Aquatic Resistome in Freshwater and Marine Environments: Interactions Between Commensal and Pathogenic in the Context of Aquaculture and One Health
Abstract
1. Introduction
2. The Aquatic Resistome: Concepts and Mechanisms
3. Commensal and Pathogenic Bacteria in Freshwater
4. Bacteria and Antibiotic Resistance
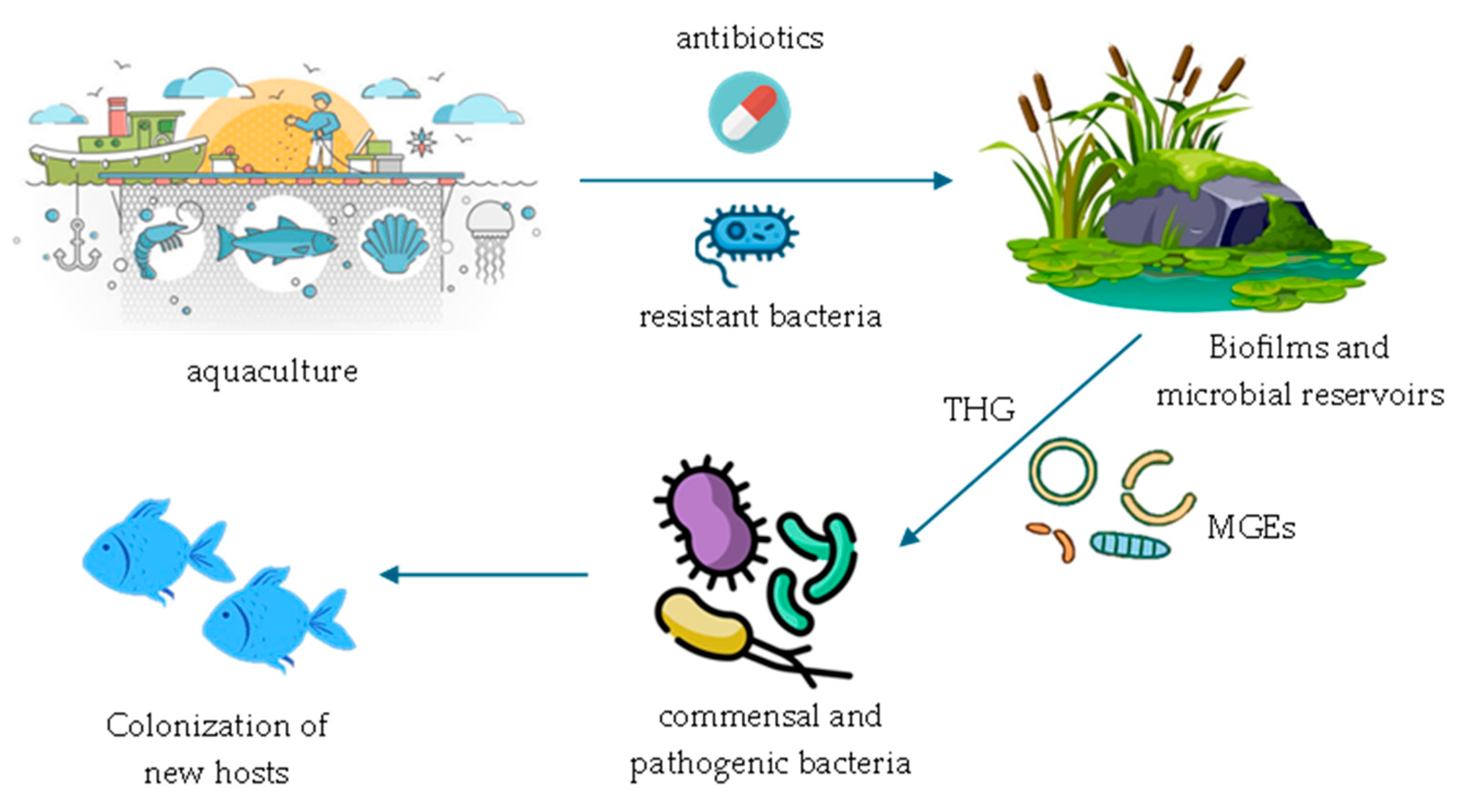
5. Aquaculture as a Vector of Dissemination
6. One Health Implications and Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acar, J.; Rostel, B. Antimicrobial resistance: An overview. Rev. Sci. Tech. 2001, 20, 797–810. [Google Scholar] [CrossRef]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.J.; Gunn, A. The One Health Concept. Br. J. Biomed. Sci. 2024, 81, 2366. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Fernandes, C.; Monteiro, S.; Cabecinha, E.; Teixeira, A.; Varandas, S.; Saavedra, M.J. The role of aquatic ecosystems (River Tua, Portugal) as reservoirs of multidrug-resistant Aeromonas spp. Water 2021, 13, 698. [Google Scholar] [CrossRef]
- Lajqi Berisha, N.; Poceva Panovska, A.; Hajrulai-Musliu, Z. Antibiotic resistance and aquatic systems: Importance in public health. Water 2024, 16, 2362. [Google Scholar] [CrossRef]
- Kim, D.W.; Cha, C.J. Antibiotic resistome from the One-Health perspective: Understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef]
- Miranda, C.D.; Concha, C.; Godoy, F.A.; Lee, M.R. Aquatic environments as hotspots of transferable low-level quinolone resistance and their potential contribution to high-level quinolone resistance. Antibiotics 2022, 11, 1487. [Google Scholar] [CrossRef]
- Saibu, S.; Perera, I.U.; Suzuki, S.; Rodó, X.; Fujiyoshi, S.; Maruyama, F. Resistomes in freshwater bioaerosols and their impact on drinking and recreational water safety: A perspective. Environ. Int. 2024, 183, 108377. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Fajardo, A.; Martinez-Martin, N.; Mercadillo, M.; Galan, J.C.; Ghysels, B.; Matthijs, S.; Cornelis, P.; Wiehlmann, L.; Tümmler, B.; Baquero, F.; et al. The neglected intrinsic resistome of bacterial pathogens. PLoS ONE 2008, 3, e1619. [Google Scholar] [CrossRef]
- Dantas, G.; Sommer, M.O.A. Context matters—the complex interplay between resistome genotypes and resistance phenotypes. Curr. Opin. Microbiol. 2012, 15, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.L.; Andrade, B.G.N.; Vicente, A.C.P. The Resistome of Low-Impacted Marine Environments Is Composed by Distant Metallo-β-Lactamases Homologs. Front. Microbiol. 2018, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Pellegrini, F.; Catalano, E.; Capozzi, L.; Del Sambro, L.; Sposato, A.; Lucente, M.S.; Vasinioti, V.I.; Catella, C.; Odigie, A.E.; et al. Acquired Bacterial Resistance to Antibiotics and Resistance Genes: From Past to Future. Antibiotics 2025, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Sandner-Miranda, L.; Vinuesa, P.; Cravioto, A.; Morales-Espinosa, R. The Genomic Basis of Intrinsic and Acquired Antibiotic Resistance in the Genus Serratia. Front. Microbiol. 2018, 9, 828. [Google Scholar] [CrossRef]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C. Editorial: Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020, 96, fiaa031. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef]
- Virolle, C.; Goldlust, K.; Djermoun, S.; Bigot, S.; Lesterlin, C. Plasmid Transfer by Conjugation in Gram-Negative Bacteria: From the Cellular to the Community Level. Genes 2020, 11, 1239. [Google Scholar] [CrossRef]
- Khedkar, S.; Smyshlyaev, G.; Letunic, I.; Maistrenko, O.M.; Coelho, L.P.; Orakov, A.; Forslund, S.K.; Hildebrand, F.; Luetge, M.; Schmidt, T.S.B.; et al. Landscape of mobile genetic elements and their antibiotic resistance cargo in prokaryotic genomes. Nucleic Acids Res. 2022, 50, 3155–3168. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, F.; Zilhao, R.; Gama, J.A. Interactions between plasmids and other mobile genetic elements affect their transmission and persistence. Plasmid 2019, 102, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, M.; Shintani, M. Microbial evolution through horizontal gene transfer by mobile genetic elements. Microb. Biotechnol. 2024, 17, e14408. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, G.; Xu, W.; Jian, S.; Peng, L.; Jia, D.; Sun, J. New estimation of antibiotic resistance genes in sediment along the Haihe River and Bohai Bay in China: A comparison between single and successive DNA extraction methods. Front. Microbiol. 2021, 12, 705724. [Google Scholar] [CrossRef] [PubMed]
- Summers, A.O. Generally overlooked fundamentals of bacterial genetics and ecology. Clin. Infect. Dis. 2002, 34 (Suppl. S3), S85–S92. [Google Scholar] [CrossRef]
- Johnson, P.T.; Paull, S.H. The ecology and emergence of diseases in fresh waters. Freshw. Biol. 2011, 56, 638–657. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Ringø, E.; Yang, D. Prevalence of Aeromonas hydrophila and Pseudomonas fluorescens and factors influencing them in different freshwater fish ponds. Iran. J. Fish. Sci. 2020, 19, 111–124. [Google Scholar]
- Salvat, M.F.; Ashbolt, N. Aeromonas. In Global Water Pathogen Project; University of Alberta: Edmonton, AB, Canada, 2019. [Google Scholar]
- Elwitigala, J.; Higgs, D.; Namnyak, S.; White, J.; Yaneza, A. Septic arthritis due to Aeromonas hydrophila: Case report and review of the literature. Int. J. Clin. Pract. 2005, 59, 121–124. [Google Scholar] [CrossRef]
- Huhulescu, S.; Simon, M.; Lubnow, M.; Kaase, M.; Wewalka, G.; Pietzka, A.T.; Stöger, A.; Ruppitsch, W.; Allerberger, F. Fatal Pseudomonas aeruginosa pneumonia in a previously healthy woman was most likely associated with a contaminated hot tub. Infection 2011, 39, 265–269. [Google Scholar] [CrossRef]
- Fujiki, Y.; Mato, N.; Watanabe, S.; Shibano, T.; Tonai, K.; Takahashi, K.; Saito, T.; Okuyama, A.; Takigami, A.; Bando, M.; et al. Virulent Pseudomonas aeruginosa pneumonia in an immunocompetent adult associated with a home whirlpool bath: A case report. Respir. Med. Case Rep. 2022, 38, 101673. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control. Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Emerging infections program healthcare-associated infections and antimicrobial use prevalence survey team. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Kosikowska, U.; Stec, J.; Andrzejczuk, S.; Mendrycka, M.; Pietras-Ożga, D.; Stępień-Pyśniak, D. Plasmid-mediated fluoroquinolone resistance genes in quinolone-susceptible Aeromonas spp. phenotypes isolated from recreational surface freshwater reservoir. Front. Cell. Infect. Microbiol. 2022, 12, 885360. [Google Scholar] [CrossRef] [PubMed]
- Milijasevic, M.; Veskovic-Moracanin, S.; Milijasevic, J.B.; Petrovic, J.; Nastasijevic, I. Antimicrobial Resistance in Aquaculture: Risk Mitigation within the One Health Context. Foods 2024, 13, 2448. [Google Scholar] [CrossRef]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Hess, W.R. Cyanobacterial genomics for ecology and biotechnology. Curr. Opin. Microbiol. 2011, 14, 608–614. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Lu, T.; Peijnenburg, W.; Gillings, M.; Yang, X.; Zhang, M.; Chen, H.; Wang, M.; Li, A.; et al. Cyanobacterial blooms contribute to the diversity of antibiotic-resistance genes in aquatic ecosystems. Commun. Biol. 2020, 3, 737. [Google Scholar] [CrossRef]
- Ji, W.; Ma, J.; Zheng, Z.; Al-Herrawy, A.Z.; Xie, B.; Wu, D. Algae blooms with resistance in fresh water: Potential interplay between Microcystis and antibiotic resistance genes. Sci. Total Environ. 2024, 940, 173528. [Google Scholar] [CrossRef] [PubMed]
- Koczura, R.; Mokracka, J.; Taraszewska, A.; Łopacinska, N. Abundance of class 1 integron-integrase and sulfonamide resistance genes in river water and sediment is affected by anthropogenic pressure and environmental factors. Microb. Ecol. 2016, 72, 909–916. [Google Scholar] [CrossRef]
- Xi, C.; Wu, J. dATP/ATP, a multifunctional nucleotide, stimulates bacterial cell lysis, extracellular DNA release and biofilm development. PLoS ONE 2010, 5, e13355. [Google Scholar] [CrossRef]
- Drudge, C.N.; Warren, L.A. Prokaryotic horizontal gene transfer in freshwater lakes: Implications of dynamic biogeochemical zonation. J. Environ. Prot. 2012, 3, 1634–1654. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dolz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Shen, X.; Jin, G.; Zhao, Y.; Shao, X. Prevalence and distribution analysis of antibiotic resistance genes in a large-scale aquaculture environment. Sci. Total. Environ. 2020, 711, 134626. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.K.; Van, T.T.H.; Nguyen, H.T.; Smooker, P.M.; Shimeta, J.; Coloe, P.J. Molecular characterization of antibiotic resistance in Pseudomonas and Aeromonas isolates from catfish of the Mekong Delta, Vietnam. Vet. Microbiol. 2014, 171, 397–405. [Google Scholar] [CrossRef]
- Salgueiro, H.S.; Ferreira, A.C.; Duarte, A.S.R.; Botelho, A. Source Attribution of Antibiotic Resistance Genes in Estuarine Aquaculture: A Machine Learning Approach. Antibiotics 2024, 13, 107. [Google Scholar] [CrossRef]
- Labella, A.; Gennari, M.; Ghidini, V.; Trento, I.; Manfrin, A.; Borrego, J.J.; Lleo, M. High incidence of antibiotic multi-resistant bacteria in coastal areas dedicated to fish farming. Mar Pollut Bull. 2013, 70, 197–203. [Google Scholar] [CrossRef]
- Saqr, S.; Khaliel, R.; Ibrahim, M.S. Antibiotic Resistance and Virulence Genes of E. Coli Isolated from Fresh Nile Tilapia (Oreochromis niloticus) in El-Behera Governorate, Egypt. Alex. J. Vet. Sci. 2016, 48. [Google Scholar] [CrossRef]
- Gao, J.; Jang, H.; Huang, L.; Matthews, K.R. Influence of product volume on water antimicrobial efficacy and cross-contamination during retail batch washing of lettuce. Int. J. Food. Microbiol. 2020, 323, 108593. [Google Scholar] [CrossRef]
- Jo, H.; Raza, S.; Farooq, A.; Kim, J.; Unno, T. Fish farm effluents as a source of antibiotic resistance gene dissemination on Jeju Island, South Korea. Environ. Pollut. 2021, 276, 116764. [Google Scholar] [CrossRef]
- Silva, D.G.; Domingues, C.P.F.; Figueiredo, J.F.; Dionisio, F.; Botelho, A.; Nogueira, T. Estuarine Aquacultures at the Crossroads of Animal Production and Antibacterial Resistance: A Metagenomic Approach to the Resistome. Biology 2022, 11, 1681. [Google Scholar] [CrossRef]
- Delpy, L.; Astbury, C.C.; Aenishaenslin, C.; Ruckert, A.; Penney, T.L.; Wiktorowicz, M.; Ciss, M.; Benko, R.; Bordier, M. Integrated surveillance systems for antibiotic resistance in a One Health context: A scoping review. BMC Public Health 2024, 24, 1717. [Google Scholar] [CrossRef]
- Han, Y.; He, J.; Li, M.; Peng, Y.; Jiang, H.; Zhao, J.; Li, Y.; Deng, F. Unlocking the Potential of Metagenomics with the PacBio High-Fidelity Sequencing Technology. Microorganisms 2024, 12, 2482. [Google Scholar] [CrossRef]
- Kumari, N.; Rai, L.C. Cyanobacterial Diversity: Molecular Insights Under Multifarious Environmental Conditions. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivistava, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 17–33. [Google Scholar]
- Sivalingam, P.; Pote, J.; Prabakar, K. Extracellular DNA (eDNA): Neglected and Potential Sources of Antibiotic Resistant Genes (ARGs) in the Aquatic Environments. Pathogens 2020, 9, 874. [Google Scholar] [CrossRef] [PubMed]
- Thamke, V.; Bezabhe, Y.H.; Jass, J.; Olsson, P.E. Preservation of Aquatic Environmental DNA Using Cationic Detergents. Environ. DNA 2024, 6, e70038. [Google Scholar] [CrossRef]
- Waskito, L.A.; Rezkitha, Y.A.A.; Vilaichone, R.K.; Wibawa, I.D.N.; Mustika, S.; Sugihartono, T.; Miftahussurur, M. Antimicrobial resistance profile by metagenomic and metatranscriptomic approach in clinical practice: Opportunity and challenge. Antibiotics 2022, 11, 654. [Google Scholar] [CrossRef]
- Vallejo-Espín, D.; Galarza-Mayorga, J.; Lalaleo, L.; Calero-Cáceres, W. Beyond clinical genomics: Addressing critical gaps in One Health AMR surveillance. Front. Microbiol. 2025, 16, 1596720. [Google Scholar] [CrossRef]
- Pruden, A.; Larsson, D.G.; Amezquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R.; et al. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One health approach. Vet. World 2022, 15, 743. [Google Scholar] [CrossRef] [PubMed]
| Key Features | Native Resistome | Acquired Resistome |
|---|---|---|
| Definition | Set of ARGs present in the bacterial genome that evolved naturally over millions of years in natural communities [9,10] | Set of ARGs recently acquired by bacteria in response to anthropogenic pressures, mainly due to the intensive and unregulated use of antibiotics in medicine, agriculture and, particularly, in aquaculture [9,11]. |
| Habitat | Associated with natural ecosystems (soils) and environments with low anthropic influence (deep marine sediments or remote marine areas) [11,12]. | Associated with environments affected by human activity, such as aquatic systems exposed to antibiotic residues (wastewater) [10]. |
| Genetic Dynamics | ARGs are integrated into the bacterial chromosome and are not associated with mobile genetic elements. They are rarely involved in horizontal gene transfer [11,12]. | ARGs are associated with mobile genetic elements (plasmids, transposons, integrons), which favors their transfer between bacterial species. They are transmitted vertically between generations and horizontally via conjugation, transformation or transduction [9,10,13,14]. |
| Mobile Genetic Element | Description |
|---|---|
| Plasmids | DNA molecules capable of autonomous replication can often carry multiple ARGs, thus increasing the spread of antibiotic resistance [20,22,23]. |
| Transposons | Mobile DNA segments capable of moving between plasmids and bacterial chromosomes through transposase activity. Transposons often contain ARGs, thus increasing the spread of these genes in bacterial populations [20,23]. |
| Integrons | These are specific genetic elements for the capture and expression of gene cassettes, often including ARGs. Class 1 integrons are the most important in the dissemination of ARGs between bacteria [20,23]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourão, A.V.; Fernandes, D.; de Sousa, T.; Calouro, R.; Saraiva, S.; Igrejas, G.; Poeta, P. Aquatic Resistome in Freshwater and Marine Environments: Interactions Between Commensal and Pathogenic in the Context of Aquaculture and One Health. Microorganisms 2025, 13, 1591. https://doi.org/10.3390/microorganisms13071591
Mourão AV, Fernandes D, de Sousa T, Calouro R, Saraiva S, Igrejas G, Poeta P. Aquatic Resistome in Freshwater and Marine Environments: Interactions Between Commensal and Pathogenic in the Context of Aquaculture and One Health. Microorganisms. 2025; 13(7):1591. https://doi.org/10.3390/microorganisms13071591
Chicago/Turabian StyleMourão, Ana V., Diana Fernandes, Telma de Sousa, Rita Calouro, Sónia Saraiva, Gilberto Igrejas, and Patrícia Poeta. 2025. "Aquatic Resistome in Freshwater and Marine Environments: Interactions Between Commensal and Pathogenic in the Context of Aquaculture and One Health" Microorganisms 13, no. 7: 1591. https://doi.org/10.3390/microorganisms13071591
APA StyleMourão, A. V., Fernandes, D., de Sousa, T., Calouro, R., Saraiva, S., Igrejas, G., & Poeta, P. (2025). Aquatic Resistome in Freshwater and Marine Environments: Interactions Between Commensal and Pathogenic in the Context of Aquaculture and One Health. Microorganisms, 13(7), 1591. https://doi.org/10.3390/microorganisms13071591









