Enhanced Natural Attenuation of Gasoline Contaminants in Groundwater: Applications and Challenges of Nitrate-Stimulating Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design
2.3. Physical and Chemical Parameter Analyses
2.4. DNA Extraction and Sequencing
2.5. Data Processing
- (1)
- The concentration–time curves were plotted using Origin software (Pro 8 SRo).
- (2)
- The apparent consumption concentrations of nitrate, nitrite, and ammonium were calculated using the added concentrations minus the detected concentration. A positive value signifies consumption and a negative value indicates generation, that is, the apparent generation quantity.
- (3)
- The CH was taken as a petroleum hydrocarbon to calculate the stoichiometric relationship between the reduction amounts of oxygen and nitrate and the degradation amount of petroleum hydrocarbons [39,40]. The reduction amount of oxygen corresponding to the complete degradation of 1 mg/kg of CH substance is 3.08 mg/kg, and the reduction amount of nitrate is 4.77 mg/kg. It should be noted that the oxygen in the experiment bottle was calculated to be 8.57 mg, and the corresponding amount of degraded CH is 2.78 mg (that is, the corresponding concentration is 278 mg/kg).
- (4)
- For microbial sequencing data, focus on the gene abundance data related to nitrogen transformation and hydrocarbon degradation. In the KEGG database, screen nitrate reductase genes, hydrocarbon oxygenase genes, genes related to benzene degradation, as well as other functional genes related to the metabolism of carbon, nitrogen, phosphorus, and sulfur. Gene abundance is expressed in ppm, that is, the number of reads containing this gene per one million reads [41]. Calculate the ratio of the abundance value of each gene sampled at different time points to the abundance value of the gene at time zero [32], and draw heat maps to evaluate the change in the relevant genes over time. The overall microbial population structure, as well as the structure of microbial populations containing certain key enzymes, was identified through comparison with the NR database and presented using bar charts [42].
3. Results and Discussion
3.1. Contaminant Degradation Characteristics
3.2. Characteristics of Concentration Changes in Inorganic Nitrogen
3.3. Microbial Functional Characteristics
3.3.1. Hydrocarbon Degradation Functional Genes
3.3.2. Functional Genes Related to Nitrate Reduction
3.3.3. Other Enhanced Functional Genes
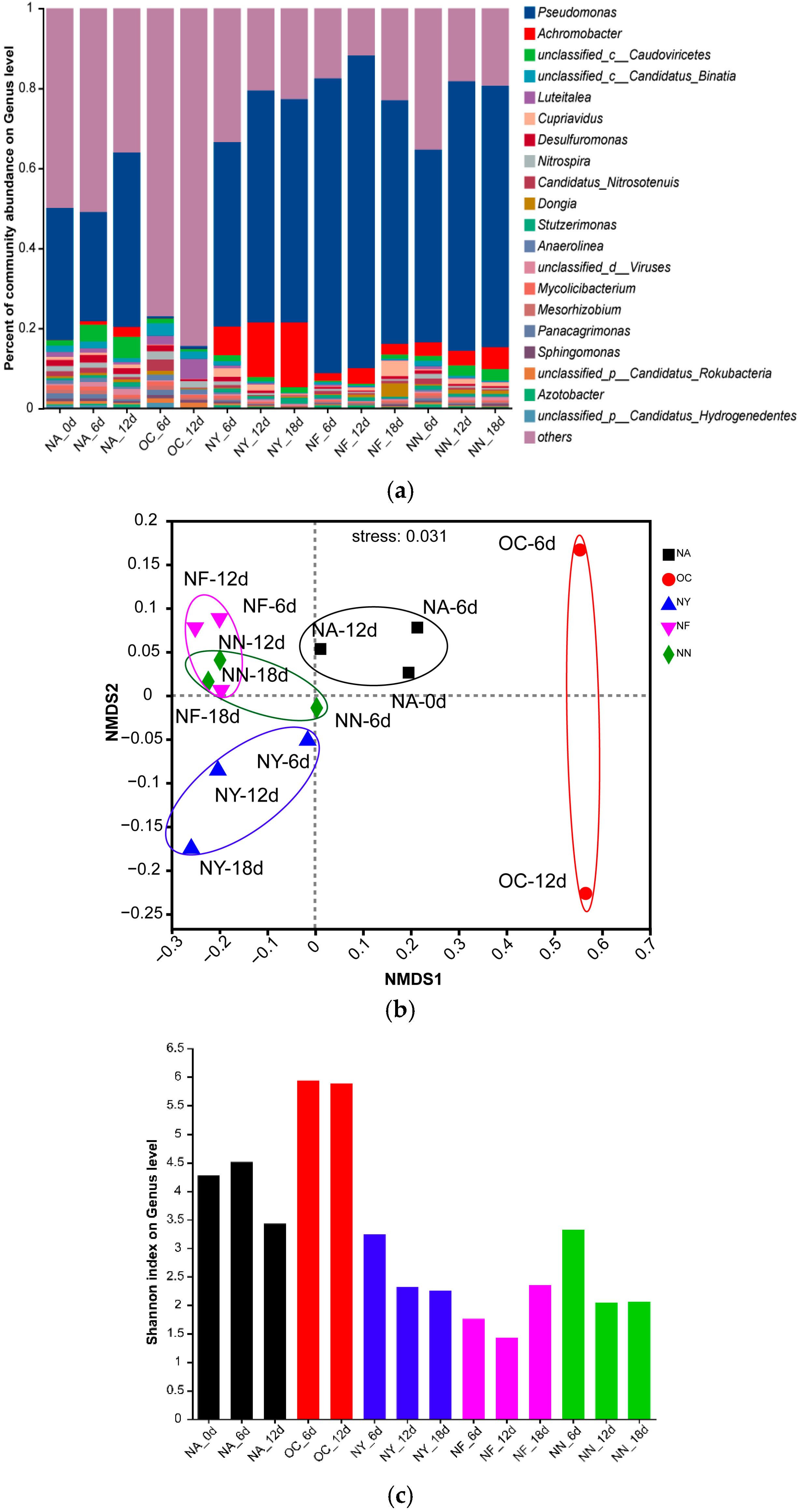
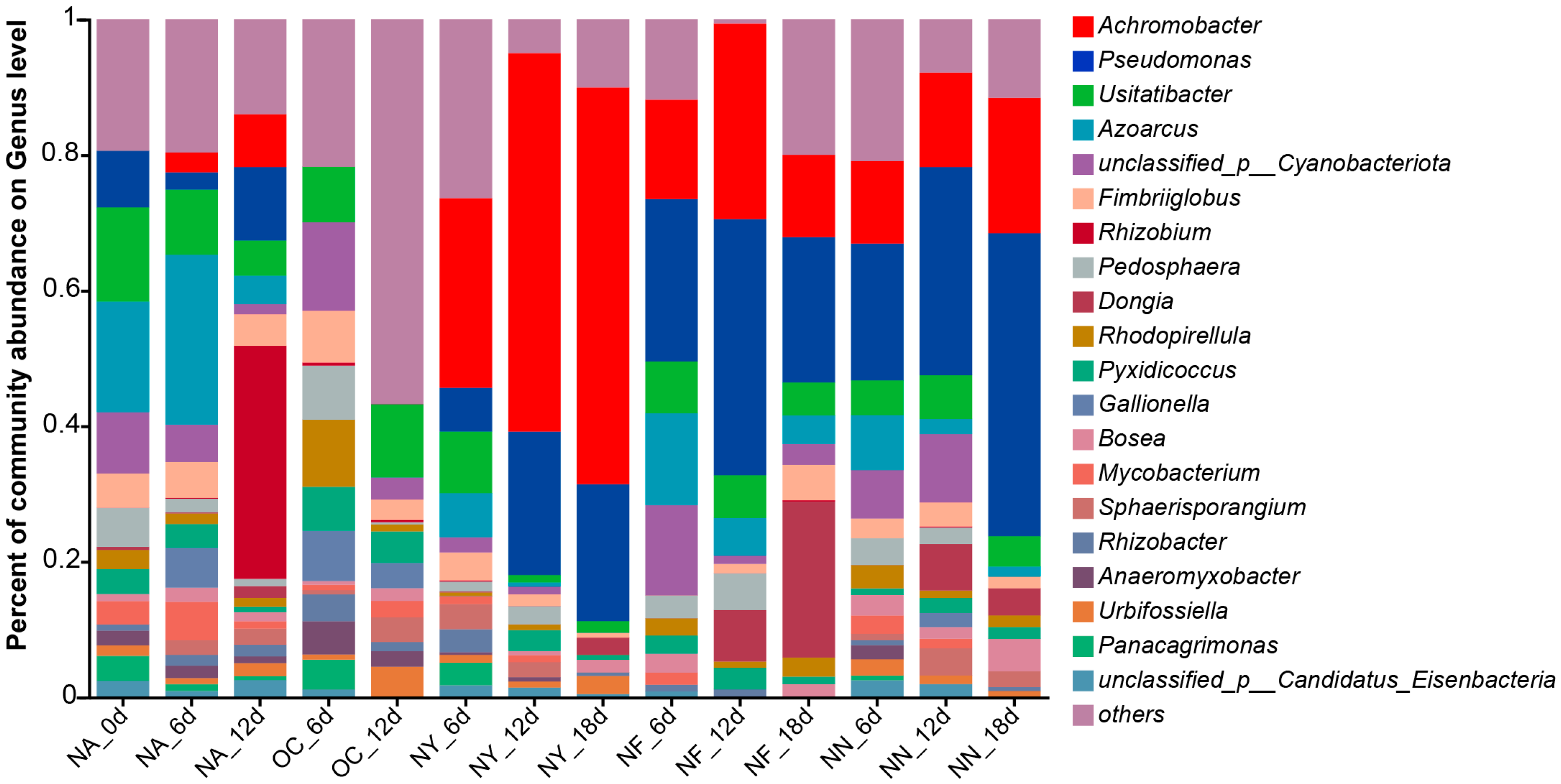
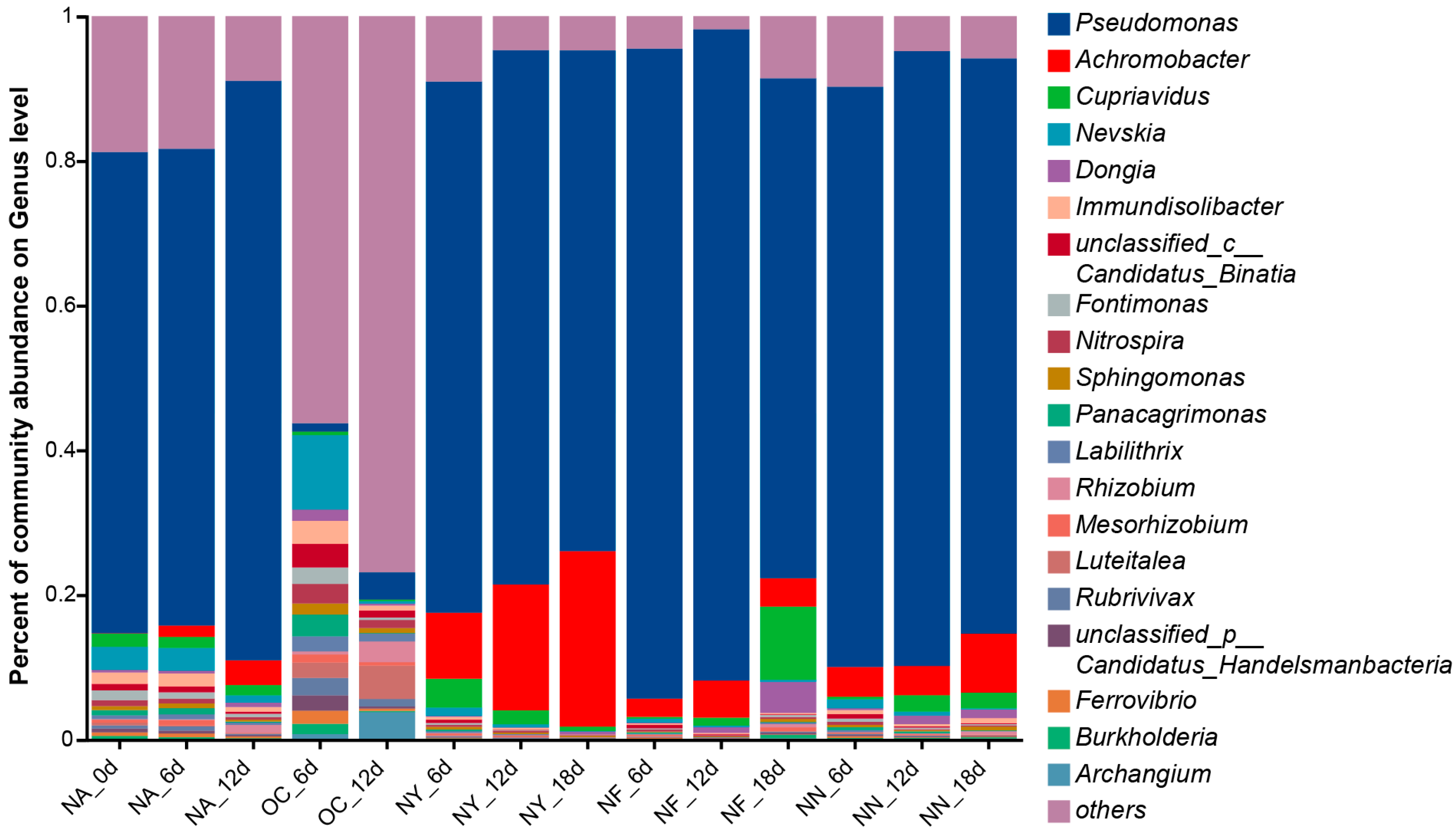
3.4. Microbial Community Structure
3.4.1. The Structural Characteristics of All Microbial Communities
3.4.2. Nitrate-Reducing Functional Microorganisms
3.4.3. Enriched Hydrocarbon-Degrading Microorganisms
3.5. Implications for ENA Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Wang, S.-w.; Guo, C.-j.; Shi, C.; Su, W.-C. Using pore-solid fractal dimension to estimate residual lnapls saturation in sandy aquifers: A column experiment. J. Groundw. Sci. Eng. 2022, 10, 87–98. [Google Scholar]
- Zhang, H.Y.; Han, X.; Wang, G.C.; Mao, H.R.; Chen, X.L.; Zhou, L.; Huang, D.D.; Zhang, F.; Yan, X. Spatial distribution and driving factors of groundwater chemistry and pollution in an oil production region in the northwest China. Sci. Total Environ. 2023, 875, 162635. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, J.; Park, J.; Yang, Y.Y.; Siddiqui, S.I.; Oh, S. A metagenome-derived artificial intelligence modeling framework advances the predictive diagnosis and interpretation of petroleum-polluted groundwater. J. Hazard. Mater. 2024, 472, 134513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, S.; Zhang, Z.F.; Guo, C.J.; Xie, Y.; Wang, X.Z.; Sun, L.; Ning, Z. Development and application of an integrated site remediation technology mix method based on site contaminant distribution characteristics. Appl. Sci. 2023, 13, 11076. [Google Scholar] [CrossRef]
- Dong, X.Q.; Yu, K.; Jia, X.S.; Zhang, Y.Q.; Peng, X.X. Perchlorate reduction kinetics and genome-resolved metagenomics identify metabolic interactions in acclimated saline lake perchlorate-reducing consortia. Water Res. 2022, 227, 119343. [Google Scholar] [CrossRef]
- Giannetta, M.G.; Soler, J.M.; Queralt, I.; Cama, J. Natural attenuation of heavy metals via secondary hydrozincite precipitation in an abandoned pb-zn mine. J. Geochem. Explor. 2023, 251, 107236. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N. Natural attenuation of contaminated soils. Environ. Int. 2004, 30, 587–601. [Google Scholar] [CrossRef]
- Lu, C.S.; Xiu, W.; Yang, B.; Zhang, H.Y.; Lian, G.X.; Zhang, T.J.; Bi, E.R.; Guo, H.M. Natural attenuation of groundwater uranium in post-neutral-mining sites evidenced from multiple isotopes and dissolved organic matter. Environ. Sci. Technol. 2024, 58, 12674–12684. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.-y.; He, Z.; Chen, L.; Zhang, F.-w.; Yin, M.-Y.; Ning, Z.; Zhen, S.-J. Application research of enhanced in-situ micro-ecological remediation of petroleum contaminated soil. J. Groundw. Sci. Eng. 2016, 4, 157. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, G.S.; Ma, J. Transport and natural attenuation of benzene vapor from a point source in the vadose zone. Chemosphere 2023, 323, 138222. [Google Scholar] [CrossRef]
- Ottosen, C.F.; Bjerg, P.L.; Kümmel, S.; Richnow, H.H.; Middeldorp, P.; Draborg, H.; Lemaire, G.G.; Broholm, M.M. Natural attenuation of sulfonamides and metabolites in contaminated groundwater—Review, advantages and challenges of current documentation techniques. Water Res. 2024, 254, 121416. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Yang, Y.; Liu, Q.Y.; Wu, S.X.; Yun, Z.C.; Xu, X.J.; Jiang, Y.H. Insights into the characteristics of changes in dissolved organic matter fluorescence components on the natural attenuation process of toluene. J. Hazard. Mater. 2024, 476, 134952. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Smith, E.; Waller, N.; Stewart, R.; Weber, J. Bioavailability of residual polycyclic aromatic hydrocarbons following enhanced natural attenuation of creosote-contaminated soil. Environ. Pollut. 2010, 158, 585–591. [Google Scholar] [CrossRef]
- Xu, F.; Bao, J.Q.; Liu, Q.; He, X.X.; Zhou, Y.Q.; Wang, H.; Xing, J.M.; Zhou, L.; Yuan, J.F. Simultaneous natural attenuation of cr(vi) and nitrate in the hyporheic zone sediments from an upstream tributary of the Jinsha River in the Sichuan Basin. Sci. Total Environ. 2024, 945, 174145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Chu, L.G.; Wang, X.H.; Fang, G.D.; Liu, C.; Chen, H.; Gu, C.; Gao, J. Roles of natural phenolic compounds in polycyclic aromatic hydrocarbons abiotic attenuation at soil-air interfaces through oxidative coupling reactions. Environ. Sci. Technol. 2023, 57, 11967–11976. [Google Scholar] [CrossRef]
- Satoh, H.; Okabe, S.; Yamaguchi, Y.; Watanabe, Y. Evaluation of the impact of bioaugmentation and biostimulation by in situ hybridization and microelectrode. Water Res. 2003, 37, 2206–2216. [Google Scholar] [CrossRef]
- Zhou, N.; Yang, Z.Y.; Zhang, J.; Zhang, Z.T.; Wang, H. The negative effects of the excessive nitrite accumulation raised by anaerobic bioaugmentation on bioremediation of pah-contaminated soil. Bioresour. Technol. 2024, 393, 130090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, C.; Shi, C.; Ning, Z.; Chen, Z. A quantitative redox zonation model for developing natural attenuation-based remediation strategy in hydrocarbon-contaminated aquifers. J. Clean. Prod. 2021, 290, 125743. [Google Scholar] [CrossRef]
- Srivastava, A.; Valsala, R.; Jagadevan, S. Biogeochemical modelling to assess benzene removal by biostimulation in aquifers containing natural reductants. Environ. Sci. Pollut. Res. 2023, 30, 88022–88035. [Google Scholar] [CrossRef]
- Komlos, J.; Kukkadapu, R.K.; Zachara, J.M.; Jaffé, P.R. Biostimulation of iron reduction and subsequent oxidation of sediment containing Fe-silicates and Fe-oxides: Effect of redox cycling on fe(iii) bioreduction. Water Res. 2007, 41, 2996–3004. [Google Scholar] [CrossRef]
- Lian, G.X.; An, Y.F.; Sun, J.; Yang, B.; Shen, Z.Y. Effects and driving mechanisms of bioremediation on groundwater after the neutral in situ leaching of uranium. Sci. Total Environ. 2024, 946, 174406. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Achal, V. Biostimulation of carbonate precipitation process in soil for copper immobilization. J. Hazard. Mater. 2019, 368, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Lin, C.W.; Cheng, Y.S.; Liu, S.H. Effects of biodegradation, biotoxicity and microbial community on biostimulation of sulfolane. Chemosphere 2023, 319, 138047. [Google Scholar] [CrossRef]
- Romantschuk, M.; Lahti-Leikas, K.; Kontro, M.; Galitskaya, P.; Talvenmäki, H.; Simpanen, S.; Allen, J.A.; Sinkkonen, A. Bioremediation of contaminated soil and groundwater by in situ biostimulation. Front. Microbiol. 2023, 14, 1258148. [Google Scholar] [CrossRef]
- Giovanella, P.; Duarte, L.D.; Kita, D.M.; De Oliveira, V.M.; Sette, L.D. Effect of biostimulation and bioaugmentation on hydrocarbon degradation and detoxification of diesel-contaminated soil: A microcosm study. J. Microbiol. 2021, 59, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.A.; Wang, R.M.; Luo, M.Y.; Xu, C.H.; Yu, D.D.; Zhan, M.J.; Long, T.; Yu, R. Coupling of biostimulation and bioaugmentation for benzene, toluene, and trichloroethylene removal from co-contaminated soil. Water Air Soil Pollut. 2024, 235, 665. [Google Scholar] [CrossRef]
- Ammeri, R.W.; Simeone, G.D.; Hidri, Y.; Abassi, M.S.; Mehri, I.; Costa, S.; Hassen, A.; Rao, M.A. Combined bioaugmentation and biostimulation techniques in bioremediation of pentachlorophenol contaminated forest soil. Chemosphere 2022, 290, 133359. [Google Scholar] [CrossRef]
- Mohammadi, M.; Bayat, Z.; Hassanshahian, M.; Mousavi, M.; Shekarchizadeh, F. Microbial community response to biostimulation and bioaugmentation in crude oil-polluted sediments of the Persian Gulf. Environ. Res. 2024, 249, 118197. [Google Scholar] [CrossRef]
- Ning, Z. Environmental Molecular Diagnosis of the Ammonium Affecting Petroleum Contaminants Degradation Mechanisms; China University of Geosciences: Beijing, China, 2019. [Google Scholar]
- Aldas-Vargas, A.; van der Vooren, T.; Rijnaarts, H.H.M.; Sutton, N.B. Biostimulation is a valuable tool to assess pesticide biodegradation capacity of groundwater microorganisms. Chemosphere 2021, 280, 130793. [Google Scholar] [CrossRef]
- Yuan, F.Z.; Zhao, Y.Y.; Dai, Y.L.; Yang, W.; Zhu, J.Y. Effects of biostimulation-bioaugmentation on coastal microbial community in an in situ mesocosm system. J. Ocean Univ. China 2024, 23, 233–246. [Google Scholar] [CrossRef]
- Ning, Z.; Zhang, M.; Zhang, N.; Guo, C.; Hao, C.; Zhang, S.; Shi, C.; Sheng, Y.; Chen, Z. Metagenomic characterization of a novel enrichment culture responsible for dehalogenation of 1, 2, 3-trichloropropane to allyl chloride. J. Environ. Chem. Eng. 2022, 10, 108907. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, Z.; Guo, C.; Shi, C.; Zhang, S.; Sheng, Y.; Chen, Z. Using compound specific isotope analysis to decipher the 1, 2, 3-trichloropropane-to-allyl chloride transformation by groundwater microbial communities. Environ. Pollut. 2023, 316, 120577. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C.; Frimmel, F.H. Application of headspace gc/ms screening and general parameters for the analysis of polycyclic aromatic hydrocarbons in groundwater samples. Fresenius J. Anal. Chem. 1998, 360, 820–823. [Google Scholar] [CrossRef]
- Lu, R. Methods for Agrochemical Analysis of Soil; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Vidyadharan, P.; Santhi, C.K.; Sethumadhavan, A.; Gopala, S.; Raghavan, C.; Urulangodi, M. Functional assessment of DNA extraction methods from frozen human blood samples for sanger sequencing analysis. Cell. Mol. Biol. 2023, 69, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Nishii, K.; Möller, M.; Foster, R.G.; Forrest, L.L.; Kelso, N.; Barber, S.; Howard, C.; Hart, M.L. A high quality, high molecular weight DNA extraction method for pacbio hifi genome sequencing of recalcitrant plants. Plant Methods 2023, 19, 41. [Google Scholar] [CrossRef]
- Ning, Z.; Sheng, Y.; Gan, S.; Guo, C.; Wang, S.; Cai, P.; Zhang, M. Metagenomic and isotopic insights into carbon fixation by autotrophic microorganisms in a petroleum hydrocarbon impacted red clay aquifer. Environ. Pollut. 2024, 361, 124824. [Google Scholar] [CrossRef]
- Gan, S.; Zhang, M.; Zhou, Y.H.; Guo, C.J.; Yang, S.; Xie, Y.; Wang, X.Z.; Sun, L.; Ning, Z. The effects of toluene mineralization under denitrification conditions on carbonate dissolution and precipitation in water: Mechanism and model. Appl. Sci. 2023, 13, 11867. [Google Scholar] [CrossRef]
- Ning, Z.; Guo, C.; Cai, P.; Zhang, M.; Chen, Z.; He, Z. Geochemical evaluation of biodegradation capacity in a petroleum contaminated aquifer. China Environ. Sci. 2018, 38, 4068–4074. [Google Scholar]
- Ning, Z.; Cai, P.; Zhang, M. Metagenomic analysis revealed highly diverse carbon fixation microorganisms in a petroleum-hydrocarbon-contaminated aquifer. Environ. Res. 2024, 247, 118289. [Google Scholar] [CrossRef]
- Gan, S.; Ning, Z.; Wang, S.; Sun, W.; Xu, Z.; Di, H.; Ti, J.; Guo, C.; Zhou, Y.; He, Z. Identification of carbon fixation microorganisms and pathways in an aquifer contaminated with long-chain petroleum hydrocarbons. Water Environ. Res. 2024, 96, e11078. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, S.C.; Ma, S.C.; Zhao, S.; Liu, Z.W. Field demonstration of in situ slow-release oxygen chemicals coupled with microbial agents for injection to remediate btex contamination. Water 2024, 16, 2815. [Google Scholar] [CrossRef]
- Zhou, L.; Qian, Y.; Chen, J.; Zhang, Y.; Zhou, X. A critical review of solid peroxides in environmental remediation and water purification: From properties to field applications. Chem. Eng. J. 2023, 465, 142424. [Google Scholar] [CrossRef]
- Tomé, D. Yeast extracts: Nutritional and flavoring food ingredients. ACS Food Sci. Technol. 2021, 1, 487–494. [Google Scholar] [CrossRef]
- van Leeuwen, J.A.; Gerritse, J.; Hartog, N.; Ertl, S.; Parsons, J.R.; Hassanizadeh, S.M. Anaerobic degradation of benzene and other aromatic hydrocarbons in a tar-derived plume: Nitrate versus iron reducing conditions. J. Contam. Hydrol. 2022, 248, 104006. [Google Scholar] [CrossRef]
- Song, B.R.; Tang, J.C.; Zhen, M.N.; Liu, X.M. Effect of rhamnolipids on enhanced anaerobic degradation of petroleum hydrocarbons in nitrate and sulfate sediments. Sci. Total Environ. 2019, 678, 438–447. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast protein as an easily accessible food source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Shi, K.; Cheng, H.; Cornell, C.R.; Wu, H.; Gao, S.; Jiang, J.; Liu, T.; Wang, A.; Zhou, J.; Liang, B. Micro-aeration assisted with electrogenic respiration enhanced the microbial catabolism and ammonification of aromatic amines in industrial wastewater. J. Hazard. Mater. 2023, 448, 130943. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, G.Y.; Lo, I.M.C. Enhancement of nitrate-induced bioremediation in marine sediments contaminated with petroleum hydrocarbons by using microemulsions. Environ. Sci. Pollut. Res. 2015, 22, 8296–8306. [Google Scholar] [CrossRef]
- Li, S.X.; Liu, F.J.; Zheng, F.Y.; Huang, X.G.; Zuo, Y.G. Risk assessment of nitrate and petroleum-derived hydrocarbon addition on Contricriba weissflogii biomass, lifetime, and nutritional value. J. Hazard. Mater. 2014, 268, 199–206. [Google Scholar]
- Di, H.; Zhang, M.; Ning, Z.; He, Z.; Liu, C.L.; Song, J.J. A conceptual model for depicting the relationships between toluene degradation and fe(iii) reduction with different fe(iii) phases as terminal electron acceptors. Appl. Sci. 2024, 14, 5017. [Google Scholar] [CrossRef]
- Wu, S.S.; Hao, W.Q.; Yao, Y.; Li, D.Q. Investigation into durability degradation and fracture of cable bolts through laboratorial tests and hydrogeochemical modelling in underground conditions. Tunn. Undergr. Space Technol. 2023, 138, 105198. [Google Scholar] [CrossRef]
- von Netzer, F.; Granitsiotis, M.S.; Szalay, A.R.; Lueders, T. Next-generation sequencing of functional marker genes for anaerobic degraders of petroleum hydrocarbons in contaminated environments. In Anaerobic Utilization of Hydrocarbons; Springer: Cham, Switzerland, 2020; pp. 257–276. [Google Scholar]
- Hammerle, M.; Hall, E.A.H.; Cade, N.; Hodgins, D. Electrochemical enzyme sensor for formaldehyde operating in the gas phase. Biosens. Bioelectron. 1996, 11, 239–246. [Google Scholar] [CrossRef]
- Ishizuka, M.; Ushio, K.; Toraya, T.; Fukui, S. Formation of thieno 3,2-g pterines from the molybdenum cofactor. Biochem. Biophys. Res. Commun. 1983, 111, 537–543. [Google Scholar] [CrossRef]
- Lambré, C.; Baviera, J.M.B.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; Mortensen, A.; et al. Safety evaluation of the food enzyme 3-phytase from the non-genetically modified Aspergillus niger strain phy93-08. EFSA J. 2024, 22, e8876. [Google Scholar]
- Pandey, A.; Szakacs, G.; Soccol, C.R.; Rodriguez-Leon, J.A.; Soccol, V.T. Production, purification and properties of microbial phytases. Bioresour. Technol. 2001, 77, 203–214. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; Rosenkrantz, T.J.; Haldimann, A.; Wanner, B.L. Escherichia coli phnn, encoding ribose 1,5-bisphosphokinase activity (phosphoribosyl diphosphate forming): Dual role in phosphonate degradation and nad biosynthesis pathways. J. Bacteriol. 2003, 185, 2793–2801. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; McSorley, F.R.; Zechel, D.L. Catabolism and detoxification of 1-aminoalkylphosphonic acids: N-acetylation by the PHNO gene product. PLoS ONE 2012, 7, e46416. [Google Scholar] [CrossRef]
- Agarwal, V.; Peck, S.C.; Chen, J.H.; Borisova, S.A.; Chekan, J.R.; van der Donk, W.A.; Nair, S.K. Structure and function of phosphonoacetaldehyde dehydrogenase: The missing link in phosphonoacetate formation. Chem. Biol. 2014, 21, 125–135. [Google Scholar] [CrossRef]
- Ruffolo, F.; Dinhof, T.; Murray, L.; Zangelmi, E.; Chin, J.P.; Pallitsch, K.; Peracchi, A. The microbial degradation of natural and anthropogenic phosphonates. Molecules 2023, 28, 6863. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Q.; Gong, Z. Microbial remediation of petroleum-contaminated soil focused on the mechanism and microbial response: A review. Environ. Sci. Pollut. Res. Int. 2024, 31, 33325–33346. [Google Scholar] [CrossRef]
- van der Ploeg, J.R.; Eichhorn, E.; Leisinger, T. Sulfonate-sulfur metabolism and its regulation in Escherichia coli. Arch. Microbiol. 2001, 176, 1–8. [Google Scholar] [CrossRef]
- Das, S. Cell surface hydrophobicity and petroleum hydrocarbon degradation by biofilm-forming marine bacterium pseudomonas furukawaii pps-19 under different physicochemical stressors. J. Hazard. Mater. 2023, 457, 131795. [Google Scholar]
- Lazzem, A.; Lekired, A.; Ouzari, H.-I.; Landoulsi, A.; Chatti, A.; El May, A. Isolation and characterization of a newly chrysene-degrading Achromobacter aegrifaciens. Int. Microbiol. 2024, 27, 857–869. [Google Scholar] [CrossRef]
- Alviz-Gazitua, P.; Durán, R.E.; Millacura, F.A.; Cárdenas, F.; Rojas, L.A.; Seeger, M. Cupriavidus metallidurans ch34 possesses aromatic catabolic versatility and degrades benzene in the presence of mercury and cadmium. Microorganisms 2022, 10, 484. [Google Scholar] [CrossRef]
- Chayabutra, C.; Ju, L.-K. Degradation of n-hexadecane and its metabolites by pseudomonas aeruginosa under microaerobic and anaerobic denitrifying conditions. Appl. Environ. Microbiol. 2000, 66, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zhao, Y.; Sun, J.; Ren, H.; Zhou, R. Btex biodegradation and its nitrogen removal potential by a newly isolated pseudomonas thivervalensis mah1. Can. J. Microbiol. 2015, 61, 691–699. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Yang, X.; Wu, D.; Li, Y.; Di, H. Isolation and characteristics of two heterotrophic nitrifying and aerobic denitrifying bacteria, achromobacter sp. Strain hnds-1 and enterobacter sp. Strain hnds-6. Environ. Res. 2023, 220, 115240. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Guo, H.; Wang, C.; Fang, T.; Rogers, M.J.; He, J.; Wang, H. Anaerobic biodegradation of phenanthrene by a newly isolated nitrate-dependent Achromobacter denitrificans strain phen1 and exploration of the biotransformation processes by metabolite and genome analyses. Environ. Microbiol. 2021, 23, 908–923. [Google Scholar] [CrossRef] [PubMed]
- Strohm, T.O.; Griffin, B.; Zumft, W.G.; Schink, B. Growth yields in bacterial denitrification and nitrate ammonification. Appl. Environ. Microbiol. 2007, 73, 1420–1424. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.W.; Zhang, C.; Tan, X.J.; Yang, X.; Wan, C.L.; Lee, D.J. Enhancement of anaerobic degradation of petroleum hydrocarbons by electron intermediate: Performance and mechanism. Bioresour. Technol. 2020, 295, 122305. [Google Scholar] [CrossRef]
- Caffrey, S.M.; Voordouw, G. Effect of sulfide on growth physiology and gene expression of desulfovibrio vulgaris hildenborough. Antonie Leeuwenhoek 2010, 97, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Korte, H.L.; Fels, S.R.; Christensen, G.A.; Price, M.N.; Kuehl, J.V.; Zane, G.M.; Deutschbauer, A.M.; Arkin, A.P.; Wall, J.D. Genetic basis for nitrate resistance in Desulfovibrio strains. Front. Microbiol. 2014, 5, 153. [Google Scholar] [CrossRef]
- Mahendran, B.; Choi, N.-C.; Choi, J.-W.; Kim, D.-J. Effect of dissolved oxygen regime on growth dynamics of Pseudomonas spp. during benzene degradation. Appl. Microbiol. Biotechnol. 2006, 71, 350–354. [Google Scholar] [CrossRef]
- Pinel-Cabello, M.; Wasmund, K.; Soder-Walz, J.M.; Vega, M.; Rosell, M.; Marco-Urrea, E. Divergent dual c-h isotopic fractionation pattern during anaerobic biodegradation of toluene within Aromatoleum species under nitrate-reducing conditions. Environ. Pollut. 2024, 361, 124823. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.; Wüensch, D.; Wöhlbrand, L.; Neumann-Schaal, M.; Schomburg, D.; Rabus, R. The catabolic network of Aromatoleum aromaticum ebn1t. Microb. Physiol. 2023, 34, 1–77. [Google Scholar] [CrossRef]
- Semenova, E.M.; Grouzdev, D.S.; Sokolova, D.S.; Tourova, T.P.; Poltaraus, A.B.; Potekhina, N.V.; Shishina, P.N.; Bolshakova, M.A.; Avtukh, A.N.; Ianutsevich, E.A.; et al. Physiological and genomic characterization of Actinotalea subterranea sp. Nov. From oil-degrading methanogenic enrichment and reclassification of the family Actinotaleaceae. Microorganisms 2022, 10, 378. [Google Scholar] [CrossRef]
- Kuznetsov, B.B.; Ivanovsky, R.N.; Keppen, O.I.; Sukhacheva, M.V.; Bumazhkin, B.K.; Patutina, E.O.; Beletsky, A.V.; Mardanov, A.V.; Baslerov, R.V.; Panteleeva, A.N.; et al. Draft genome sequence of the anoxygenic filamentous phototrophic bacterium Oscillochloris trichoides subsp. Dg-6. J. Bacteriol. 2011, 193, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Mechelke, M.; Koeck, D.E.; Broeker, J.; Roessler, B.; Krabichler, F.; Schwarz, W.H.; Zverlov, V.V.; Liebl, W. Characterization of the arabinoxylan-degrading machinery of the thermophilic bacterium Herbinix hemicellulosilytica-six new xylanases, three arabinofuranosidases and one xylosidase. J. Biotechnol. 2017, 257, 122–130. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.; Cao, X.; Xiang, M.; Huang, Y.; Li, H. A critical review of groundwater table fluctuation: Formation, effects on multifields, and contaminant behaviors in a soil and aquifer system. Environ. Sci. Technol. 2024, 58, 2185–2203. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.; Ojha, C.S.P.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Martínez, P. Toxicity of hydrocarbons to microorganisms. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2018; p. 335. [Google Scholar]
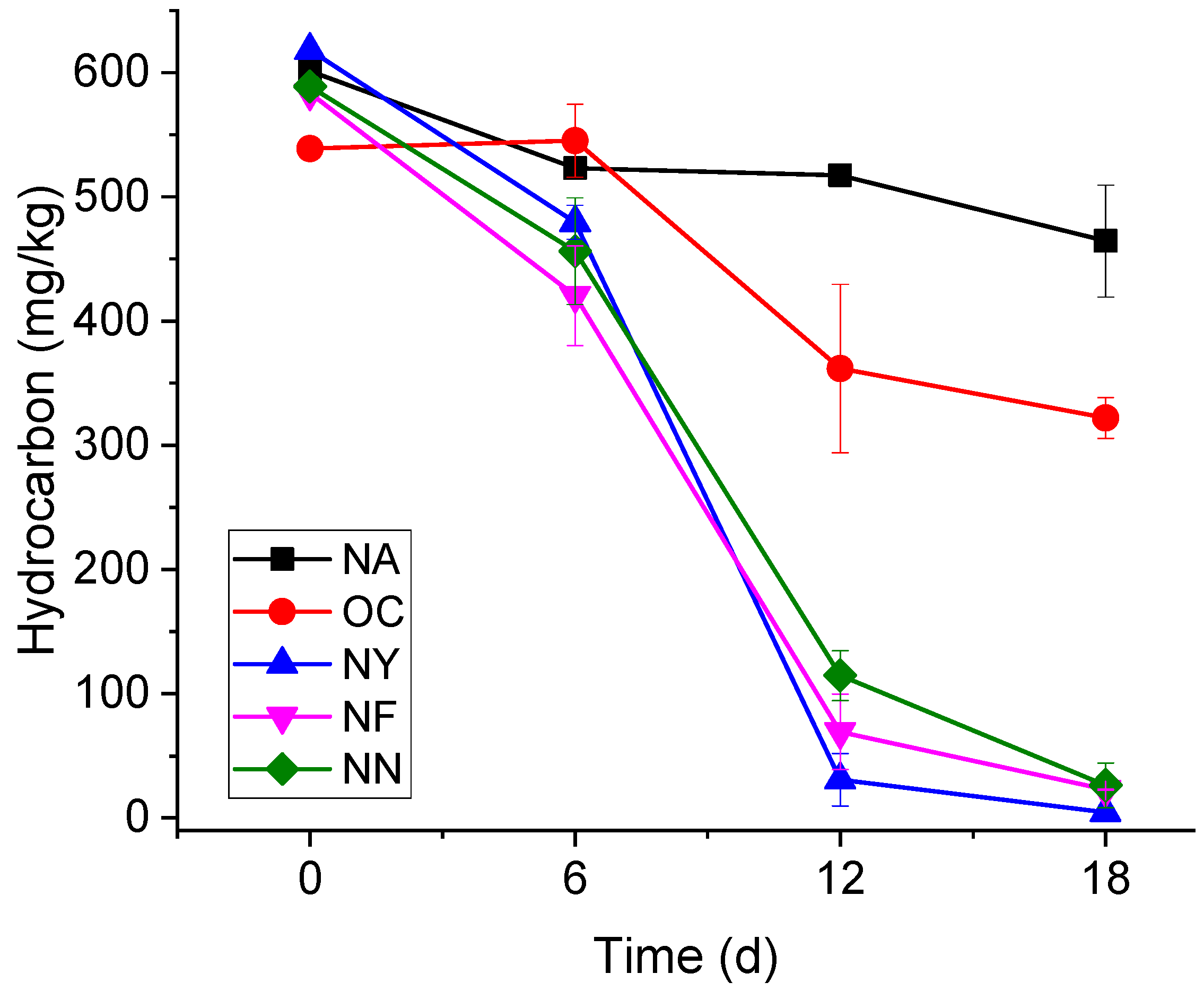
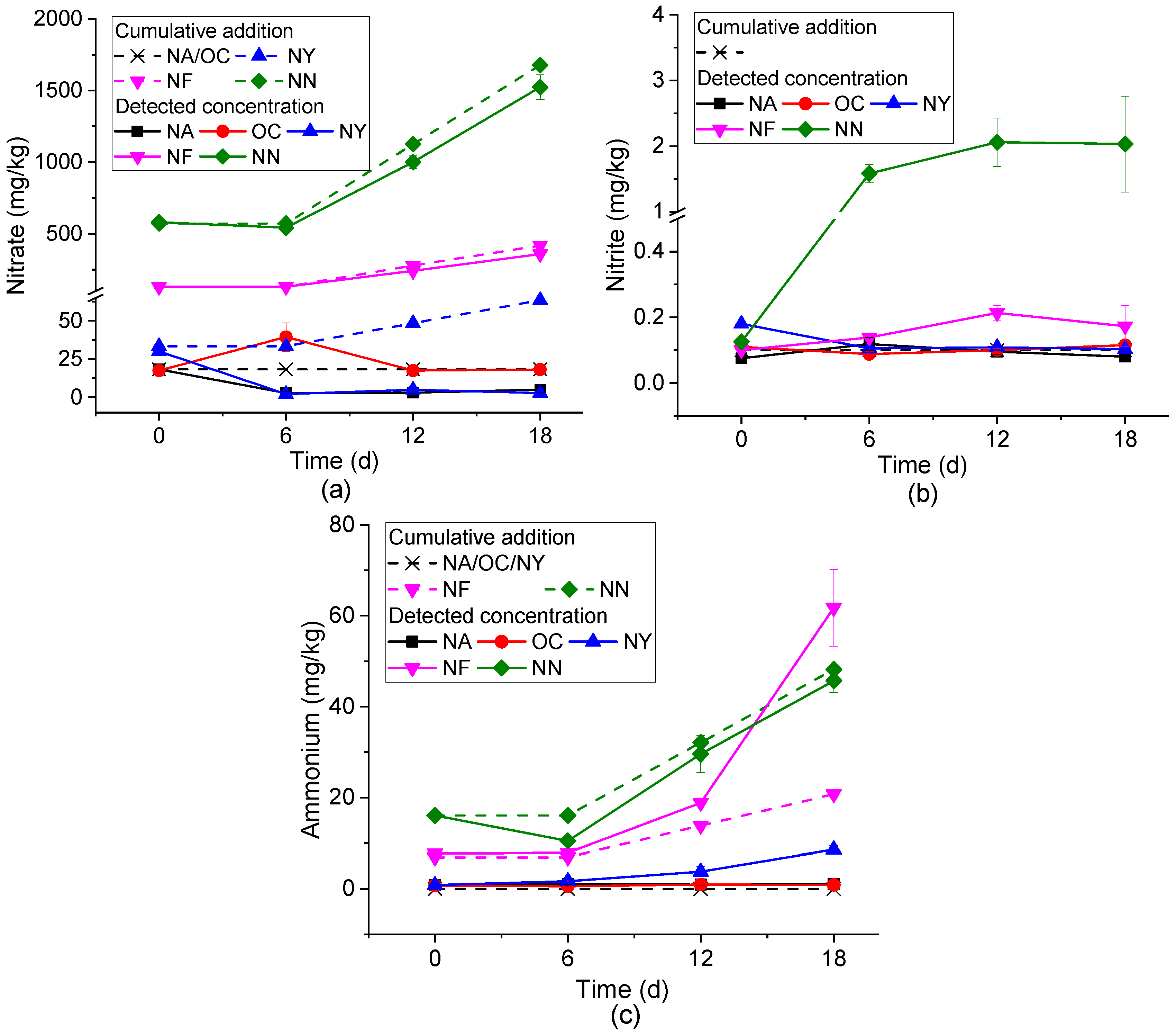
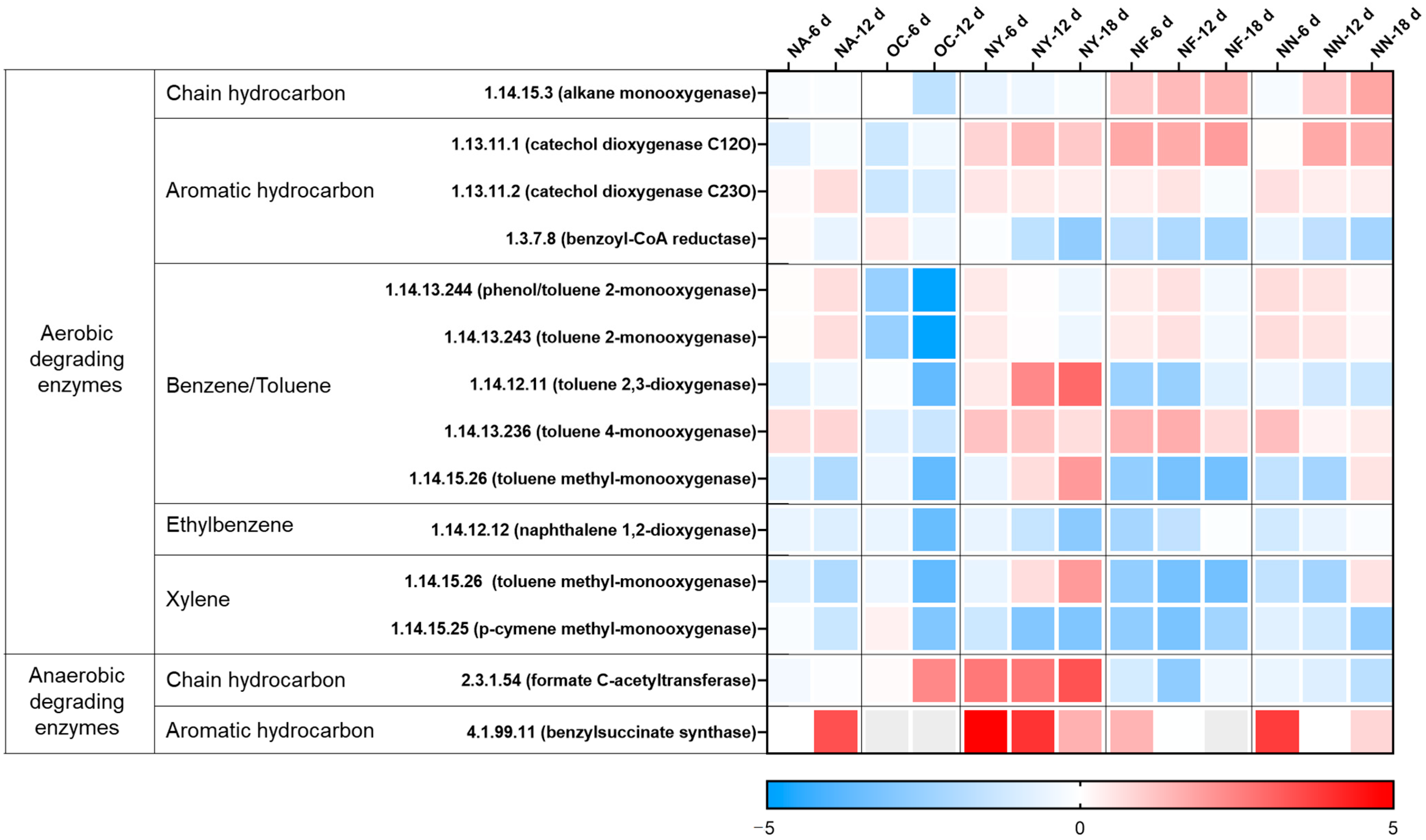
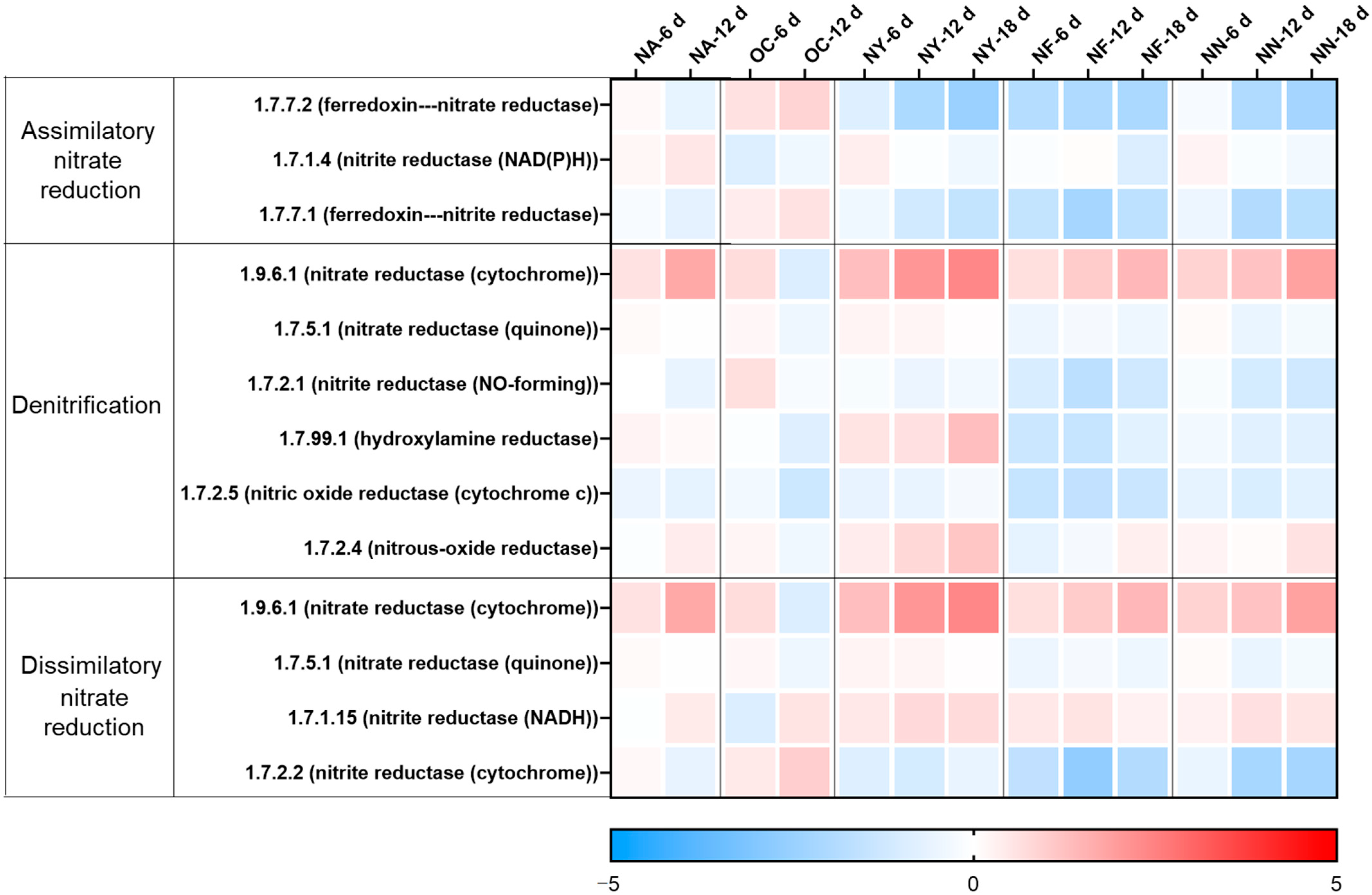
| Group | Material Categories | Treatment Performed | Manufacturer | Dosage to Be Added Each Time |
|---|---|---|---|---|
| NA | Natural attenuation | None | -- | -- |
| OC | Oxygen supply | Calcium peroxide | Nong xin ke ji | 1600 mg/kg a |
| NY | Nitrate supply | Yeast extract | Solarbio® (Cat#Y8020) | 100 mg/kg [32] |
| NF | Nitrogenous fertilizer | Dugao® Water-soluble fertilizer containing macronutrients (#25-10-20) | 464 mg/kg b | |
| NN | Self-compounding reagent containing Nitrate | In the study (see Table S1 for the specific formula) | 20 mL/kg c |
| Time (d) | 6 | 12 | 18 | |||
|---|---|---|---|---|---|---|
| TD | AD | TD | AD | TD | AD | |
| NA | 3.27 ± 0.17 | 77.95 ± 6.55 | 3.21 ± 0.06 | 83.76 ± 4.77 | 2.79 ± 0.38 | 136.71 ± 45.1 |
| OC | −4.42 ± 1.94 | −6.37 ± 29.33 | 0.18 ± 0.14 | 177.12 ± 67.8 | 0.02 ± 0 | 216.92 ± 16.27 |
| NY | 6.55 ± 0.04 | 138.61 ± 13.64 | 9.12 ± 0.33 | 587.16 ± 21.13 | 12.75 ± 0.04 | 613.43 ± 3.56 |
| NF | 0.01 ± 0.98 | 163 ± 40.14 | 7.86 ± 0.71 | 514.03 ± 30.5 | 12.32 ± 1.19 | 560.58 ± 0.41 |
| NN | 6.16 ± 2.77 | 132.61 ± 42.92 | 26.14 ± 9.1 | 474.27 ± 20.12 | 32.29 ± 17.95 | 562.53 ± 17.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Z.; Liang, J.; Ti, J.; Zhang, M.; Cai, C. Enhanced Natural Attenuation of Gasoline Contaminants in Groundwater: Applications and Challenges of Nitrate-Stimulating Substances. Microorganisms 2025, 13, 1575. https://doi.org/10.3390/microorganisms13071575
Ning Z, Liang J, Ti J, Zhang M, Cai C. Enhanced Natural Attenuation of Gasoline Contaminants in Groundwater: Applications and Challenges of Nitrate-Stimulating Substances. Microorganisms. 2025; 13(7):1575. https://doi.org/10.3390/microorganisms13071575
Chicago/Turabian StyleNing, Zhuo, Jiaqing Liang, Jinjin Ti, Min Zhang, and Chao Cai. 2025. "Enhanced Natural Attenuation of Gasoline Contaminants in Groundwater: Applications and Challenges of Nitrate-Stimulating Substances" Microorganisms 13, no. 7: 1575. https://doi.org/10.3390/microorganisms13071575
APA StyleNing, Z., Liang, J., Ti, J., Zhang, M., & Cai, C. (2025). Enhanced Natural Attenuation of Gasoline Contaminants in Groundwater: Applications and Challenges of Nitrate-Stimulating Substances. Microorganisms, 13(7), 1575. https://doi.org/10.3390/microorganisms13071575






