1. Introduction
Ormosia henryi Prain (
Figure 1), as a species of the
Ormosia genus of Leguminosae in China, has high ecological and economic value, and is prized as both precious timber and an ornamental landscape tree.
Ormosia henryi seeds are pod-type seeds. Each pod contains approximately four to eight seeds, exhibiting a distinct masting phenomenon [
1]. Their 1000-seed weight ranges from approximately 350 to 450 g, and seeds measure 0.75–1.27 cm in length and 0.72–1.04 cm in width. Due to their large size and weight, the seeds are primarily dispersed near the parent tree, relying on natural germination for regeneration [
2]. A single
O. henryi tree produces around 2000 pods, with a seed extraction rate of approximately 49%, indicating low seed set and utilization efficiency. Under natural conditions, the seed germination rate and seedling regeneration exhibit significant variability [
3]. Furthermore, artificially cultivated seedlings demonstrate poor stress tolerance, reduced root nodulation, and drought sensitivity, resulting in weak, spindly growth and low survival rates.
In sowing practices, the seed dormancy of
O. henryi is broken through scarification using either 80 °C hot water or 95% concentrated sulfuric acid. However, these methods often cause physical damage and nutrient loss, resulting in reduced seed vigor, compromised seedling development, and diminished stress resistance [
4]. While seed germination is a critical phase in plant propagation, the hard-seeded characteristics of
O. henryi, including impermeable seed coats and structural barriers, inherently limit germination rates and hinder regeneration. Therefore, there is an urgent need to find a suitable means to solve the problems of a low germination rate, as well as the vitality and poor stress resistance of
O. henryi seeds, which are of great significance for expanding the germplasm resources of
O. henryi and improving the seedling system.
The spermosphere (
Figure 2), scientifically defined as the 1~10 mm microbial hotspot surrounding germinating seeds, represents a critical ecological niche where seed-derived metabolites stimulate enhanced biological activity during germination [
5]. This dynamic interface hosts complex microbial communities whose metabolic interactions are fundamentally shaped by rhizodeposition processes and cross-kingdom signaling exchanges, ultimately exerting profound impacts on plant developmental trajectories [
6]. Germinating seeds release exudates within the spermosphere, creating a suitable environment for microbial growth [
7]. Similarly, germinating seeds recruit specific microbes into the spermosphere based on changes in these exudates [
8,
9,
10]. Seed exudates constitute a significant component of the spermosphere alongside its microbial community, influencing the growth and development of surrounding soil microorganisms. Sugars serve as crucial energy sources for both plants and soil microbes, while amino acids play vital roles in microbial colonization, biomass accumulation, phosphate solubilization activity, and biofilm formation. Amino acids also act as mediators in plant–bacteria interactions, as many bacterial species possess genes enabling them to sense specific amino acids, thereby inducing chemotaxis in beneficial microbial communities. For instance, Dong et al. [
11] reported that amino acids such as arginine, alanine, and lysine in cotton root exudates significantly promote the aggregation of the
Bacillus subtilis strain NCD−2. Increased abundance and the colonization intensity of NCD−2 correlate with enhanced biocontrol efficacy against cotton Verticillium wilt. Consequently, the interactions between seed exudates and spermosphere microorganisms significantly impact seed germination and the recruitment of beneficial microbes within the spermosphere [
6].
Contemporary research has elucidated the functional significance of specific spermosphere microbiota in modulating seed physiology across major agricultural systems:
Bacillus velezensis enhances maize (
Zea mays) germination through dual mechanisms of antifungal metabolite production and phytohormone regulation [
12].
Methylobacterium oryzae orchestrates tomato (
Solanum lycopersicum) seed activation via ACC deaminase-mediated ethylene modulation and auxin biosynthesis [
13].
Sphingomonas melonis demonstrates rice (
Oryza sativa) spermosphere colonization efficiency through biofilm-mediated pathogen exclusion and ROS-scavenging enzymatic systems [
14]. Notably, diverse microbial taxa including
Pseudomonas spp. [
15], Bacteroidetes strains [
16], and
Azospirillum brasilense [
17] have been shown to exhibit growth-promoting effects through nitrogen fixation, nutrient solubilization, and stress tolerance enhancement. This accumulating evidence strongly supports the paradigm that spermosphere microbiota serve as biochemical catalysts initiating critical germination processes. Despite these advances in herbaceous species, significant knowledge gaps persist regarding the microbial drivers of germination in perennial woody plants.
Seed germination, constituting a pivotal yet vulnerable phase in plant ontogeny, represents a metabolically intensive process susceptible to environmental perturbations and biotic challenges. To address the instability of this developmental transition, the biopriming method has been developed as an ecologically sustainable approach for synchronizing germination schedules while enhancing stress resilience [
18]. Seed priming is a pre-sowing seed treatment technique that prepares the plant for future adversity by pre-exposing it to a known initiator, leading to greater survival under adverse environmental conditions. The seed priming technique involves two principal modalities: biotic and abiotic priming; the technique includes beneficial microorganisms that are inoculated the surface of a seed to establish antagonistic effects and achieve a promotion of the germination rate, seed vigor, and emergence uniformity, which is often called “biopriming” [
19].
Empirical evidence has demonstrated the biostimulatory potential of a spermosphere microbial community. Rehman A, et al. [
20] found that wheat yield was improved by the method of seed priming through a combination of microorganisms and Zn. Cyanobacterial inoculants enhanced maize (
Zea mays) germination indices through the dual modulation of nitrogen metabolism and micronutrient bioavailability [
21].
Pseudomonas fluorescens elevates East Indian sandalwood seeds’ germination efficiency via synergistic production of the plant growth hormone, organic acid, a fixation of atmospheric nitrogen, the solubilization of phosphate, and antibiotics production [
18]. These findings underscore the potential for spermosphere microorganisms to reprogram seed metabolism through reactive oxygen homeostasis modulation, reserve mobilization enzyme activation, and plant growth hormone production.
Based on the above studies, whether the inoculated indigenous or non-indigenous spermosphere strains played an important role in seed germination still needs to be determined. Previous research has confirmed that spermosphere bacteria promote the germination of
O. henryi seeds by regulating seed metabolic pathways [
22]. Furthermore, by investigating the differences in spermosphere microbial communities across various soil media, the dynamics of spermosphere bacterial communities during different germination stages, functional predictions, and changes in the spermosphere bacterial network structure over germination time, we elucidated key bacterial species promoting
O. henryi seed germination in natural soil. It was also revealed that the spermosphere bacterial community exhibits a functional group transition pattern across germination stages: shifting from decomposer-dominant, to antagonist-dominant, and finally to nutrient provider-dominant communities [
3]. These findings demonstrate that spermosphere bacteria collectively regulate the natural germination of
O. henryi seeds through synergistic interactions. However, the specific physiological and biochemical mechanisms by which individual bacteria promote germination remain poorly understood.
Therefore, this study focuses on hard-seeded O. henryi, using functionally superior spermosphere bacterial strains isolated and screened from wild O. henryi spermosphere soil as inoculants. The aims of this study are (1) to characterize and identify the functional traits and molecular taxonomy of the spermosphere bacterial isolates, (2) to investigate microscopic structural changes in the seed coat induced by spermosphere bacteria during the early germination phase, (3) to determine the differences in hormone levels, storage compounds, energy status, and seed germination-related enzyme activities between bioprimed seeds and sterilely-primed seeds at various germination stages, and (4) to elucidate the internal physiological and biochemical changes triggered by spermosphere bacterial priming, thereby revealing the underlying mechanisms driving O. henryi seed germination. This research will provide a theoretical basis for the chemotaxis and long-term colonization of functional spermosphere bacteria, ultimately aiming to develop these beneficial strains into green bio-fertilizers for practical application.
2. Materials and Methods
2.1. Isolation and Purification of Spermosphere Strains
The spermosphere soils of the two functional strains isolated and purified were, respectively, from Pingtang County and Guanling Buyi and Miao Autonomous County in Guizhou Province, China. Spermosphere soil suspension was prepared by homogenizing 10 g of soil with 90 mL of sterile water and six glass beads in a 250 mL conical flask. The mixture was agitated at 180 rpm for 30 min using a magnetic stirrer. Serial dilutions (10−3, 10−4, 10−5, 10−6, 10−7) of the suspension were prepared. Aliquots (0.1 mL) from each dilution were spread-plated onto a beef extract peptone agar medium. After incubation at 28 °C for 2–4 days, morphologically distinct colonies were aseptically subcultured onto fresh beef extract peptone agar plates using an inoculating loop. Pure strains were obtained through iterative streak-plate isolation until stable colony phenotypes were observed.
2.2. Determination of Functional Activity of Spermosphere Strains
The purified strains were inoculated on solid mediums [
23] for amylase production, cellulase production, phosphate-solubilizing, potassium-solubilizing, and nitrogen-fixing, respectively. The inoculated strains were cultured at 30 °C for 5 days, with three biological replicates per treatment. The capacities of starch hydrolysis, cellulose hydrolysis, phosphate-solubilization, nitrogen-fixation, and potassium-solubilization were quantified by calculating the ratios of functional zone diameters to the colony diameters.
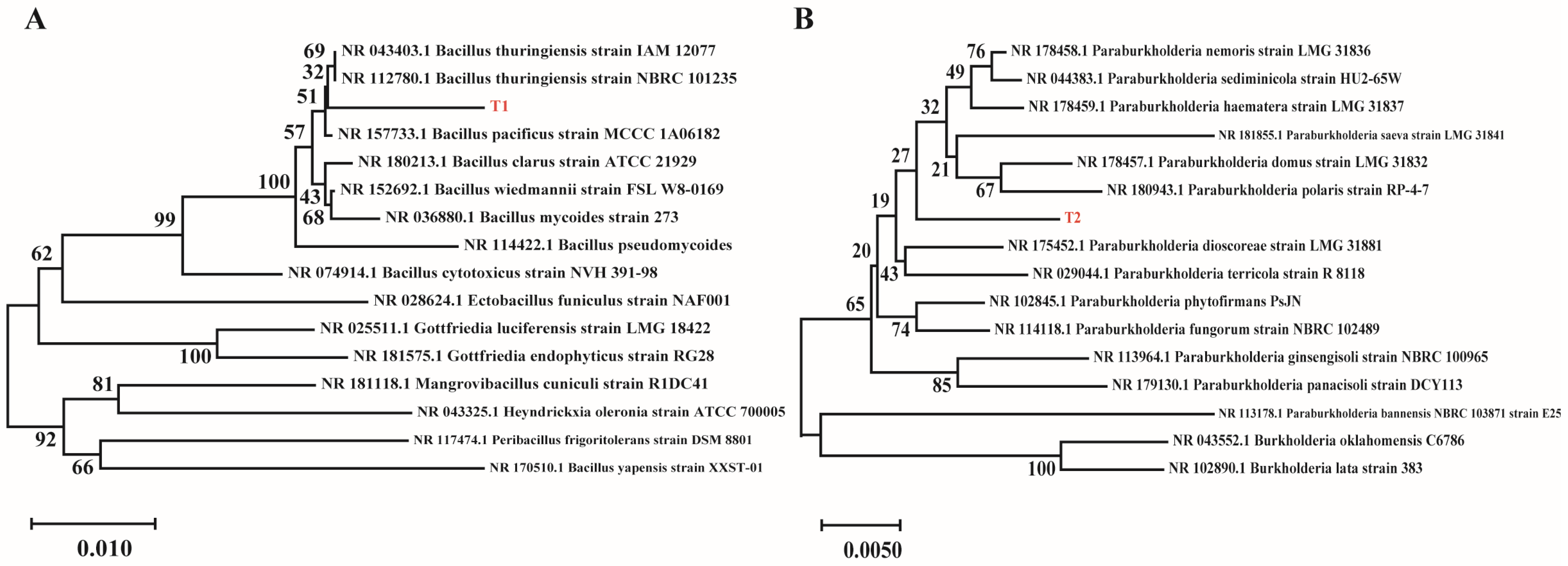
2.3. Molecular Identification of Spermosphere Strains
Genomic DNA was extracted from purified functional bacterial strains using a Bacterial Genomic DNA Extraction Kit (Sangon Biotech Co., Ltd., Shanghai, China). DNA samples (1–2 μL) were electrophoresed on 1% agarose gels containing GelRed at 110 V for 30 min, with the DL2000 DNA Marker as a reference. All samples exhibited clear, singular high-molecular-weight bands without degradation or low-molecular-weight smearing. The 16S rRNA gene was amplified by PCR using the universal primers 27F and 1492R [
24] (5′-GGTTACCTTGTTACGACTT-3′), and the PCR products were purified by the 2× BenchTop™ Taq Master Mix (BIOMIGA, San Diego, CA, USA). The 50 μL reaction mixture contained the following: 25 μL of Master Mix, 2 μL of template DNA, 2 μL of each primer, and 19 μL of ddH
2O. The procedure conditions of PCR amplification were as follows: pre-denaturation (1 min 30 s at 94 °C), denaturation (30 s at 94 °C), annealing (30 s at 57 °C), extension (60 s at 72 °C), and final elongation (5 min at 72 °C). The final PCR amplified products were stored at 4 °C. Among them, the steps of denaturation, annealing, and extension were continuously cycled 30 times. Purified PCR products underwent bidirectional Sanger sequencing using the dideoxy chain termination method (ABI 3730 xl, Sangon Biotech, Shanghai, China). The sequences were blasted in the GenBank database to obtain accession numbers. Phylogenetic trees were constructed using MEGA 7.0 to determine taxonomic classification.
2.4. Biopriming Experimental Design
The seeds used for the biopriming experiment were collected in late November 2021 from the single mother tree of
O. henryi in Qinglong County (105°13′23.07″ W, 26°0′53.97″ N), Guizhou Province, China, with a 1000-grain weight of 362.35 ± 8.38 g. The surface dirt was cleaned with sterile water, and healthy, full, and uniform size seeds were selected for the test. The two strains (T1 and T2) were isolated and purified from the soil and inoculated onto
O. henryi seeds to assess physiological indices and germination rates. The strains were transferred into a beef extract–peptone liquid medium [
23] and cultured overnight at 30 °C with continuous shaking at 150 rpm. The bacterial suspension was adjusted to an OD
600 of 1.0 (about 10
8 CFU·mL
−1).
The seeds were soaked in 4% sodium hypochlorite (NaClO) and 75% ethanol for 10 min and 30 s, respectively, and rinsed 2–3 times with sterile water for surface sterilization. The surface-sterilized seeds were then immersed in respective bacterial suspensions (different strains) for 4 h under controlled conditions. Following surface sterilization, the seeds were transferred to germination chambers lined with pre-saturated sterilized absorbent cotton (pre-treated with sterilized distilled water). The control groups included (1) seeds were soaked in sterilized water (CK1), and (2) seeds immersed in a sterilized beef extract–peptone liquid medium (CK2). Each chamber contained 70 seeds, with six biological replicates arranged in a split-plot design—with three replicates allocated for destructive sampling, and the other three replicates reserved for germination rate quantification and physiological index measurements. The germination conditions were maintained at 25 °C with a 12 h photoperiod during daytime, and 20 °C with a 12 h dark period at night. All treatments were inoculated twice during the experiment, with 10-day intervals between inoculations. Throughout the experimental period, the seeds received regular quantitative water supplementation.
Germination was defined as the period spanning from initial seed imbibition to epicotyl extension. Samples were collected at four developmental stages: (I) imbibition, (II) radicle protrusion, (III) radicle elongation, and (IV) epicotyl extension. All collected samples were processed uniformly for subsequent analysis.
2.5. Determination of Germination Indicators
The germination rate (GR) was counted after 20 days, and the germination index (GI) and germination potential (GP) were calculated through the following formulas.
In Formula (2), the percentage of the number of seeds germinated within the first 1/3 period (7 d) specified in the germination test to the number of seeds tested is generally taken as the standard.
In Formula (3) [
25], Gt represents the number of germination seeds on day t; Dt represents the number of days of germination. Take the arithmetic mean of the parallel measurement results as the final analysis result, and retain the calculation result to two decimal places.
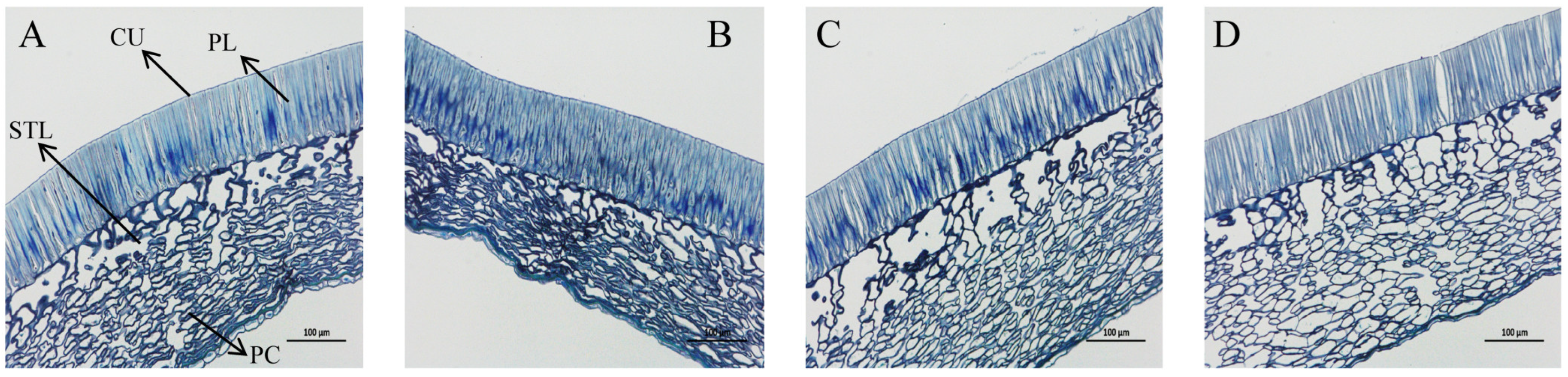
2.6. Observation of Seed Coat Microstructure
Samples of the seed coats (inoculated and uninoculated with the strain of T1) from the same part in the discoloration stage were taken. The imbibition stage of seeds was respectively selected, then the seeds were fixed with an FAA fixative solution (48 h), and pumped. The preparation method and steps of paraffin sections were as follows: The fixed seed coats were dehydrated in a series of concentrations of ethanol 75%, 85%, 90%, 95%, 100%, and 100% for 4 h, 2 h, 2 h, 1 h, 30 min, and 30 min, respectively. Then, they were placed in a room temperature setting to obtain transparency, with ratios of ethanol to xylene of 1:1, 0:1, 0:1, and 0:1, respectively, and the transparency time was 30 min for each. After that, the xylene in the tissue was slowly replaced with xylene and paraffin in a solution with volume ratios of 1:1, 0:1, and 0:1 at 60 °C~62 °C, and each concentration was treated for 3 h. After the waxing was completed, the tissue was embedded and trimmed, and cut into 8 μm thick sections in a paraffin sectioning machine (Laica RM2235, Wetzlar, Germany). After pasting, spreading, and baking, the dried sections were immersed in xylene for dewaxing for 40 min, and rehydrated in ethanol series concentration gradients (100%, 100%, 75%) for 5 min. After rehydration, the sections were stained with 1% toluidine blue for 2–5 min, immersed in tap water for 30 s, then 95% ethanol for about 20 s, and finally, dehydrated with anhydrous ethanol 2 times, for 5 min each; they were also made transparent with xylene after being exposed 2 times, for 5 min each. The transparent sections were permanently sealed with neutral gum and coverslips. Finally, the sections were observed under an optical microscope (Laica DM2500, Wetzlar, Germany) and typical images were selected for photographing.
2.7. Determination of Physiological and Biochemical Indexes of Seeds
Germinating seed samples were collected at four stages: imbibition (I), radicle protrusion (II), radicle elongation (III), and epicotyl extension (IV). All samples (including the seed coat and cotyledon) were ground with liquid nitrogen and mortar, and sieved through 20 mesh to determine physiological and biochemical indexes. All samples were collected and stored at −80 °C, and finally determined uniformly.
Gibberellin and abscisic acid levels were determined by an enzyme-linked immunosorbent kit (ELISA) (Jiangsu Meimian Industrial Co., Ltd., Yancheng, China); glucose content was determined by the hexokinase method (HK). Soluble protein content was determined by the BCA (bicinchoninic acid) method, the cellulose content was determined by sulfuric acid-anthrone colorimetry, and amylase activity was determined by the 3,5-dinitrosalicylic acid method. All physiological and biochemical activity indicators except endogenous hormones were determined using the kits of Jiangsu Grace Biotechnology (Suzhou, China) [
26].
2.8. Statistical Analysis
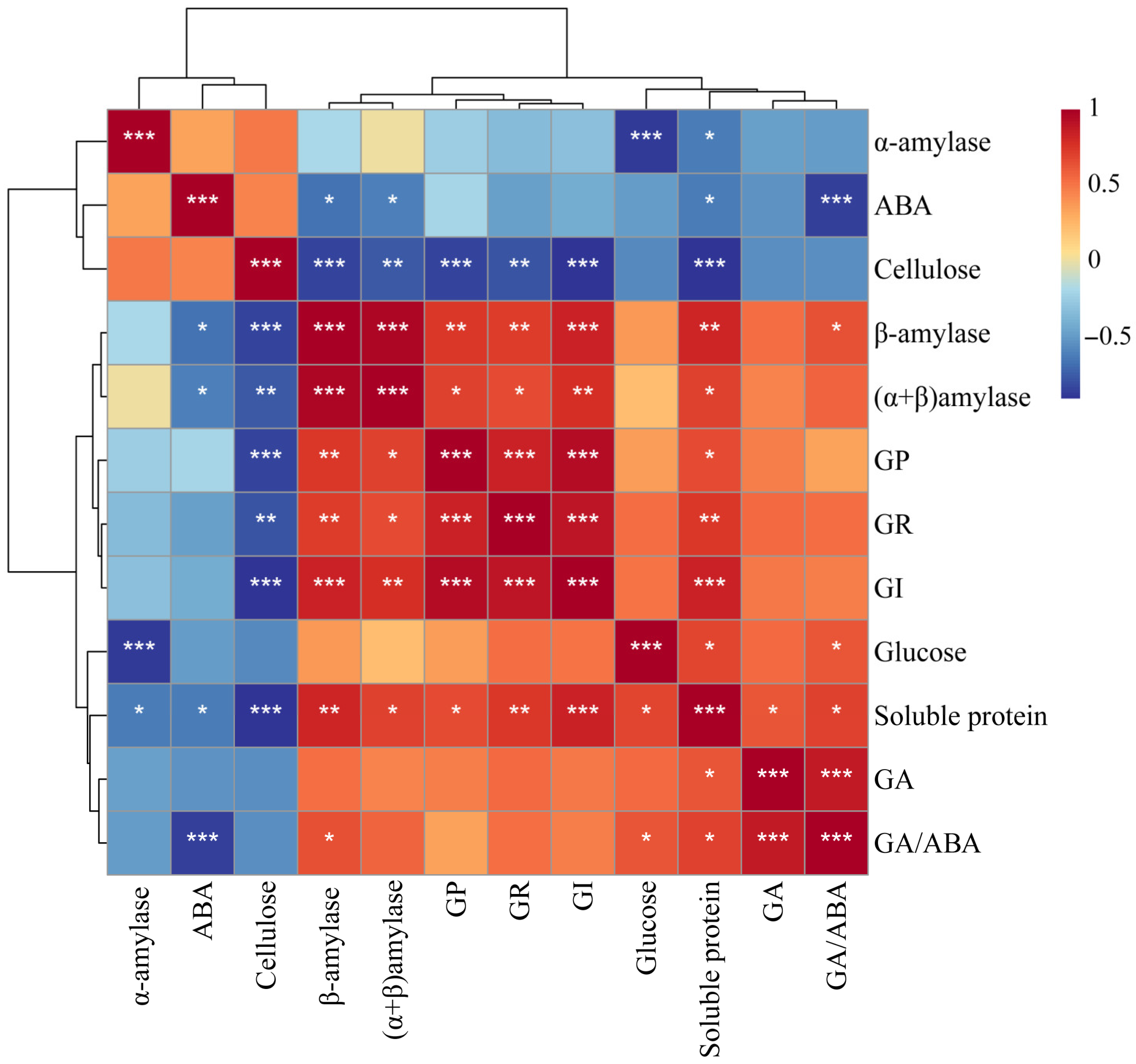
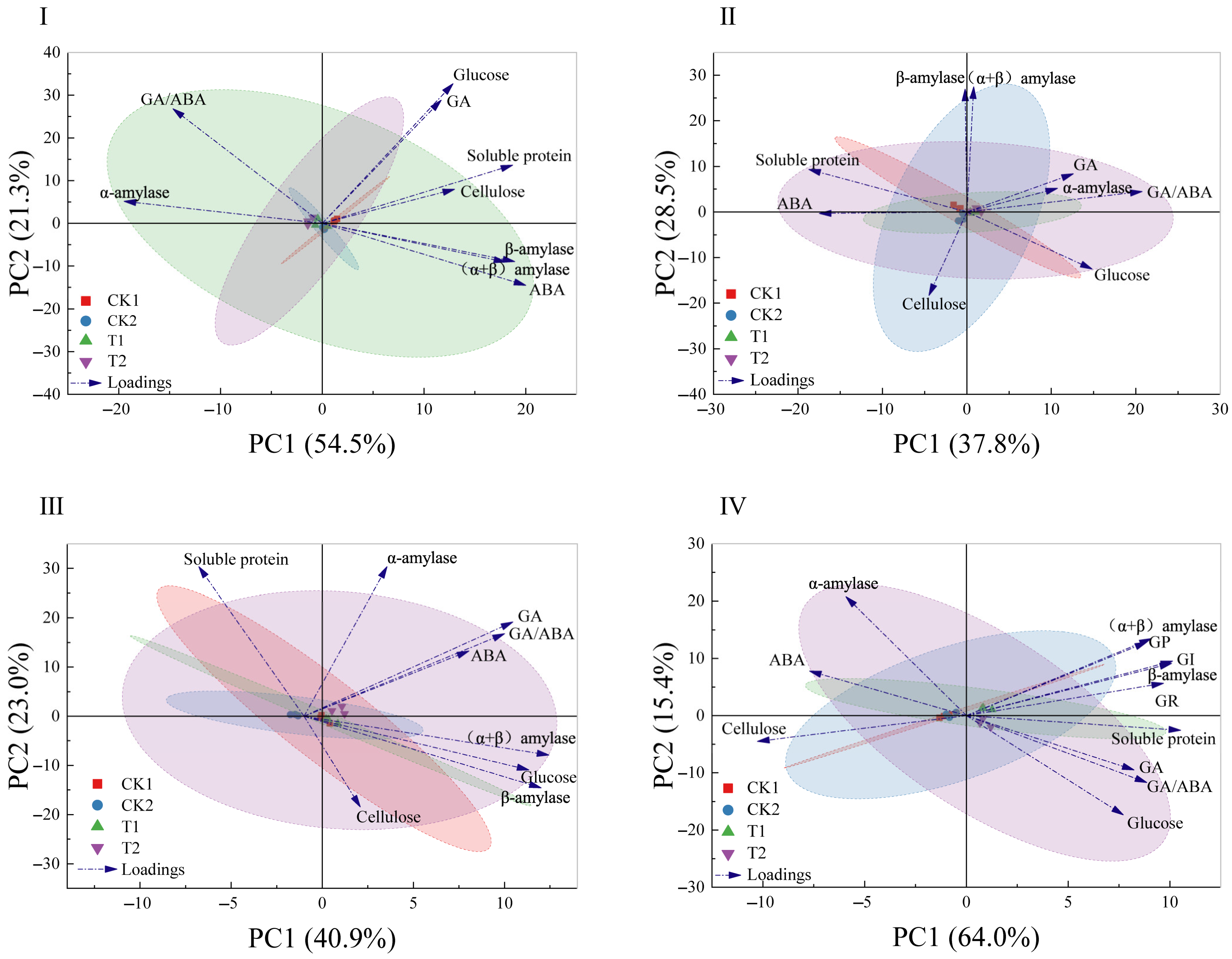
Excel table and SPSS 26.0 were used for data processing. Mean ± SE was used for seed germination indicators and physiological and biochemical indexes at different stages of seed development after inoculation with a strain. Prior to performing one-way ANOVA, data should undergo normality testing and a homogeneity of variance assessment. If the normality assumption is violated, apply logarithmic transformation to approximate normal distribution. When homogeneity of variance is confirmed, use LSD post hoc testing following ANOVA. If heterogeneity of variance is detected, employ Dunnett’s T3 nonparametric analysis. Two-way ANOVA was used to study the primary effects of the germination stages and biopriming treatments and their interactions on various physiological and biochemical indexes. The Pearson correlation coefficient was used to estimate the correlation between germination indexes and physiological and biochemical indexes. A principal component analysis (PCA) was employed to further analyze the physiological and biochemical indicators of O. henryi seeds in different germination stages. A difference was considered statistically significant at the probability level of p < 0.05. Figures were produced using OriginPro Version 2024 (OriginLab Co., Northampton, MA, USA).
4. Discussion
O. henryi is classified as a rare and endangered species under second-class national protection in China, and serves as a key timber species for national strategic forest reserves. This status results from its narrow, fragmented distribution, low pod yield and seed germination rates, severe anthropogenic disturbances, and limited wild populations [
27]. Seed germination represents a critical prerequisite for population continuity, enabling range expansion and the colonization of new territories, while providing the foundation for maintaining genetic diversity and adaptive evolution. Given its low vegetative propagation success,
O. henryi primarily relies on seedling propagation. However, cultivation faces significant constraints: low germination rates, poor stress tolerance in seedlings induced through physical/chemical methods, and irregular growth patterns collectively impede population regeneration and expansion.
Recent research on dormancy-breaking mechanisms reveals two primary germination barriers: the impermeable seed coat characterized by a thick cuticle, palisade and sclerenchymatous tissues, surface wax and lipid deposits, and the presence of endogenous inhibitory hormones. These structural and biochemical features collectively cause low permeability and, consequently, minimal germination in untreated seeds. As intimate partners during germination, spermosphere microorganisms play crucial roles in mediating environmental stress responses. Being a rhizobia-symbiotic legume, O. henryi likely benefits from spermosphere bacteria during germination. Seed priming—a key technique for enhancing vigor through rapid germination and optimized field establishment—may be revolutionized using functionally superior spermosphere bacteria as biopriming agents. Such biopriming could positively regulate germination physiology through metabolic, hormonal, and energetic pathways.
4.1. Effects of Spermosphere Microorganisms on Seed Germination of Ormosia henryi
In the previous research on spermosphere bacteria in
O. henryi seed germination, it was demonstrated that during interactions between spermosphere microbiota and seed exudates, key bacterial taxa exhibit enriched abundance and functional roles in promoting hard-seed germination, including
Bacillus sp., norank_o__Acidobacteriales,
Nitrospira sp. [
22]. Furthermore, the following critical seed exudates were identified: L-lysine, L-isoleucine, α-D-glucose, and raffinose. These bacteria likely facilitate germination by modulating hormone- and energy-related metabolic pathways through their functional traits, exemplified by the following: galactose metabolism, lysine degradation, valine, leucine, and isoleucine degradation [
3]. Concurrently, they mobilize seed energy reserves, accelerating germination kinetics. In the soils supporting high germination rates, an enriched recruitment of functional spermosphere bacteria was observed, notably
Mesorhizobium sp.,
Massilia sp., and
Burkholderia-Caballeronia-Paraburkholderia sp., which showed significant positive correlations with germination rates. These taxa contribute to nitrogen fixation, biocontrol, and stress tolerance.
The current findings validate previous studies. Herein, the physiological mechanism of
Bacillus sp. T1—an indigenous multifunctional strain exhibiting phosphate solubilization, potassium release, nitrogen fixation, amylase production, and cellulase production—was revealed. This bacterium primarily enhances germination by biodegrading the impermeable seed coat, modulating the ratio of GA to ABA, and balancing amylase synthesis to release physiological dormancy. This is consistent with the research results of Hu et al. [
28]; in this study,
Bacillus QM3 significantly promoted the expression of TaGA3ox-B2 in wheat seeds, thereby inducing the biosynthesis of GA and further promoting germination. This study elucidates the biochemical basis of spermosphere bacteria-mediated germination in
O. henryi. 4.2. Advantages of Biopriming and Functional Characteristics of Spermosphere Strains
As an innovative approach, seed biopriming demonstrates significant potential in improving seed health through alleviating biotic and abiotic stresses, which effectively enhances seed uniformity and seedling quality [
29]. This method has been proven to be particularly beneficial for functional applications in both spermosphere and rhizosphere environments. Spermosphere microorganisms, being the primary responders to seed exudates, actively participate in metabolic dynamics during germination. Their interactions with seeds provide critical insights into the microbial-mediated metabolic mechanisms that stimulate germination [
22]. Effective colonization by beneficial bacteria, particularly those originating from the host’s spermosphere, ensures sustained functional impacts on plant development [
30]. Notably, the spermosphere harbors numerous beneficial bacteria exhibiting multifaceted capabilities including phosphorus solubilization, potassium mobilization, enzyme synthesis, as well as the production of iron carriers and hormones. These functional attributes collectively influence seed germination and subsequent seedling development.
The two spermosphere bacterial strains investigated in this study demonstrate distinct beneficial characteristics: both exhibit phosphorus and potassium solubilization along with nitrogen fixation capabilities, while strain T1 additionally produces cellulase and amylase. This enzymatic profile proves particularly relevant for
O. henryi seeds, whose impermeable seed coat contains palisade tissue and stone cells containing excessive cellulose and lignin. Cellulases play a crucial role in degrading these structural components [
31,
32], whereas amylases facilitate starch mobilization during germination, directly impacting seedling establishment [
33].
Beyond these specific functions, beneficial bacteria contribute to plant health through nutrient provisioning, phytohormone synthesis, biocontrol activities, and soil structure improvement [
34,
35]. Molecular identification confirmed that strain T1 belongs to the
Bacillus sp. and T2 belongs to the
Paraburkholderia sp.
Bacillus species, which are widely recognized for their multifunctional applications in agriculture, particularly in crop growth promotion and yield enhancement [
36].
Paraburkholderia strains demonstrate dual efficacy in fungal pathogen biocontrol [
37] and soybean yield improvement [
38], while also harboring the biodegradation genes critical for soil remediation [
39]. Therefore, these two spermosphere strains will have great application value in the field of hard-seed germination.
4.3. Effects of Biopriming on Seed Germination Indexes
Seed priming serves as a crucial pretreatment technique in plant propagation. Research demonstrates that this method effectively enhances seed vigor and improves stress resistance [
40]. However, during
O. henryi seed germination, structural damage to cellular membranes can lead to a substantial leakage of intracellular organic compounds, consequently diminishing seed vigor [
3,
22]. Seed vigor is typically assessed through three key parameters: germination potential (reflecting germination speed), germination rate (indicating overall viability), and germination index (quantifying both speed and uniformity). Notably, seeds with elevated germination potential exhibit stronger vigor, synchronized germination patterns, and consistent seedling emergence. In our experimental findings, the
O. henryi seeds inoculated with two spermosphere strains significantly outperformed both control groups (CK1 and CK2) across all germination metrics. The
Bacillus-treated seeds achieved optimal performance with the highest germination parameters indicating maximum vigor and germination efficiency. The
Paraburkholderia-treated seeds ranked second in germination effectiveness. These results confirm the superiority of biopriming over conventional treatments, aligning with previous reports on microbial-enhanced seed germination and seedling development [
37,
41]. This study establishes that biopriming effectively enhances both germination vigor and efficiency for
O. henryi seeds, providing a robust theoretical foundation and practical framework for artificial cultivation.
4.4. Effects of Biopriming on Seed Hormones
Hormones serve as central regulators in plant growth and developmental processes. Seeds are regulated by hormones from maturation to subsequent germination [
42,
43]. Particularly, the antagonistic interaction between gibberellic acid (GA) and abscisic acid (ABA) constitutes a fundamental regulatory mechanism controlling seed dormancy and germination. GA functions as a dormancy-breaking agent that stimulates germination through the transcriptional activation of hydrolase genes and the synthesis of germination-related enzymes, while simultaneously counteracting ABA effects [
44,
45]. Conversely, ABA maintains dormancy by inhibiting GA-mediated mobilization of seed reserves during germination [
46]. Scientific research has found that the ratio of GA/ABA will produce a dynamic balance during the growth cycle of plants. Elevated cellular GA levels correlate with accelerated growth, whereas ABA predominance results in growth retardation or arrest [
47]. This hormonal balance has consequently been established as a reliable indicator of germination progression.
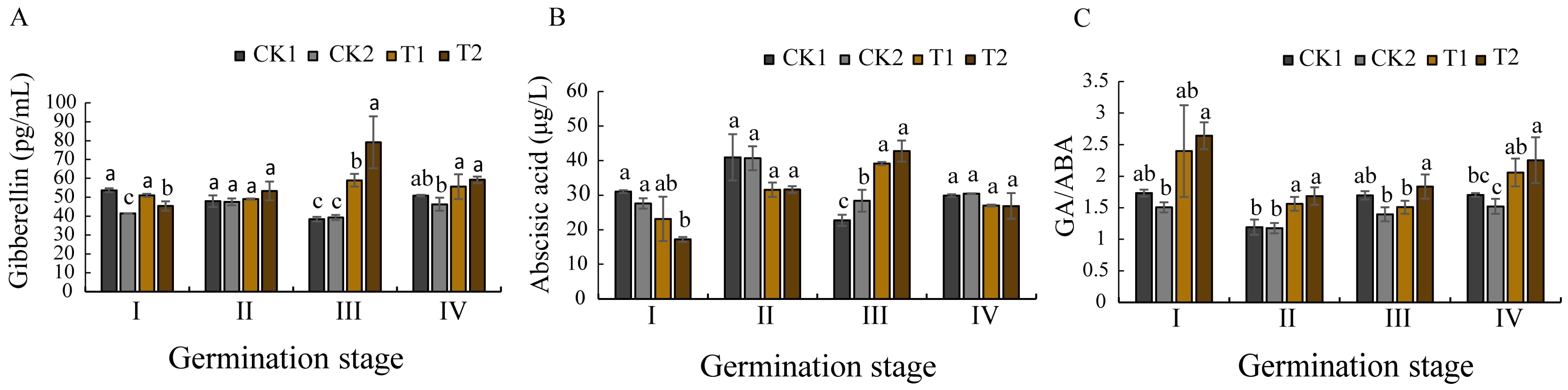
These experimental data revealed significant hormonal modulation in the bioprimed
O. henryi seeds. Both T1 (
Bacillus sp.) and T2
(Paraburkholderia sp.) treatments induced marked GA accumulation from germination stage III on, peaking at this phase while maintaining levels substantially higher than the control groups (CK1 and CK2). Conversely, ABA concentrations in the treated seeds were significantly reduced during stages I–II, reaching minimal values at stage I. Moreover, the value of GA/ABA was significantly higher for T1 and T2 than CK1 and CK2 at germination stage I. The results showed that the biopriming method elicited a characteristic biphasic hormonal response: an initial suppression of ABA synthesis during the early germination stages (I–II), followed by progressive GA upregulation, peaking at stage III. This coordinated regulation accelerated germination efficiency, as evidenced by the enhanced germination rates and seed vigor across the germination stages. The observed effects align with the established mechanisms of microbial-mediated hormonal regulation [
39], confirming that biopriming enhances germination efficiency through GA/ABA modulation.
4.5. Effects of Biopriming on Storage Substances and the Enzyme Activity of Seeds
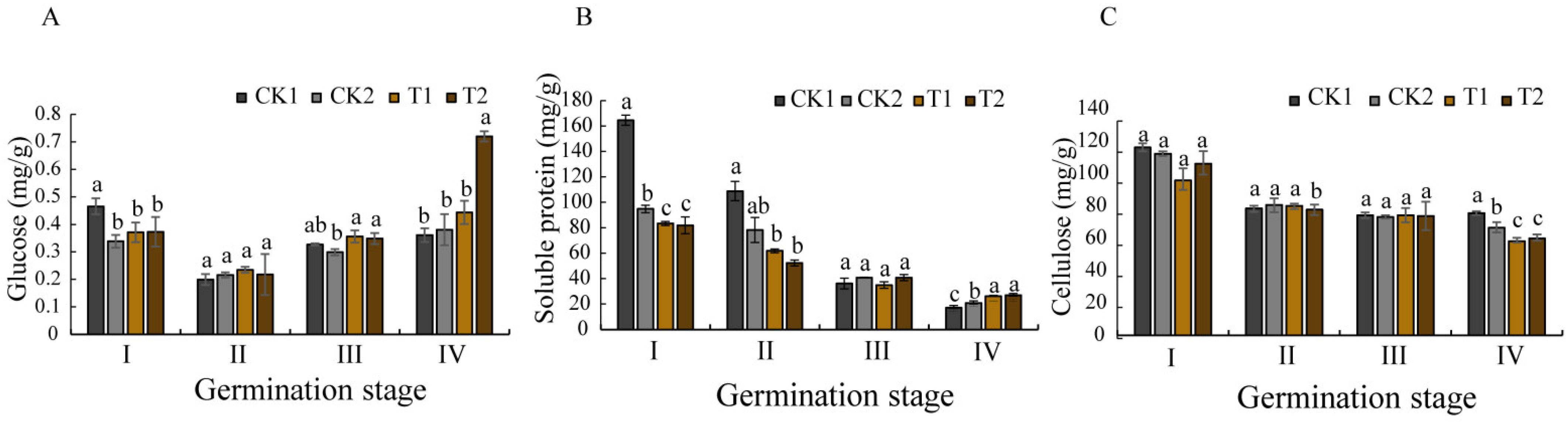
Seed germination requires substantial energy and nutrient resources to support seedling establishment [
48]. Glucose is a soluble sugar, which is the basis of plant metabolism and an important energy source for plants in the growth and development process. Research has demonstrated that glucose availability directly influences seed germination efficiency and early seedling vigor. Some studies have found that amylase activity in germinating seeds is positively correlated with soluble sugar content [
49], techniques enhance this process by stimulating amylase production, thereby accelerating starch hydrolysis into metabolically available monosaccharides. This study revealed that biopriming induced synchronized fluctuations in glucose content and total (α + β) amylase activity, both parameters displayed decreases first and then increases, peaking at germination stage IV. This enzymatic regulation appears interconnected with phytohormonal control mechanisms, as amylase activity is known to be modulated by the antagonistic actions of gibberellins (GAs) and abscisic acid (ABA) [
50,
51]. Specifically, ABA accumulation suppresses GAMYB protein expression, subsequently inhibiting GA-mediated α-amylase production [
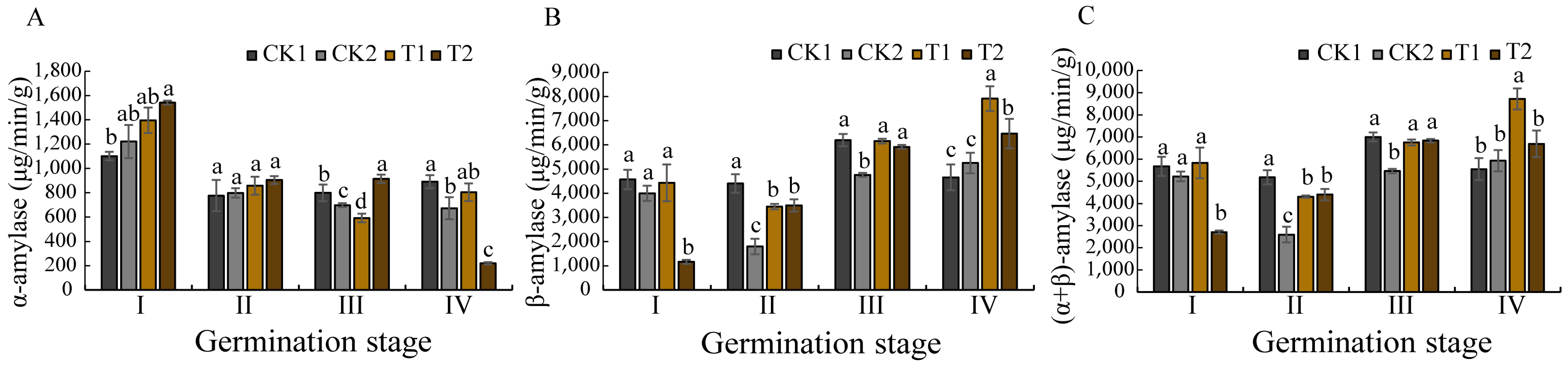
52], establishing an inverse relationship between ABA levels and α-amylase activity. Consistent with this model, our biopriming treatment effectively suppressed ABA biosynthesis during the early germination phases, resulting in enhanced α-amylase activation. Notably, we observed a temporal shift in the different amylases, with α-amylase activity progressively declining while β-amylase activity increased during the later germination stages.
The physiological impacts of biopriming extended to other critical germination parameters. Soluble protein content, a key indicator of embryonic metabolic activity, exhibited stage-dependent dynamics, initially decreasing below control levels (CK1 and CK2) during stages I–II, then surpassing the control values by stage IV. Cell wall metabolism showed similar biopriming effects, with significantly reduced cellulose content in the bioprimed seeds, which was particularly evident in the T1-treated groups at stages I and IV. This cellulose degradation is likely caused by cellulase production by the inoculated
Bacillus strain, potentially addressing the water impermeability issues associated with
O. henryi seed coats [
4]. The enzymatic softening of sclerenchymatous cell walls appears crucial for dormancy release in this species.
In conclusion, inoculation with Bacillus sp. strain T1 effectively alleviates both physical and embryonic dormancy of O. henryi seeds. Functioning as a biopriming agent, this bacterial strain coordinates multiple physiological enhancements, including modulating the endogenous phytohormone balance to enhance amylase activity, promoting storage substance mobilization, and facilitating structural modifications in seed coat architecture. These synergistic effects collectively improve germination efficiency, enhance seed vigor, and provide essential energetic reserves for post-germinative growth.
5. Conclusions
Spermosphere-mediated biopriming significantly enhanced germination performance in O. henryi. The comprehensive effect of biopriming with Bacillus sp. is the best, as the germination rate reached 76.19%, and it had the ability to decompose cellulose during the early germination period, hydrolyzing starch, decomposing organophosphorus, causing nitrogen fixation, and solubilizing potassium, which played an important role in breaking the dormancy of hard seeds and improving seed vigor. This functional synergy effectively overcame both physical and physiological dormancy constraints, establishing it as a sustainable solution for propagating recalcitrant legume species.
Building upon previous findings, this study selected two functionally superior bacterial strains for biopriming treatment. Through a microscopic structural analysis and physiological assessments, we elucidated how spermosphere bacteria enhance O. henryi seed germination. However, the following limitations remain: the current inoculation methods employed single-strain applications only on O. henryi seeds, mixed inoculation strategies and stage-specific bacterial consortia were not investigated, and the sustained effects on seedling development remain unverified. Future research directions will address these gaps. An experimental validation of spermosphere bacteria (single/combined strains) needs to be conducted including determining the long-term effects on seedling growth, chemotactic mobility, and colonization capacity, and an exploration of the potential symbiotic traits in O. henryi seedlings.