li, Q. Do All Fungi Have Ancestors with Endophytic Lifestyles? Fungal Divers. 2023, 125, 73–98. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms Underlying the Protective Effects of Beneficial Fungi against Plant Diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- De Marco, A.; Vittozzi, P.; Virzo De Santo, A. Elements Dynamics, from Leaf to Stable Leaf Litter Residue and Soil, for Two Functional Types of Tree Planted on Volcanic Deposits. Plant Soil 2023, 482, 127–140. [Google Scholar] [CrossRef]
- Yang, X.; Yi, X.; Ni, K.; Zhang, Q.; Shi, Y.; Chen, L.; Zhao, Y.; Zhang, Y.; Ma, Q.; Cai, Y.; et al. Patterns and Abiotic Drivers of Soil Organic Carbon in Perennial Tea (Camellia sinensis L.) Plantation System of China. Environ. Res. 2023, 237, 116925. [Google Scholar] [CrossRef]
- Zou, Z.; Mi, W.; Li, X.; Hu, Q.; Zhang, L.; Zhang, L.; Fu, J.; Li, Z.; Han, W.; Yan, P. Biochar Application Method Influences Root Growth of Tea (Camellia sinensis L.) by Altering Soil Biochemical Properties. Sci. Hortic. 2023, 315, 111960. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Ou, X.; Chen, D.; Li, Y.; McMillan, C.; Ge, T.; Liu, J.; Xue, M.; Wang, C.; Shen, W. Shifts in Fungal Communities and Potential Functions Under Masson Pine Forest-to-Tea Plantation Conversion in Subtropical China. Microorganisms 2025, 13, 1614. https://doi.org/10.3390/microorganisms13071614
Ma X, Ou X, Chen D, Li Y, McMillan C, Ge T, Liu J, Xue M, Wang C, Shen W. Shifts in Fungal Communities and Potential Functions Under Masson Pine Forest-to-Tea Plantation Conversion in Subtropical China. Microorganisms. 2025; 13(7):1614. https://doi.org/10.3390/microorganisms13071614
Chicago/Turabian StyleMa, Xiaofang, Xiaofang Ou, Dan Chen, Yong Li, Cameron McMillan, Tida Ge, Ji Liu, Min Xue, Cong Wang, and Weijun Shen. 2025. "Shifts in Fungal Communities and Potential Functions Under Masson Pine Forest-to-Tea Plantation Conversion in Subtropical China" Microorganisms 13, no. 7: 1614. https://doi.org/10.3390/microorganisms13071614
APA StyleMa, X., Ou, X., Chen, D., Li, Y., McMillan, C., Ge, T., Liu, J., Xue, M., Wang, C., & Shen, W. (2025). Shifts in Fungal Communities and Potential Functions Under Masson Pine Forest-to-Tea Plantation Conversion in Subtropical China. Microorganisms, 13(7), 1614. https://doi.org/10.3390/microorganisms13071614







