Cooperative Interplay Between PGPR and Trichoderma longibrachiatum Reprograms the Rhizosphere Microecology for Improved Saline Alkaline Stress Resilience in Rice Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Microbial Inoculants and Soil
2.2. Pot Experiment
2.3. Plant Growth Parameters and Rhizosphere Soil Sampling
2.4. Soil Physicochemical Property Analysis
2.5. DNA Extraction, Amplicon Sequencing, and Data Processing
2.6. Data Analysis
3. Results
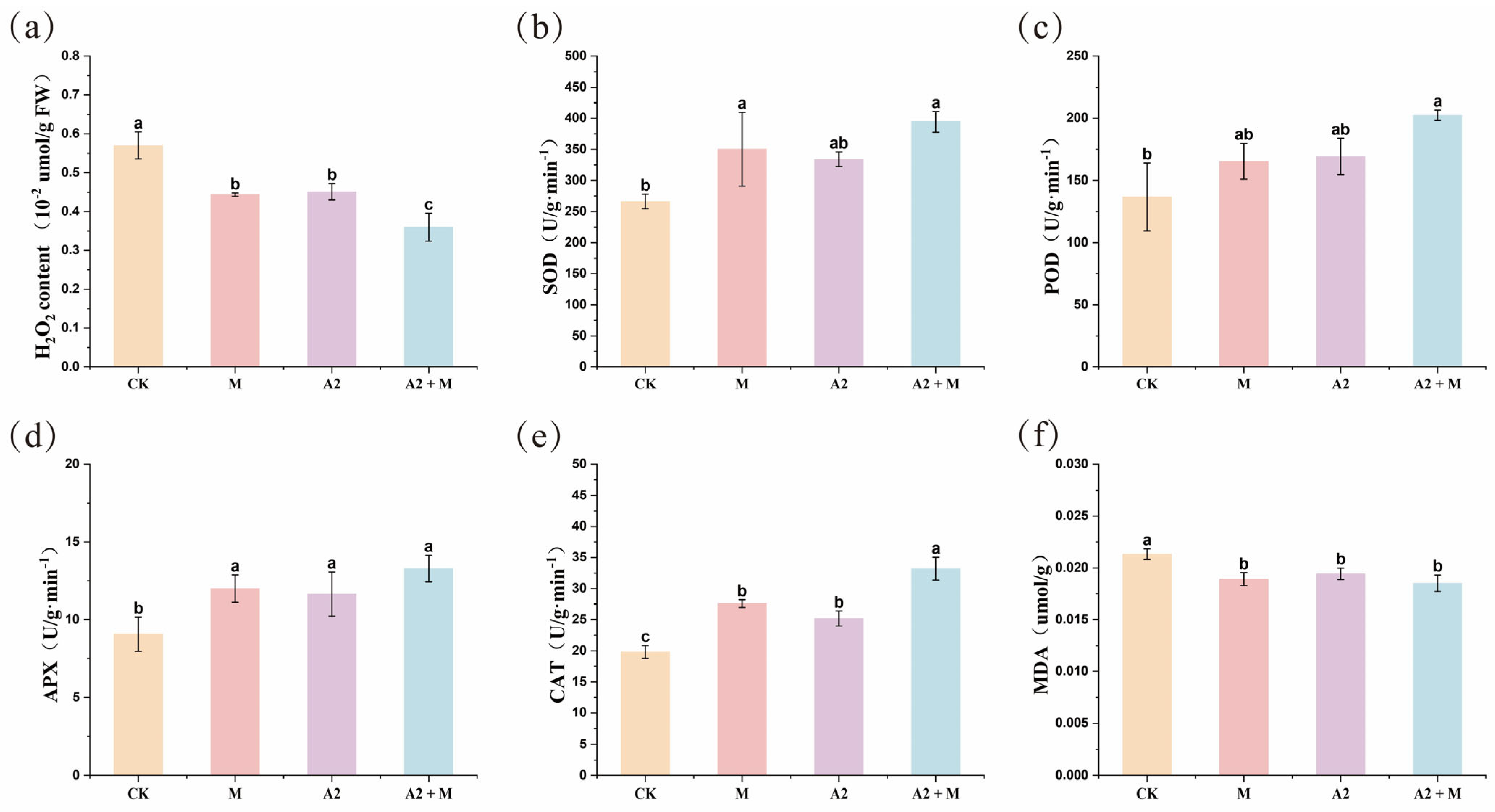
3.1. Effects of Microbial Inoculation on Rice Physiological and Biochemical Parameters
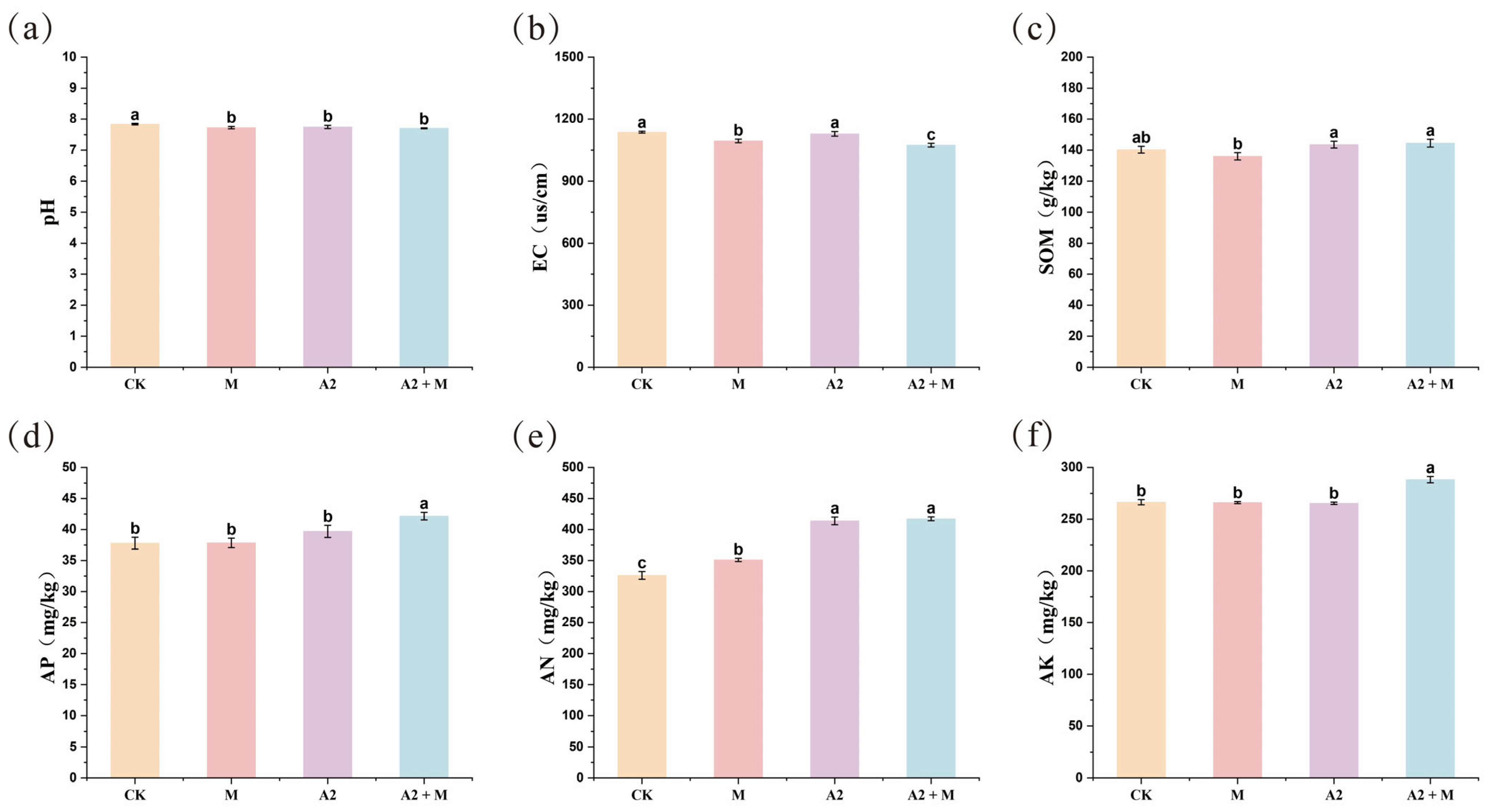
3.2. Effects of Microbial Inoculation on Rhizosphere Soil Physicochemical Properties
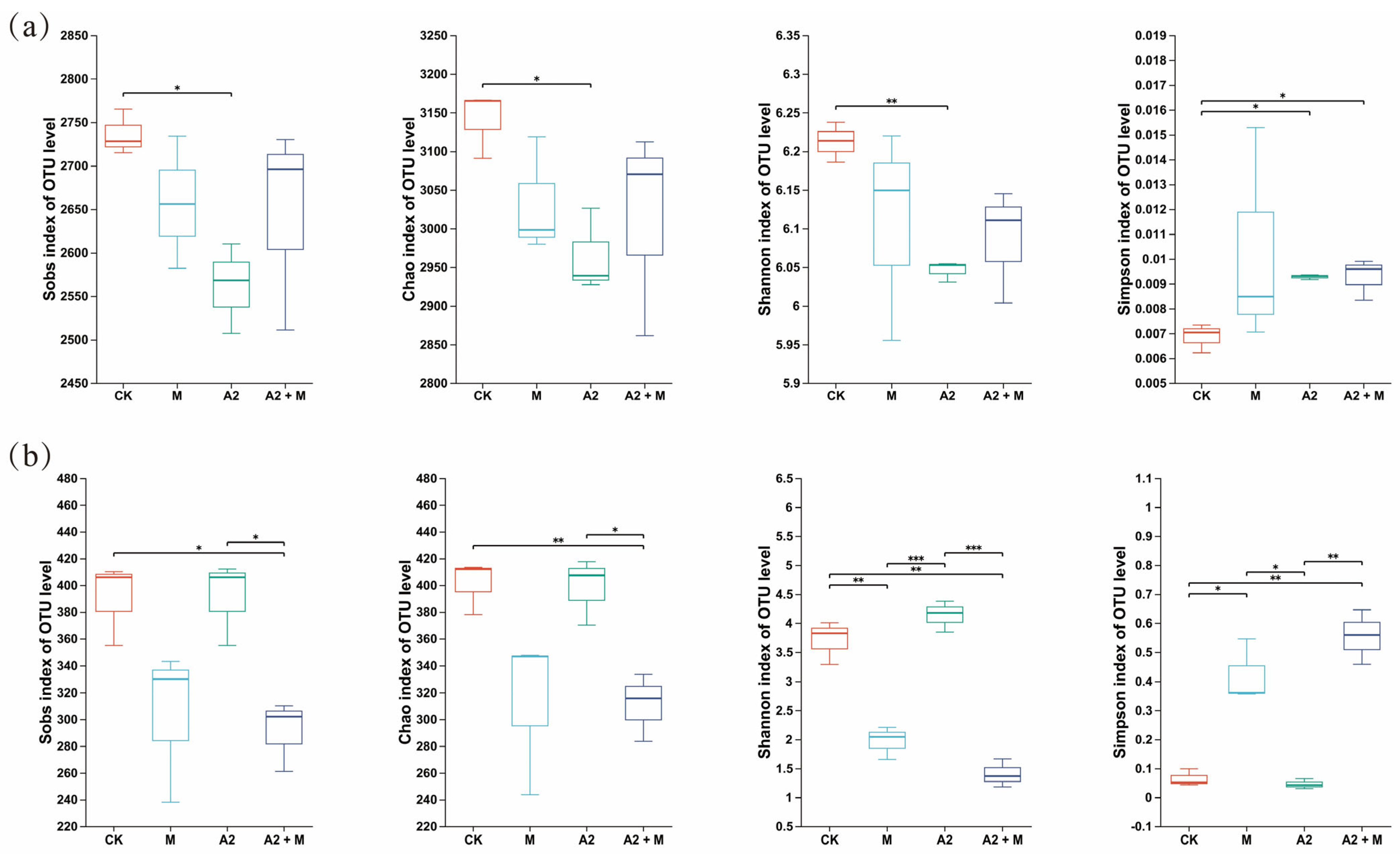
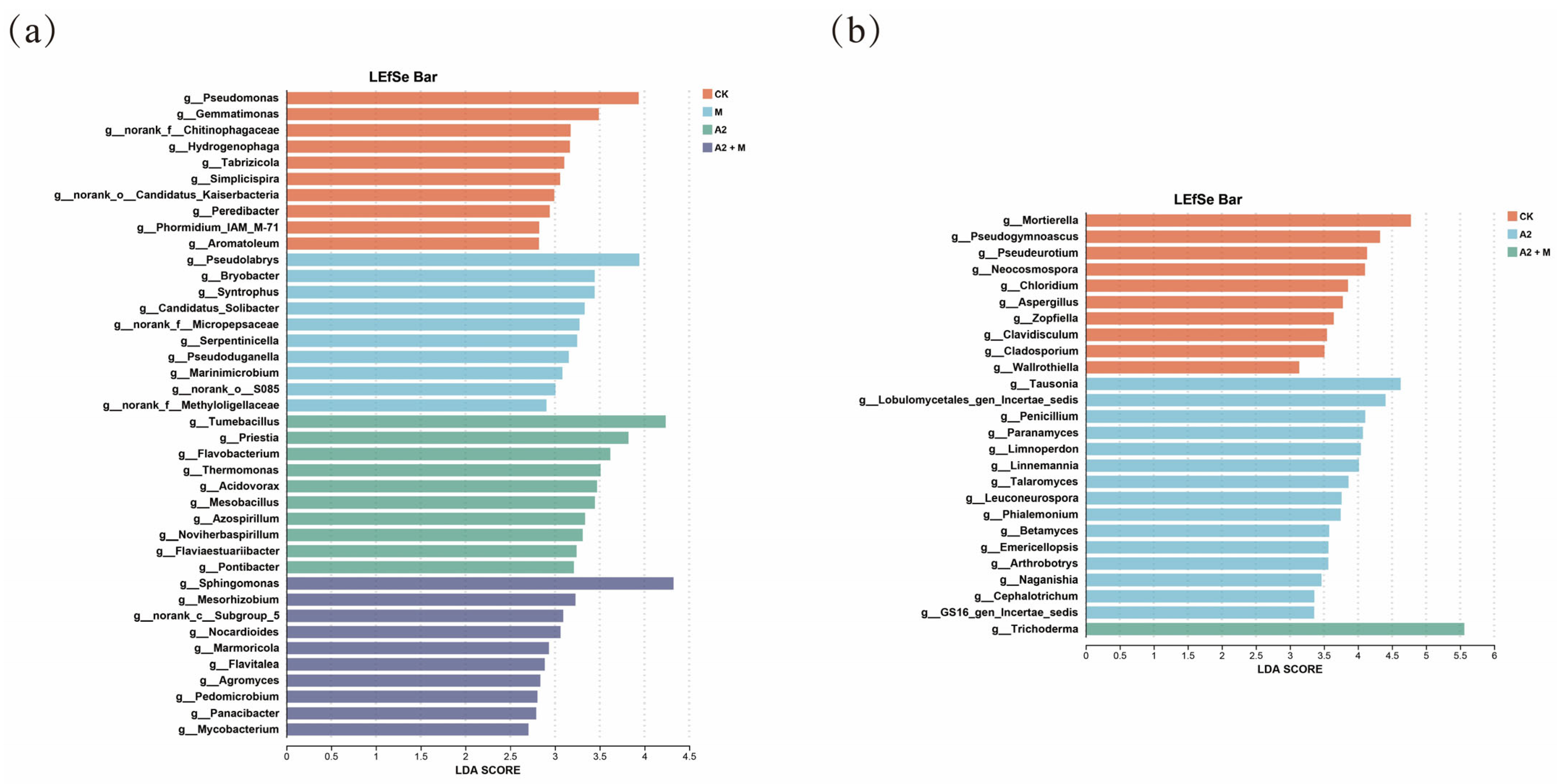
3.3. Effects of Different Inoculation Treatments on Rhizosphere Microbial Community Diversity
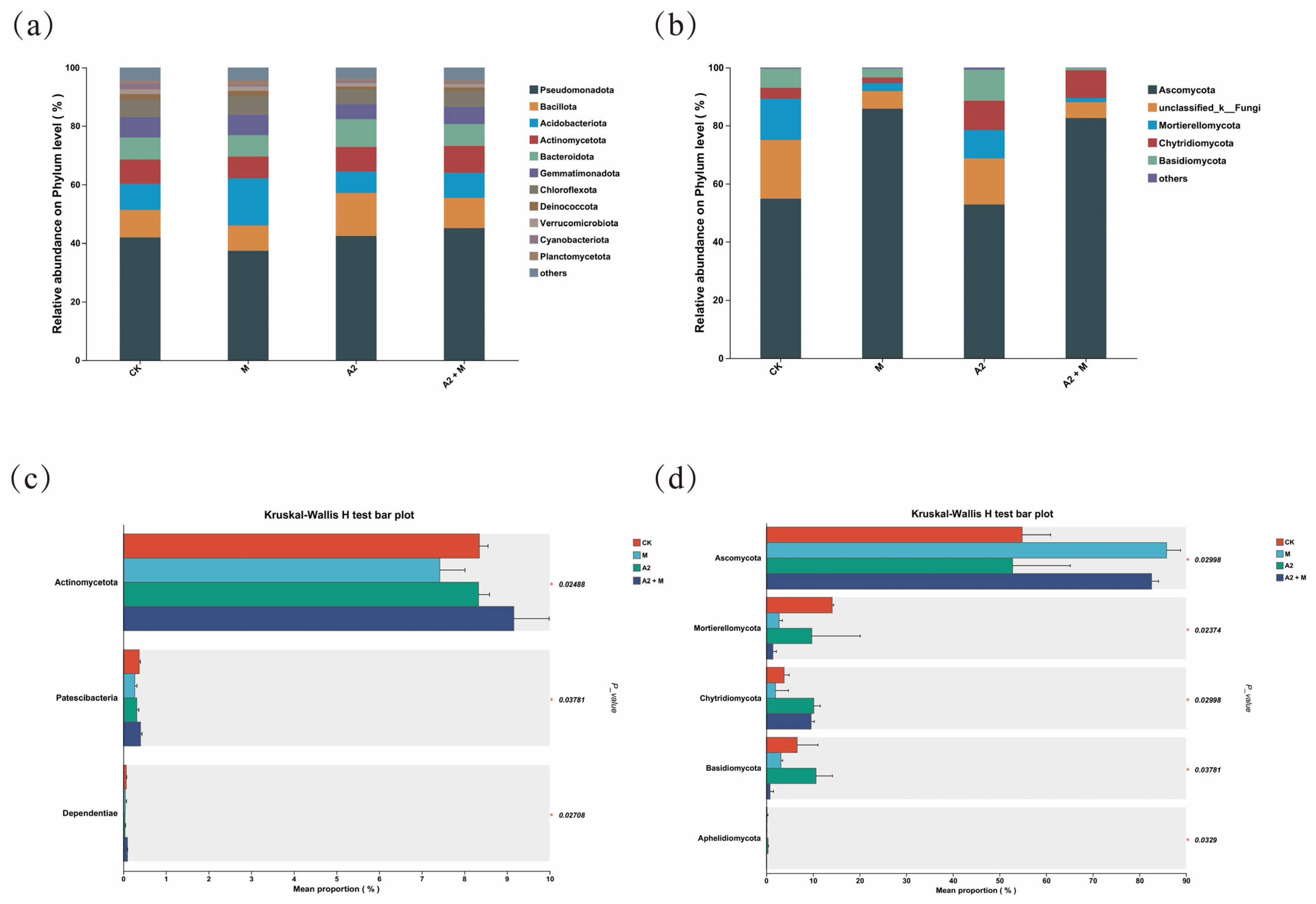
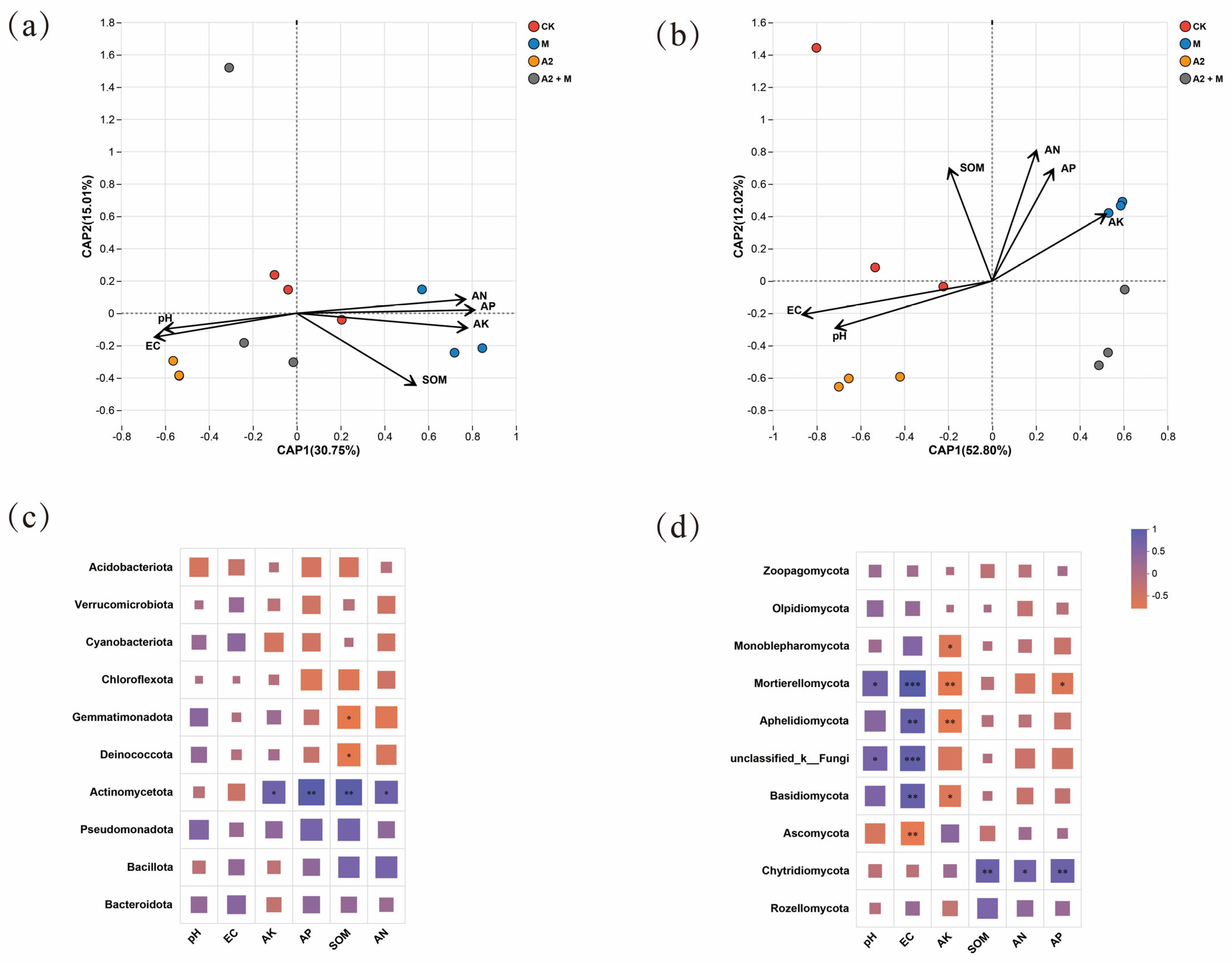
3.4. Effects of Inoculation Treatments on Rhizosphere Microbial Community Structure
3.5. Interactions of Rice Rhizosphere Microbial Communities with Environmental Variables and Physiology
4. Discussion
4.1. Effects of Microbial Inoculation on Rice Physiological and Biochemical Characteristics
4.2. Effects of Microbial Inoculation on Soil Nutrients
4.3. Effects of Microbial Inoculation on Soil Microbial Diversity and Composition
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Z.; Bian, F.; Wang, Z.; Zhu, J.; Zhang, X.; Wang, J.; Gai, X.; Zhong, Z. Microorganisms facilitated the saline-alkali soil remediation by biochar: Soil properties, microbial communities, and plant responses. Land Degrad. Dev. 2024, 35, 3567–3578. [Google Scholar] [CrossRef]
- Oelviani, R.; Adiyoga, W.; Suhendrata, T.; Bakti, I.G.M.Y.; Sutanto, H.A.; Fahmi, D.A.; Chanifah, C.; Jatuningtyas, R.K.; Samijan, S.; Malik, A.; et al. Effects of soil salinity on rice production and technical efficiency: Evidence from the northern coastal region of Central Java, Indonesia. Case Stud. Chem. Environ. Eng. 2024, 10, 101010. [Google Scholar] [CrossRef]
- Xing, J.; Li, X.; Li, Z.; Wang, X.; Hou, N.; Li, D. Remediation of soda-saline-alkali soil through soil amendments: Microbially mediated carbon and nitrogen cycles and remediation mechanisms. Sci. Total Environ. 2024, 924, 171641. [Google Scholar] [CrossRef]
- Sun, Z.; Ge, J.; Li, C.; Wang, Y.; Zhang, F.; Lei, X. Enhanced improvement of soda saline-alkali soil by in-situ formation of super-stable mineralization structure based on CaFe layered double hydroxide and its large-scale application. Chemosphere 2022, 300, 134543. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Wang, J.; Han, L.; Tan, J.; Ge, X. Ameliorating saline-sodic soils: A global meta-analysis of field studies on the influence of exogenous amendments on crop yield. Land Degrad. Dev. 2024, 35, 3330–3343. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D.; Schubert, S.; Noble, A.D.; Sahrawat, K.L. Phytoremediation of Sodic and Saline-Sodic Soils. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2007; Volume 96, pp. 197–247. [Google Scholar]
- Zhang, W.-W.; Wang, C.; Xue, R.; Wang, L.-J. Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Nie, Y.; Jiang, L.; Liu, X.; Feng, L.; Li, Z. Study on the Use of Soda Saline–Alkali Soil as a Rice-Seedling-Raising Soil After Short-Term Improvement. Appl. Sci. 2025, 15, 4638. [Google Scholar] [CrossRef]
- Peng, W.; Zhu, X.; Zheng, W.; Xie, Q.; Wang, M.; Ran, E. Rice cultivation can mitigate soil salinization and alkalization by modifying the macropore structure in saline–sodic paddy fields. Agric. Water Manag. 2025, 313, 109473. [Google Scholar] [CrossRef]
- Anam, G.B.; Reddy, M.S.; Ahn, Y.-H. Characterization of Trichoderma asperellum RM-28 for its sodic/saline-alkali tolerance and plant growth promoting activities to alleviate toxicity of red mud. Sci. Total Environ. 2019, 662, 462–469. [Google Scholar] [CrossRef]
- Korres, N.E.; Loka, D.A.; Gitsopoulos, T.K.; Varanasi, V.K.; Chachalis, D.; Price, A.; Slaton, N.A. Salinity effects on rice, rice weeds, and strategies to secure crop productivity and effective weed control. A review. Agron. Sustain. Dev. 2022, 42, 58. [Google Scholar] [CrossRef]
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective strategies for reclamation of saline-alkali soil and response mechanisms of the soil-plant system. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef]
- de Andrade, L.A.; Santos, C.H.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Al Tawaha, A.R.M.; Karnwal, A.; Pati, S.; Al-Tawaha, A.R.; Upadhyay, A.K.; Singh, A.; Rajput, V.D.; Ghazaryan, K.; Minkina, T.; Ali, I.; et al. Chapter 14—Biofertilizers: A sustainable solution for enhancing soil fertility and crop productivity. In Sustainable Agriculture under Drought Stress; Etesami, H., Chen, Y., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 209–217. [Google Scholar]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, J.; Arora, N.K. Plant Growth-Promoting Rhizobacteria: Diversity and Applications. In Environmental Biotechnology: For Sustainable Future; Sobti, R.C., Arora, N.K., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 129–173. [Google Scholar]
- Gul, F.; Khan, I.U.; Rutherford, S.; Dai, Z.C.; Li, G.; Du, D.L. Plant growth promoting rhizobacteria and biochar production from Parthenium hysterophorus enhance seed germination and productivity in barley under drought stress. Front. Plant Sci. 2023, 14, 1175097. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Liu, H. Modification of Rhizosphere Microbial Communities: A Possible Mechanism of Plant Growth Promoting Rhizobacteria Enhancing Plant Growth and Fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, K.; Liu, Z.; Liu, Y.; Yang, X.; Du, B.; Li, X.; Li, N.; Zhou, B.; Zhu, X.; et al. Bacillus paralicheniformis SYN-191 isolated from ginger rhizosphere soil and its growth-promoting effects in ginger farming. BMC Microbiol. 2025, 25, 75. [Google Scholar] [CrossRef]
- Wang, R.; Huo, B.; Chen, L.; Li, K.; Yi, G.; Wang, E.; Mi, G.; Sui, X. Rhizobia modulate the peanut rhizobacterial community and soil metabolites depending on nitrogen availability. Biol. Fertil. Soils 2023, 59, 887–900. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, L.; Wang, Y.; Mei, P.; Zhou, F.; Rozhkova, T.; Li, C. Effects of Streptomyces sp. HU2014 inoculation on wheat growth and rhizosphere microbial diversity under hexavalent chromium stress. Ecotoxicol. Environ. Saf. 2024, 276, 116313. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xiao, Y.; Wang, Y.-F.; Liu, Z.-H.; Yang, K.-J. Trichoderma affects the physiochemical characteristics and bacterial community composition of saline–alkaline maize rhizosphere soils in the cold-region of Heilongjiang Province. Plant Soil 2019, 436, 211–227. [Google Scholar] [CrossRef]
- Jian’ai, C.; Youchen, D.; Feng, G.U.O.; Wuhan, Y.; Weijing, C.; Shubo, W.A.N. Ecological effect of Trichoderma agent on platform field soil improvement in saline coastal area. Chin. J. Eco-Agric. 2016, 24, 90–97. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystems. Microbiol. Res. 2024, 281, 127621. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Shahraki-Mojahed, L.; Piri, R.; Sobhanizadeh, A. 20-Trichoderma: Improving growth and tolerance to biotic and abiotic stresses in plants. In Trends of Applied Microbiology for Sustainable Economy; Soni, R., Suyal, D.C., Yadav, A.N., Goel, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 525–564. [Google Scholar]
- Sarsan, S.; Pandiyan, A.; Rodhe, A.V.; Jagavati, S. Synergistic Interactions Among Microbial Communities. In Microbes in Microbial Communities: Ecological and Applied Perspectives; Singh, R.P., Manchanda, G., Bhattacharjee, K., Panosyan, H., Eds.; Springer: Singapore, 2021; pp. 1–37. [Google Scholar]
- Sun, Y.; Tang, L.; Cui, Y.; Yang, D.; Gao, H.; Chen, J.; Zheng, Z.; Guo, C. Inoculation of plant growth-promoting rhizobacteria and rhizobia changes the protist community of alfalfa rhizosphere soil under saline-alkali environment. Appl. Soil Ecol. 2025, 206, 105775. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Z.; Feng, S.; Guo, P.; Wang, Y.; Hao, B.; Guo, W.; Li, F.Y. Synergistic effects of AMF and PGPR on improving saline-alkaline tolerance of Leymus chinensis by strengthening the link between rhizosphere metabolites and microbiomes. Environ. Technol. Innov. 2024, 36, 103900. [Google Scholar] [CrossRef]
- Chaves, N.P.; Pocasangre, L.E.; Elango, F.; Rosales, F.E.; Sikora, R. Combining endophytic fungi and bacteria for the biocontrol of Radopholus similis (Cobb) Thorne and for effects on plant growth. Sci. Hortic. 2009, 122, 472–478. [Google Scholar] [CrossRef]
- Neelipally, R.T.; Anoruo, A.O.; Nelson, S. Effect of Co-Inoculation of Bradyrhizobium and Trichoderma on Growth, Development, and Yield of Arachis hypogaea L. (Peanut). Agronomy 2020, 10, 1415. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control 2022, 176, 105100. [Google Scholar] [CrossRef]
- Han, Z.; Chen, L.; Wang, W.; Guan, X.; Song, J.; Ma, S. Biochemical and Transcriptomic Analyses Reveal Key Salinity and Alkalinity Stress Response and Tolerance Pathways in Salix linearistipularis Inoculated with Trichoderma. Agronomy 2024, 14, 2358. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef]
- Malar, S.; Shivendra Vikram, S.; Jc Favas, P.; Perumal, V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths (Eichhornia crassipes (Mart.)). Bot. Stud. 2014, 55, 54. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Pavuk, M.; Päpke, O.; Malisch, R. The use of potassium dichromate and ethyl alcohol as blood preservatives for analysis of organochlorine contaminants. Chemosphere 2004, 57, 1–7. [Google Scholar] [CrossRef]
- Fulford, A.M.; Roberts, T.L. Soil total carbon and nitrogen as indicators of rice response to supplemental fertilizer nitrogen. Soil Sci. Soc. Am. J. 2022, 86, 1665–1676. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Jiao, R. Soil phosphorus fractions, phosphatase activity, and the abundance of phoC and phoD genes vary with planting density in subtropical Chinese fir plantations. Soil Tillage Res. 2021, 209, 104946. [Google Scholar] [CrossRef]
- Wei, H.; Deng, Y.; Lin, L.; Wang, J.; Huang, J. Improved soil composition promotes nutrient recovery during vegetation restoration in karst peak-cluster depressions. CATENA 2023, 222, 106769. [Google Scholar] [CrossRef]
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil salinization in agriculture: Mitigation and adaptation strategies combining nature-based solutions and bioengineering. iScience 2024, 27, 108830. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.J.; Wang, W.Y.; Li, Y.; Liu, L.; Ma, S.R. Effects of Trichoderma longibrachiatum on Salinity and Alkali Tolerance in Rice Seedlings. For. Eng. 2023, 39, 37–45. [Google Scholar]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.-F.; Liu, Z.-H.; Li, Z.-T.; Yang, K.-J. Trichoderma asperellum alleviates the effects of saline–alkaline stress on maize seedlings via the regulation of photosynthesis and nitrogen metabolism. Plant Growth Regul. 2018, 85, 363–374. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Sharma, P.; Manokaran, R.; Lakshman, D.K.; Rokadia, P.; Jambhulkar, N. Assessing synergism of combined applications of Trichoderma harzianum and Pseudomonas fluorescens to control blast and bacterial leaf blight of rice. Eur. J. Plant Pathol. 2018, 152, 747–757. [Google Scholar] [CrossRef]
- Akbari, S.I.; Prismantoro, D.; Permadi, N.; Rossiana, N.; Miranti, M.; Mispan, M.S.; Mohamed, Z.; Doni, F. Bioprospecting the roles of Trichoderma in alleviating plants’ drought tolerance: Principles, mechanisms of action, and prospects. Microbiol. Res. 2024, 283, 127665. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ali, M.; Jing, H.; Hussain, A.; Manghwar, H.; Ali, M.; Raza, W.; Mundra, S. Power of plant microbiome: A sustainable approach for agricultural resilience. Plant Stress 2024, 14, 100681. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Zhang, H. The Mechanisms of Trichoderma Species to Reduce Drought and Salinity Stress in Plants. Phyton Int. J. Exp. Bot. 2023, 92, 2261–2281. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Arora, N.K. Plant growth promoting bacteria for combating salinity stress in plants—Recent developments and prospects: A review. Microbiol. Res. 2021, 252, 126861. [Google Scholar] [CrossRef]
- Santoyo, G.; Orozco-Mosqueda, M.C.; Afridi, M.S.; Mitra, D.; Valencia-Cantero, E.; Macías-Rodríguez, L. Trichoderma and Bacillus multifunctional allies for plant growth and health in saline soils: Recent advances and future challenges. Front. Microbiol. 2024, 15, 1423980. [Google Scholar] [CrossRef]
- Wahab, A.; Batool, F.; Abdi, G.; Muhammad, M.; Ullah, S.; Zaman, W. Role of plant growth-promoting rhizobacteria in sustainable agriculture: Addressing environmental and biological challenges. J. Plant Physiol. 2025, 307, 154455. [Google Scholar] [CrossRef]
- Cheng, L.; Xu, Z.; Zhou, X. Application of Trichoderma species increases plant salinity resistance: A bibliometric analysis and a meta-analysis. J. Soils Sediments 2023, 23, 2641–2653. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wang, X.; Feng, Z.; Yang, E.; Wu, M.; Jiang, Y.; Huang, J.; Gao, Z.; Du, Y. The synergistic effect of extracellular polysaccharide-producing salt-tolerant bacteria and biochar promotes grape growth under saline-alkaline stress. Environ. Technol. Innov. 2025, 38, 104070. [Google Scholar] [CrossRef]
- Wen, Y.; Wu, R.; Qi, D.; Xu, T.; Chang, W.; Li, K.; Fang, X.; Song, F. The effect of AMF combined with biochar on plant growth and soil quality under saline-alkali stress: Insights from microbial community analysis. Ecotoxicol. Environ. Saf. 2024, 281, 116592. [Google Scholar] [CrossRef]
- Zheng, C.; Kong, K.; Zhang, Y.; Yang, W.; Wu, L.; Munir, M.Z.; Ji, B.; Muneer, M.A. Differential response of bacterial diversity and community composition to different tree ages of pomelo under red and paddy soils. Front. Microbiol. 2022, 13, 958788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, X.; Wang, G.; Wu, B.; Xiao, Y. Medicago sativa and soil microbiome responses to Trichoderma as a biofertilizer in alkaline-saline soils. Appl. Soil Ecol. 2020, 153, 103573. [Google Scholar] [CrossRef]
- Velmourougane, K.; Prasanna, R.; Singh, S.; Chawla, G.; Kumar, A.; Saxena, A.K. Modulating rhizosphere colonisation, plant growth, soil nutrient availability and plant defense enzyme activity through Trichoderma viride-Azotobacter chroococcum biofilm inoculation in chickpea. Plant Soil 2017, 421, 157–174. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-Based Biostimulants Optimize N Use Efficiency and Stimulate Growth of Leafy Vegetables in Greenhouse Intensive Cropping Systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Ladha, J.K.; Peoples, M.B.; Reddy, P.M.; Biswas, J.C.; Bennett, A.; Jat, M.L.; Krupnik, T.J. Biological nitrogen fixation and prospects for ecological intensification in cereal-based cropping systems. Field Crops Res. 2022, 283, 108541. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Singh, K.; Chandra, R. Recent advances of plant growth promoting rhizobacteria (PGPR) for eco-restoration of polluted Soil. Clean. Eng. Technol. 2024, 23, 100845. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Chen, J.; Wang, R.; Zhang, J.; Hou, J.; Liu, T. Insights into the molecular mechanism of Trichoderma stimulating plant growth and immunity against phytopathogens. Physiol. Plant 2023, 175, e14133. [Google Scholar] [CrossRef]
- Chamkhi, I.; El Omari, N.; Balahbib, A.; El Menyiy, N.; Benali, T.; Ghoulam, C. Is the rhizosphere a source of applicable multi-beneficial microorganisms for plant enhancement? Saudi J. Biol. Sci. 2022, 29, 1246–1259. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, K.; Geisen, S.; Yang, W.; Yan, Y.; Gu, D.; Liu, N.; Borisjuk, N.; Luo, Y.; Friman, V.-P. The effect of microbial inoculant origin on the rhizosphere bacterial community composition and plant growth-promotion. Plant Soil 2020, 452, 105–117. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Z.; Li, K.; Sha, Y.; Wang, E.; Sui, X.; Mi, G.; Tian, C.; Chen, W. Effects of growth-promoting rhizobacteria on maize growth and rhizosphere microbial community under conservation tillage in Northeast China. Microb. Biotechnol. 2021, 14, 535–550. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, C.; Zheng, K.; Sun, Y.; Ru, J.; Ma, Y.; Zhang, X.; Zhou, Y.; Zhuang, J. Native mixed microbe inoculants (M1H) optimize soil health to promote Cajanus cajan growth: The soil fungi are more sensitive than bacteria. Front. Microbiol. 2025, 16, 1521064. [Google Scholar] [CrossRef]
- Nie, H.; Shi, Y.; Yang, X.; Zeng, J.; Tang, Y.; Liu, X.; Sun, L.; Zhou, Y.; Xu, X.; Liu, M.; et al. Microbial inoculant-induced modifications of rhizospheric metabolites and microbial communities enhance plant growth. Plant Soil 2024, 1–19. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, H.; Hu, J.; Li, H.; Zhao, Z.; Wu, Y.; Li, J.; Zhou, Y.; Yang, K.; Yang, H. Trichoderma harzianum inoculation promotes sweet sorghum growth in the saline soil by modulating rhizosphere available nutrients and bacterial community. Front. Plant Sci. 2023, 14, 1258131. [Google Scholar] [CrossRef]
- Zhang, R.; Vivanco, J.M.; Shen, Q. The unseen rhizosphere root–soil–microbe interactions for crop production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Liu, R.; Liang, B.; Zhao, H.; Zhao, Y. Impacts of various amendments on the microbial communities and soil organic carbon of coastal saline–alkali soil in the Yellow River Delta. Front. Microbiol. 2023, 14, 1239855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, Z.; Lin, Q.; Lin, Y.; Long, K.; Xie, Z.; Hu, W. Salt stress affects the bacterial communities in rhizosphere soil of rice. Front. Microbiol. 2024, 15, 1505368. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Kang, S.-M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.-Y.; et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef]

| Treatments | Plant Height (cm) | Root Length (cm) | Dry Weight (g) | Fresh Weight (g) |
|---|---|---|---|---|
| CK | 23.69 ± 1.63 f | 5.93 ± 1.13 e | 0.06 ± 0.01 f | 0.32 ± 0.10 d |
| M | 33.41 ± 1.72 c | 7.62 ± 0.90 d | 0.09 ± 0.02 de | 0.54 ± 0.14 b |
| A1 | 29.80 ± 0.55 e | 7.93 ± 0.68 cd | 0.09 ± 0.01 de | 0.54 ± 0.06 b |
| A2 | 30.89 ± 0.95 de | 7.31 ± 0.84 de | 0.08 ± 0.02 e | 0.49 ± 0.10 bc |
| B3 | 32.09 ± 1.06 cd | 8.10 ± 1.83 cd | 0.11 ± 0.03 cd | 0.57 ± 0.15 b |
| E2 | 32.86 ± 0.82 c | 8.29 ± 1.28 bcd | 0.09 ± 0.01 de | 0.56 ± 0.07 b |
| A1 + M | 35.38 ± 1.18 b | 11.43 ± 2.53 a | 0.13 ± 0.02 bc | 0.81 ± 0.12 a |
| A2 + M | 39.74 ± 1.44 a | 9.82 ± 0.48 b | 0.18 ± 0.03 a | 0.93 ± 0.12 a |
| B3 + M | 36.61 ± 2.44 b | 9.35 ± 0.40 bc | 0.14 ± 0.03 b | 0.89 ± 0.18 a |
| E2 + M | 29.92 ± 1.30 e | 7.56 ± 1.20 d | 0.07 ± 0.01 ef | 0.40 ± 0.06 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Guan, X.; Chen, L.; Han, Z.; Cui, H.; Ma, S. Cooperative Interplay Between PGPR and Trichoderma longibrachiatum Reprograms the Rhizosphere Microecology for Improved Saline Alkaline Stress Resilience in Rice Seedlings. Microorganisms 2025, 13, 1562. https://doi.org/10.3390/microorganisms13071562
Song J, Guan X, Chen L, Han Z, Cui H, Ma S. Cooperative Interplay Between PGPR and Trichoderma longibrachiatum Reprograms the Rhizosphere Microecology for Improved Saline Alkaline Stress Resilience in Rice Seedlings. Microorganisms. 2025; 13(7):1562. https://doi.org/10.3390/microorganisms13071562
Chicago/Turabian StyleSong, Junjie, Xueting Guan, Lili Chen, Zhouqing Han, Haojun Cui, and Shurong Ma. 2025. "Cooperative Interplay Between PGPR and Trichoderma longibrachiatum Reprograms the Rhizosphere Microecology for Improved Saline Alkaline Stress Resilience in Rice Seedlings" Microorganisms 13, no. 7: 1562. https://doi.org/10.3390/microorganisms13071562
APA StyleSong, J., Guan, X., Chen, L., Han, Z., Cui, H., & Ma, S. (2025). Cooperative Interplay Between PGPR and Trichoderma longibrachiatum Reprograms the Rhizosphere Microecology for Improved Saline Alkaline Stress Resilience in Rice Seedlings. Microorganisms, 13(7), 1562. https://doi.org/10.3390/microorganisms13071562






