Short-Chain Fatty Acid Utilization in Cyberlindnera jadinii for Single-Cell Protein and Odd-Chain Fatty Acid Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Cultures and Media
2.2. Small-Scale Cultivation
2.3. Stirred Tank Bioreactor Fermentation
2.4. Optical Density and Cell Dry Weight
2.5. Short-Chain Fatty Acid Quantification
2.6. Crude Protein Analysis
2.7. Lipid Analysis
3. Results
3.1. Small-Scale Cultivation with Increasing SCFA Concentrations
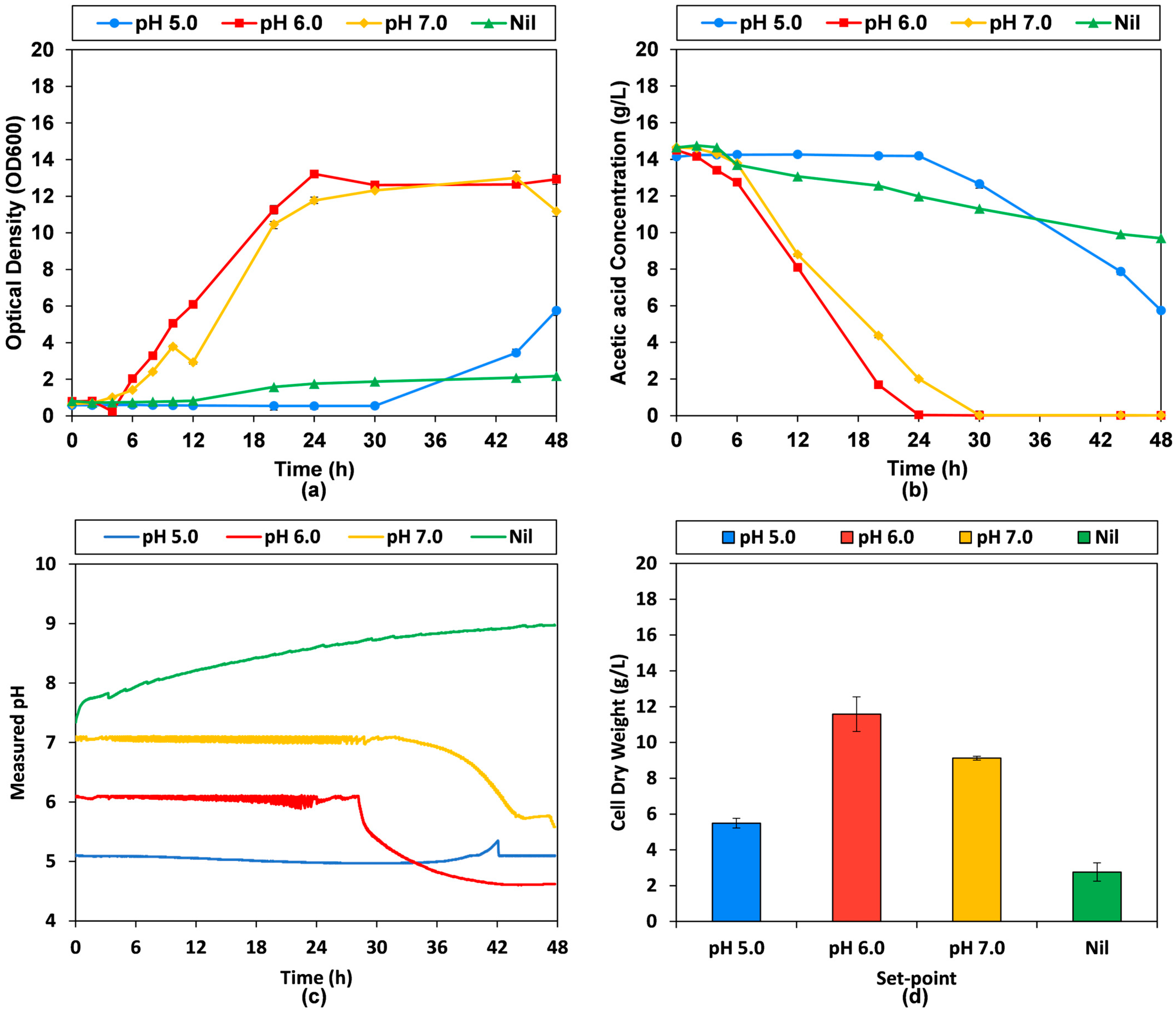
3.2. Stirred Tank Bioreactor Fermentation with Different pH Control Settings
3.3. The Stirred Tank Bioreactor pH-Stat Bioprocess
3.4. Bioprocess Titer, Rate, Yield and Biomass Composition
3.5. Single-Cell Protein Characterization
4. Discussion
4.1. SCFA Metabolism in Cyberlindnera jadinii
4.2. Single-Cell Protein from Short-Chain Fatty Acids
4.3. Lipid Production from Short-Chain Fatty Acids
4.4. C. jadinii as a Chassis for Odd-Chain Fatty Acid Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Fed-Batch pH-Stat Fermentation 10g/L Sodium SCFA and 3M SCFA Feed
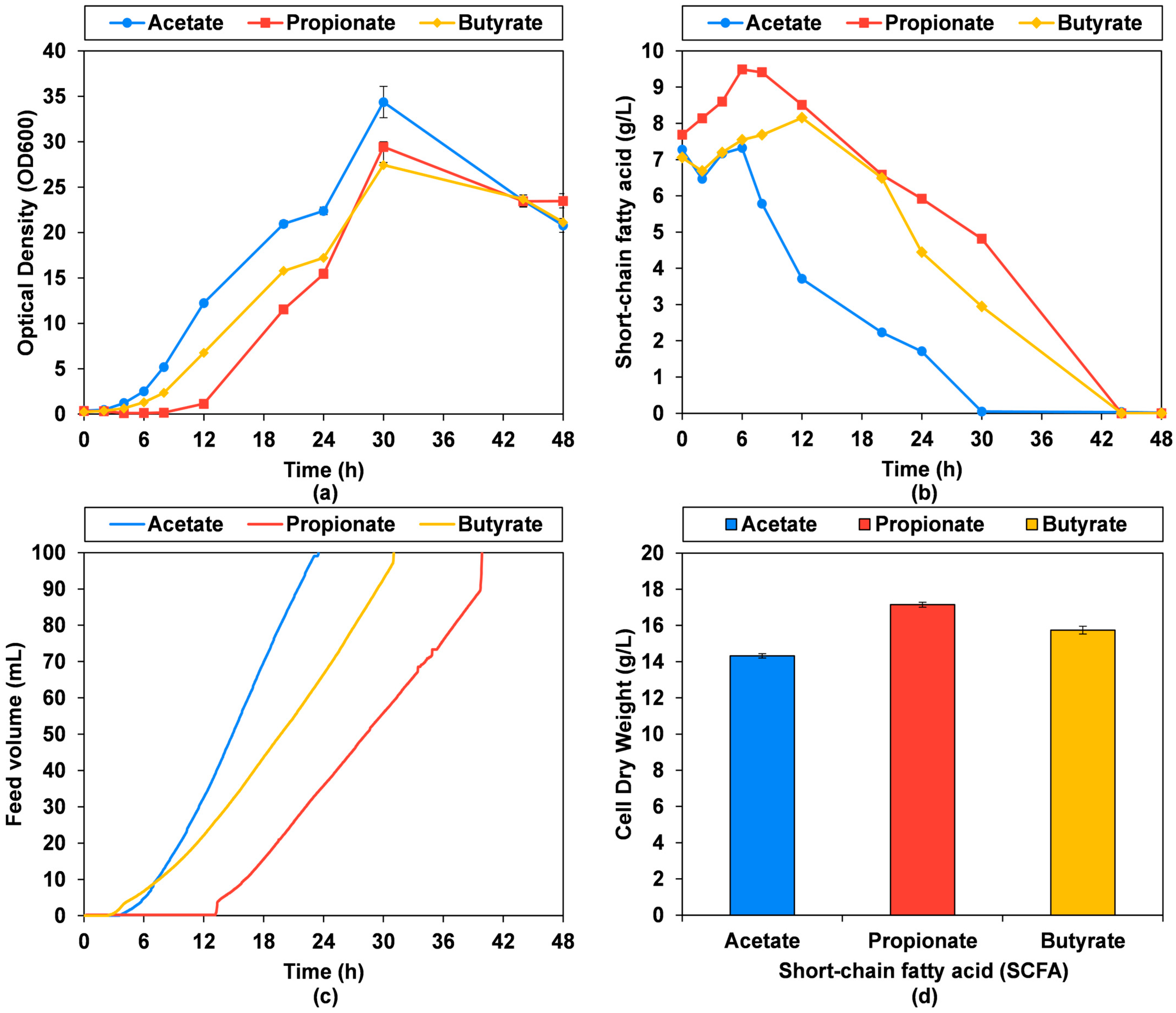
| Fed-Batch pH-Stat Fermentation 10 g/L Sodium SCFA, 3 M SCFA Feed | |||
|---|---|---|---|
| pH-stat parameters (dwb) | Acetate | Propionate | Butyrate |
| pH set-point | 6.0 ± 0.1 | 6.0 ± 0.1 | 6.0 ± 0.1 |
| Time (t) | 48 h | 48 h | 48 h |
| Batch volume (VBatch) | 200 mL | 200 mL | 200 mL |
| Feed volume (VFeed) | 100 mL | 100 mL | 100 mL |
| Batch concentration (SCFABatch) | 7.3 g/L | 7.7 g/L | 8.0 g/L |
| Feed concentration (SCFAFeed) | 180 g/L | 222 g/L | 264 g/L |
| Final concentration (SCFAFinal) | 0 g/L | 0 g/L | 0 g/L |
| pH-stat TRY (dwb) | Acetate | Propionate | Butyrate |
| Biomass titer (X) | 14.3 ± 0.12 g/L | 17.1 ± 0.1 g/L | 15.7 ± 0.2 g/L |
| Biomass rate (RX) | 0.30 ± 0.00 g/L/h | 0.36 ± 0.00 g/L/h | 0.33 ± 0.00g/L/h |
| Biomass yield (YX/S) | 0.22 ± 0.00 g/g | 0.22 ± 0.00 g/g | 0.17 ± 0.00 g/g |
| Fed-Batch pH-Stat Fermentation 10 g/L Sodium SCFA, 3 M SCFA Feed | |||
|---|---|---|---|
| C. jadinii composition (dwb) | Acetate | Propionate | Butyrate |
| Crude protein | 41.8% ± 0.7% | - | - |
| Crude lipid | 11.50% | 13.50% | 12.50% |
| C. jadinii fatty acids (dwb) | Acetate | Propionate | Butyrate |
| Pentadecanoic acid (C15:0) | 0% | 1% | 0% |
| Palmitic acid (C16:0) | 11% | 6% | 9% |
| Palmitoleic acid (C16:1) | 1% | 1% | 1% |
| Margaric acid (C17:0) | 0% | 7% | 0% |
| Margaroleic acid (C17:1) | 0% | 32% | 0% |
| Stearic acid (C18:0) | 1% | 0% | 2% |
| Oleic acid (C18:1) | 33% | 22% | 35% |
| Linoleic acid (C18:2) | 43% | 24% | 46% |
| Linolenic acid (C18:3) | 10% | 7% | 7% |
| Total saturated | 12% | 13% | 11% |
| Total unsaturated | 88% | 87% | 89% |
| Total odd-chain | 0% | 39% | 0% |
| Total even-chain | 100% | 61% | 100% |
References
- Hoa, T.; Wang, X.; Utomo, D.; Gage, E.; Xu, B. Cleaner and Circular Bioeconomy Circular Bioeconomy and Sustainable Food Systems: What Are the Possible Mechanisms? Clean. Circ. Bioeconomy 2025, 11, 100145. [Google Scholar] [CrossRef]
- Sabaghi, M.; Seyedalmoosavi, M.M. Applications of Sustainable Proteins in Food and Feed, and Perspectives on Health and Circular Bioeconomy. Int. J. Biol. Macromol. 2025, 309, 143193. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.J.O.; Chan, S. Future Production of Yeast Biomass for Sustainable Proteins: A Critical Review. Sustain. Food Technol. 2024, 2, 1592–1609. [Google Scholar] [CrossRef]
- Chan, A.M.Z.; Hau, V.J.H.; Haberkorn, I.; Mathys, A.; Liu, S.-Q. Strategies to Overcome Challenges in Upcycling Industrial Side-Streams into Microalgae-Based Foods: Auxenochlorella Protothecoides as a Case Study. Curr. Opin. Food Sci. 2025, 63, 101310. [Google Scholar] [CrossRef]
- Zhang, C.; Ottenheim, C.; Weingarten, M.; Ji, L.H. Microbial Utilization of Next-Generation Feedstocks for the Biomanufacturing of Value-Added Chemicals and Food Ingredients. Front. Bioeng. Biotechnol. 2022, 10, 874612. [Google Scholar] [CrossRef] [PubMed]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein-State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Park, Y.K.; González-Fernández, C.; Robles-Iglesias, R.; Vidal, L.; Fontanille, P.; Kennes, C.; Tomás Pejó, E.; Nicaud, J.M.; Fickers, P. Bioproducts Generation from Carboxylate Platforms by the Non-Conventional Yeast Yarrowia Lipolytica. FEMS Yeast Res. 2021, 21, foab047. [Google Scholar] [CrossRef]
- Žganjar, M.; Ogrizović, M.; Matul, M.; Čadež, N.; Gunde-Cimerman, N.; González-Fernández, C.; Gostinčar, C.; Tomás-Pejó, E.; Petrovič, U. High-Throughput Screening of Non-Conventional Yeasts for Conversion of Organic Waste to Microbial Oils via Carboxylate Platform. Sci. Rep. 2024, 14, 14233. [Google Scholar] [CrossRef]
- Feito, R.F.; Guwy, A.J.; Dinsdale, R.M.; Massanet-Nicolau, J. Real-Time Monitoring of Volatile Fatty Acids Using a Novel VFAs Analyser and Automated High Solids Separation System. J. Water Process Eng. 2025, 76, 108157. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Liu, H.; Zhou, T.; Qin, Z.; Mou, J.; Sun, J.; Huang, S.; Chaves, A.V.; Gao, L.; et al. Bioproduction and Applications of Short-Chain Fatty Acids from Secondary Sludge Anaerobic Fermentation: A Critical Review. Renew. Sustain. Energy Rev. 2023, 183, 113502. [Google Scholar] [CrossRef]
- Leone, S.; Sannino, F.; Tutino, M.L.; Parrilli, E.; Picone, D. Acetate: Friend or Foe? Efficient Production of a Sweet Protein in Escherichia Coli BL21 Using Acetate as a Carbon Source. Microb. Cell Fact. 2015, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Liu, J.N.; Lu, L.J.; Peng, K.M.; Yang, G.X.; Liu, J. Culture Strategies for Lipid Production Using Acetic Acid as Sole Carbon Source by Rhodosporidium Toruloides. Bioresour. Technol. 2016, 206, 141–149. [Google Scholar] [CrossRef]
- Xu, J.; Liu, N.; Qiao, K.; Vogg, S.; Stephanopoulos, G. Application of Metabolic Controls for the Maximization of Lipid Production in Semicontinuous Fermentation. Proc. Natl. Acad. Sci. USA 2017, 114, E5308–E5316. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, Q. Engineering Escherichia Coli to Convert Acetic Acid to β-Caryophyllene. Microb. Cell Fact. 2016, 15, 74. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; De Wever, H. Acetic Acid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock. Appl. Sci. 2018, 8, 1416. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Zhang, Z.Z.; Tan, T.W.; Li, Z.J. Metabolic Engineering of Escherichia Coli for the Synthesis of Polyhydroxyalkanoates Using Acetate as a Main Carbon Source. Microb. Cell Fact. 2018, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Marang, L.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Butyrate as Preferred Substrate for Polyhydroxybutyrate Production. Bioresour. Technol. 2013, 142, 232–239. [Google Scholar] [CrossRef]
- Qin, N.; Li, L.; Wang, Z.; Shi, S. Microbial Production of Odd-Chain Fatty Acids. Biotechnol. Bioeng. 2023, 120, 917–931. [Google Scholar] [CrossRef]
- Park, Y.K.; Dulermo, T.; Ledesma-Amaro, R.; Nicaud, J.M. Optimization of Odd Chain Fatty Acid Production by Yarrowia Lipolytica. Biotechnol. Biofuels 2018, 11, 158. [Google Scholar] [CrossRef]
- de Vicente, M.; Gonzalez-Fernández, C.; Nicaud, J.M.; Tomás-Pejó, E. Turning Residues into Valuable Compounds: Organic Waste Conversion into Odd-Chain Fatty Acids via the Carboxylate Platform by Recombinant Oleaginous Yeast. Microb. Cell Fact. 2025, 24, 32. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; González-Fernández, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzonella, D.; Müller, B.; Passoth, V. Production of Short-Chain Fatty Acids (SCFAs) as Chemicals or Substrates for Microbes to Obtain Biochemicals. Biotechnol. Biofuels Bioprod. 2023, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Azmat, U.; Rick, O.; Stanley, B.; Smits, G.J. Quantitative Analysis of the Modes of Growth Inhibition by Weak Organic Acids in Saccharomyces Cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8377–8387. [Google Scholar] [CrossRef]
- Sousa-Silva, M.; Vieira, D.; Soares, P.; Casal, M.; Soares-Silva, I. Expanding the Knowledge on the Skillful Yeast Cyberlindnera Jadinii. J. Fungi 2021, 7, 36. [Google Scholar] [CrossRef]
- Kurcz, A.; Błażejak, S.; Kot, A.M.; Bzducha-Wróbel, A.; Kieliszek, M. Application of Industrial Wastes for the Production of Microbial Single-Cell Protein by Fodder Yeast Candida Utilis. Waste Biomass Valorization 2018, 9, 57–64. [Google Scholar] [CrossRef]
- Stork, E.; Ekeberg, D.; Devle, H.M.; Umu, Ö.C.O.; Porcellato, D.; Olsen, M.A.; Vhile, S.G.; Kidane, A.; Devold, T.; Skeie, S.B. Substituting Imported Soybean Meal with Locally Produced Novel Yeast Protein in Concentrates for Norwegian Red Dairy Cows: Implications for Rumen Microbiota and Fatty Acid Composition. J. Dairy Res. 2024, 91, 156–163. [Google Scholar] [CrossRef]
- Lagos, L.; Bekkelund, A.K.; Skugor, A.; Ånestad, R.; Åkesson, C.P.; Press, C.M.L.; Øverland, M. Cyberlindnera Jadinii Yeast as a Protein Source for Weaned Piglets—Impact on Immune Response and Gut Microbiota. Front. Immunol. 2020, 11, 1924. [Google Scholar] [CrossRef] [PubMed]
- Sitepu, I.; Selby, T.; Lin, T.; Zhu, S.; Boundy-Mills, K. Carbon Source Utilization and Inhibitor Tolerance of 45 Oleaginous Yeast Species. J. Ind. Microbiol. Biotechnol. 2014, 41, 1061–1070. [Google Scholar] [CrossRef]
- Mota, M.N.; Múgica, P.; Sá-Correia, I. Exploring Yeast Diversity to Produce Lipid-Based Biofuels from Agro-Forestry and Industrial Organic Residues. J. Fungi 2022, 8, 687. [Google Scholar] [CrossRef]
- Lapeña, D.; Olsen, P.M.; Arntzen, M.; Kosa, G.; Passoth, V.; Eijsink, V.G.H.; Horn, S.J. Spruce Sugars and Poultry Hydrolysate as Growth Medium in Repeated Fed-Batch Fermentation Processes for Production of Yeast Biomass. Bioprocess Biosyst. Eng. 2020, 43, 723–736. [Google Scholar] [CrossRef]
- Lapeña, D.; Kosa, G.; Hansen, L.D.; Mydland, L.T.; Passoth, V.; Horn, S.J.; Eijsink, V.G.H. Production and Characterization of Yeasts Grown on Media Composed of Spruce-Derived Sugars and Protein Hydrolysates from Chicken by-Products. Microb. Cell Fact. 2020, 19, 19. [Google Scholar] [CrossRef]
- Camargo Guarnizo, A.F.; Woiciechowski, A.L.; Noseda, M.D.; Zevallos Torres, L.A.; Zandona Filho, A.; Pereira Ramos, L.; Letti, L.A.J.; Soccol, C.R. Pentose-Rich Hydrolysate from Oil Palm Empty Fruit Bunches for $β$-Glucan Production Using Pichia Jadinii and Cyberlindnera Jadinii. Bioresour. Technol. 2021, 320, 124212. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.C.; Hermansen, C.; Yong, A.; Siao, R.; Chua, G.G.; Ho, S.; Busran, C.T.; Teo, M.; Thong, A.; Weingarten, M. Two-Stage Bioconversion of Cellulose to Single-Cell Protein and Oil via a Cellulolytic Consortium. Fermentation 2025, 11, 72. [Google Scholar] [CrossRef]
- Woods, V.B.; Fearon, A.M. Dietary Sources of Unsaturated Fatty Acids for Animals and Their Transfer into Meat, Milk and Eggs: A Review. Livest. Sci. 2009, 126, 1–20. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, N.; Xin, H.; Yu, L.; Xu, Q.; Cui, Y. Unsaturated Fatty Acids in Natural Edible Resources, a Systematic Review of Classification, Resources, Biosynthesis, Biological Activities and Application. Food Biosci. 2023, 53, 102790. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of Dietary Odd-Chain Saturated Fatty Acid Pentadecanoic Acid Parallels Broad Associated Health Benefits in Humans: Could It Be Essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; West, J.A.; Koulman, A. A Review of Odd-Chain Fatty Acid Metabolism and the Role of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) in Health and Disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef]
- Petersen, K.S.; Maki, K.C.; Calder, P.C.; Belury, M.A.; Messina, M.; Kirkpatrick, C.F.; Harris, W.S. Perspective on the Health Effects of Unsaturated Fatty Acids and Commonly Consumed Plant Oils High in Unsaturated Fat. Br. J. Nutr. 2024, 132, 1039–1050. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Konopka, I. Update on Food Sources and Biological Activity of Odd-Chain, Branched and Cyclic Fatty Acids––A Review. Trends Food Sci. Technol. 2022, 119, 514–529. [Google Scholar] [CrossRef]
- Duan, G.; Li, M.; Zheng, C.; Wan, M.; Yu, J.; Cao, B.; Yin, Y.; Duan, Y.; Cong, F. Odd-Chain Fatty Acid-Enriched Fats Improve Growth and Intestinal Morphology and Function in Milk Replacer-Fed Piglets. J. Nutr. 2025, 155, 1298–1310. [Google Scholar] [CrossRef]
- ISO 16634-2:2016; Food Products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content—Part 2: Cereals, Pulses and Milled Cereal Products. International Organization for Standardization: Geneva, Switzerland, 2016.
- Kasemets, K.; Kahru, A.; Laht, T.-M.; Paalme, T. Study of the Toxic Effect of Short- and Medium-Chain Monocarboxylic Acids on the Growth of Saccharomyces Cerevisiae Using the CO2-Auxo-Accelerostat Fermentation System. Int. J. Food Microbiol. 2006, 111, 206–215. [Google Scholar] [CrossRef]
- Leão, C.; van Uden, N. Transport of Lactate and Other Short-Chain Monocarboxylates in the Yeast Candida Utilis. Appl. Microbiol. Biotechnol. 1986, 23, 389–393. [Google Scholar] [CrossRef]
- Sousa-Silva, M.; Soares, P.; Alves, J.; Vieira, D.; Casal, M.; Soares-Silva, I. Uncovering Novel Plasma Membrane Carboxylate Transporters in the Yeast Cyberlindnera Jadinii. J. Fungi 2022, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Krivoruchko, A.; Zhang, Y.; Siewers, V.; Chen, Y.; Nielsen, J. Microbial Acetyl-CoA Metabolism and Metabolic Engineering. Metab. Eng. 2015, 28, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty Acid Synthesis and Elongation in Yeast. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.; Schneiter, R. FIT2 Proteins and Lipid Droplet Emergence, an Interplay between Phospholipid Synthesis, Surface Tension, and Membrane Curvature. Front. Cell Dev. Biol. 2024, 12, 1422032. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. Lipid Droplets And Cellular Lipid Metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef]
- Kurihara, T.; Ueda, M.; Okada, H.; Kamasawa, N.; Naito, N.; Osumi, M.; Tanaka, A. β-Oxidation of Butyrate, the Short-Chain-Length Fatty Acid, Occurs in Peroxisomes in the Yeast Candida Tropicalis. J. Biochem. 1992, 111, 783–787. [Google Scholar] [CrossRef]
- Elmaleh, S.; Defrance, M.B.; Ghommidh, C. Organic Acids Oxidation by Candida Utilis: Application to Industrial Waste Water Treatment. Process Biochem. 1999, 35, 441–449. [Google Scholar] [CrossRef]
- Schwab, M.A.; Sauer, S.W.; Okun, J.G.; Nijtmans, L.G.J.; Rodenburg, R.J.T.; van den Heuvel, L.P.; Dröse, S.; Brandt, U.; Hoffmann, G.F.; Ter Laak, H.; et al. Secondary Mitochondrial Dysfunction in Propionic Aciduria: A Pathogenic Role for Endogenous Mitochondrial Toxins. Biochem. J. 2006, 398, 107–112. [Google Scholar] [CrossRef]
- Brock, M.; Buckel, W. On the Mechanism of Action of the Antifungal Agent Propionate. Eur. J. Biochem. 2004, 271, 3227–3241. [Google Scholar] [CrossRef]
- Dittrich, F.; Zajonc, D.; Hühne, K.; Hoja, U.; Ekici, A.; Greiner, E.; Klein, H.; Hofmann, J.; Bessoule, J.J.; Sperling, P.; et al. Fatty Acid Elongation in Yeast-Biochemical Characteristics of the Enzyme System and Isolation of Elongation-Defective Mutants. Eur. J. Biochem. 1998, 252, 477–485. [Google Scholar] [CrossRef]
- Heijnen, J.J.; van Dijken, J.P. In Search of a Thermodynamic Description of Biomass Yields for the Chemotropic Growth of Microorganisms. Biotechnol. Bioeng. 1992, 39, 833–858. [Google Scholar] [CrossRef]
- Verduyn, C.; Stouthamer, A.H.; Scheffers, W.A.; van Dijken, J.P. A Theoretical Evaluation of Growth Yields of Yeasts. Antonie Van Leeuwenhoek 1991, 59, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Verduyn, C. Physiology of Yeasts in Relation to Biomass Yields. Antonie Van Leeuwenhoek 1991, 60, 325–353. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Horne, T.; Hollomon, D.W.; Wood, P.M. Fungal Respiration: A Fusion of Standard and Alternative Components. Biochim. Biophys. Acta Bioenerg. 2001, 1504, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Lara, M.A.; Warmerdam, M.; de Hulster, E.A.F.; van den Broek, M.; Daran, J.-M.; Pronk, J.T. Quantitative Physiology and Biomass Composition of Cyberlindnera Jadinii in Ethanol-Grown Cultures. Biotechnol. Biofuels Bioprod. 2024, 17, 142. [Google Scholar] [CrossRef]
- Garay, L.A.; Sitepu, I.R.; Cajka, T.; Chandra, I.; Shi, S.; Lin, T.; German, J.B.; Fiehn, O.; Boundy-Mills, K.L. Eighteen New Oleaginous Yeast Species. J. Ind. Microbiol. Biotechnol. 2016, 43, 887–900. [Google Scholar] [CrossRef]
- Peterson, E.C.; Siao, R.; Chua, G.G.; Busran, C.T.; Pavlovic, R.; Thong, A.; Hermansen, C.; Sofeo, N.; Kanagasundaram, Y.; Weingarten, M.; et al. Single Cell Protein and Oil Production from Solid Cocoa Fatty Acid Distillates Co-Fed Ethanol. Bioresour. Technol. 2023, 387, 129630. [Google Scholar] [CrossRef]
- Sofeo, N.; Toi, M.G.; Ee, E.Q.G.; Ng, J.Y.; Busran, C.T.; Lukito, B.R.; Thong, A.; Hermansen, C.; Peterson, E.C.; Glitsos, R.; et al. Sustainable Production of Lipids from Cocoa Fatty Acid Distillate Fermentation Driven by Adaptive Evolution in Yarrowia Lipolytica. Bioresour. Technol. 2024, 394, 130302. [Google Scholar] [CrossRef]
- Christman, Z. Growing Torula Yeast (Candida Utilis) For Food Grade Fatty Acids; University of Nebraska: Lincoln, NE, USA, 2020. [Google Scholar]
- McMurrough, I.; Rose, A.H. Effects of Temperature Variation on the Fatty Acid Composition of Candida Utilis. J. Bacteriol. 1971, 107, 753–758. [Google Scholar] [CrossRef]
- Brown, C.M.; Rose, A.H. Fatty-Acid Composition of Candida Utilis as Affected by Growth Temperature and Dissolved-Oxygen Tension. J. Bacteriol. 1969, 99, 371–378. [Google Scholar] [CrossRef]
- Øverland, M.; Skrede, A. Yeast Derived from Lignocellulosic Biomass as a Sustainable Feed Resource for Use in Aquaculture. J. Sci. Food Agric. 2017, 97, 733–742. [Google Scholar] [CrossRef]
- Suutari, M.; Liukkonen, K.; Laakso, S. Temperature Adaptation in Yeasts: The Role of Fatty Acids. J. Gen. Microbiol. 1990, 136, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Suutari, M.; Rintamäki, A.; Laakso, S. Membrane Phospholipids in Temperature Adaptation of Candida Utilis: Alterations in Fatty Acid Chain Length and Unsaturation. J. Lipid Res. 1997, 38, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, B.; Valdivié, M.; Lezcano, P.; Herrera, M. Evaluation of Torula Yeast (Candida Utilis) Grown on Distillery Vinasse for Broilers. Cuba. J. Agric. Sci. 2013, 47, 183–188. [Google Scholar]
- Nicolas, O.; Aly, S.; Marius, K.S.; François, T.; Cheikna, Z.; Alfred, S.T. Effect of Mineral Salts and Nitrogen Source on Yeast (Candida Utilis NOY1) Biomass Production Using Tubers Wastes. African J. Biotechnol. 2017, 16, 359–365. [Google Scholar] [CrossRef]
- Øverland, M.; Karlsson, A.; Mydland, L.T.; Romarheim, O.H.; Skrede, A. Evaluation of Candida Utilis, Kluyveromyces Marxianus and Saccharomyces Cerevisiae Yeasts as Protein Sources in Diets for Atlantic Salmon (Salmo Salar). Aquaculture 2013, 402–403, 1–7. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Nosek, K.; Synowiec, A.; Chlebowska-Śmigiel, A.; Pobiega, K. Magnesium Binding by Cyberlindnera Jadinii Yeast in Media from Potato Wastewater and Glycerol. Microorganisms 2023, 11, 1923. [Google Scholar] [CrossRef]
- Raita, S.; Kusnere, Z.; Spalvins, K.; Blumberga, D. Optimization of Yeast Cultivation Factors for Improved SCP Production. Environ. Clim. Technol. 2022, 26, 848–861. [Google Scholar] [CrossRef]
- Hueting, S.; Tempest, D.W. Influence of Acetate on the Growth of Candida Utilis in Continuous Culture. Arch. Microbiol. 1977, 115, 73–78. [Google Scholar] [CrossRef]
- Edwards, V.H.; Gottschalk, M.J.; Noojin III, A.Y.; Tuthill, L.B.; Tannahill, A.L. Extended Culture: The Growth of Candida Utilis at Controlled Acetate Concentrations. Biotechnol. Bioeng. 1970, 12, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.D.; Kang, J.Y.; Kim, H.J.; Moon, C. A Critical Overview of HPLC-MS-Based Lipidomics in Determining Triacylglycerol and Phospholipid in Foods. Foods 2023, 12, 3177. [Google Scholar] [CrossRef] [PubMed]
- Suhr, K.I.; Nielsen, P. V Effect of Weak Acid Preservatives on Growth of Bakery Product Spoilage Fungi at Different Water Activities and PH Values. Int. J. Food Microbiol. 2004, 95, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Pattison, T.-L.; Von Holy, A. Effect of Selected Natural Antimicrobials on Baker’s Yeast Activity. Lett. Appl. Microbiol. 2001, 33, 211–215. [Google Scholar] [CrossRef]
- Pronk, J.T.; Van der Linden-Beuman, A.; Verduyn, C.; Scheffers, W.A.; Van Dijken, J.P. Propionate Metabolism in Saccharomyces Cerevisiae: Implications for the Metabolon Hypothesis. Microbiology 1994, 140, 717–722. [Google Scholar] [CrossRef]
- Graybill, E.R.; Rouhier, M.F.; Kirby, C.E.; Hawes, J.W. Functional Comparison of Citrate Synthase Isoforms from S. Cerevisiae. Arch. Biochem. Biophys. 2007, 465, 26–37. [Google Scholar] [CrossRef]
- Wiese, J. Propionate Metabolism in Yeast and Plants; Heinrich-Heine-Universität Düsseldorf: Düsseldorf, Germany, 2014. [Google Scholar]
- Micalizzi, E.W.; Golshani, A.; Smith, M.L. Propionic Acid Disrupts Endocytosis, Cell Cycle, and Cellular Respiration in Yeast. BMC Res. Notes 2021, 14, 335. [Google Scholar] [CrossRef]
- Lourenço, A.B.; Ascenso, J.R.; Sá-Correia, I. Metabolic Insights into the Yeast Response to Propionic Acid Based on High Resolution 1H NMR Spectroscopy. Metabolomics 2011, 7, 457–468. [Google Scholar] [CrossRef]
- Xu, X.; Williams, T.C.; Divne, C.; Pretorius, I.S.; Paulsen, I.T. Biotechnology for Biofuels Evolutionary Engineering in Saccharomyces Cerevisiae Reveals a TRK1-Dependent Potassium Influx Mechanism for Propionic Acid Tolerance. Biotechnol. Biofuels 2019, 12, 97. [Google Scholar] [CrossRef]
- Luttik, M.A.H.; Kotter, P.; Salomons, F.A.; van der Klei, I.J.; van Dijken, J.P.; Pronk, J.T. The Saccharomyces Cerevisiae ICL2 Gene Encodes a Mitochondrial 2-Methylisocitrate Lyase Involved in Propionyl-Coenzyme A Metabolism. J. Bacteriol. 2000, 182, 7007–7013. [Google Scholar] [CrossRef]
- Giannattasio, S.; Guaragnella, N.; Ždralević, M.; Marra, E. Molecular Mechanisms of Saccharomyces Cerevisiae Stress Adaptation and Programmed Cell Death in Response to Acetic Acid. Front. Microbiol. 2013, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.N.; Matos, M.; Bahri, N.; Sá-Correia, I. Shared and More Specific Genetic Determinants and Pathways Underlying Yeast Tolerance to Acetic, Butyric, and Octanoic Acids. Microb. Cell Fact. 2024, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Mira, N.P.; Teixeira, M.C.; Sá-Correia, I. Adaptive Response and Tolerance to Weak Acids in Saccharomyces Cerevisiae: A Genome-Wide View. Omi. A J. Integr. Biol. 2010, 14, 525–540. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Han, Y.H.; Park, Y.L.; Jung, H.R.; Yang, S.Y.; Song, H.S.; Yang, Y.H. A Clean and Green Approach for Odd Chain Fatty Acids Production in Rhodococcus Sp. YHY01 by Medium Engineering. Bioresour. Technol. 2019, 286, 121383. [Google Scholar] [CrossRef]
- Park, Y.K.; Nicaud, J.M. Screening a Genomic Library for Genes Involved in Propionate Tolerance in Yarrowia Lipolytica. Yeast 2020, 37, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, N.T.; El Kantar, S.; De Souza, C.P.; Khelfa, A.; Nicaud, J.; Louka, N.; Koubaa, M. Improving the Synthesis of Odd-Chain Fatty Acids in the Oleaginous Yeast Yarrowia Lipolytica. Fermentation 2024, 10, 597. [Google Scholar] [CrossRef]
- Park, Y.K.; Bordes, F.; Letisse, F.; Nicaud, J.M. Engineering Precursor Pools for Increasing Production of Odd-Chain Fatty Acids in Yarrowia Lipolytica. Metab. Eng. Commun. 2021, 12, e00158. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, R.; Fan, X.; Wang, Y.; Ma, K.; Jiang, J.; Li, G.; Wang, H.; Fan, F.; Zhang, X. Development of CRISPR/Cas9-Based Genome Editing Tools for Polyploid Yeast Cyberlindnera Jadinii and Its Application in Engineering Heterologous Steroid-Producing Strains. ACS Synth. Biol. 2023, 12, 2947–2960. [Google Scholar] [CrossRef]
- Bonzanini, V.; Haddad Momeni, M.; Olofsson, K.; Olsson, L.; Geijer, C. Impact of Glucose and Propionic Acid on Even and Odd Chain Fatty Acid Profiles of Oleaginous Yeasts. BMC Microbiol. 2025, 25, 79. [Google Scholar] [CrossRef]

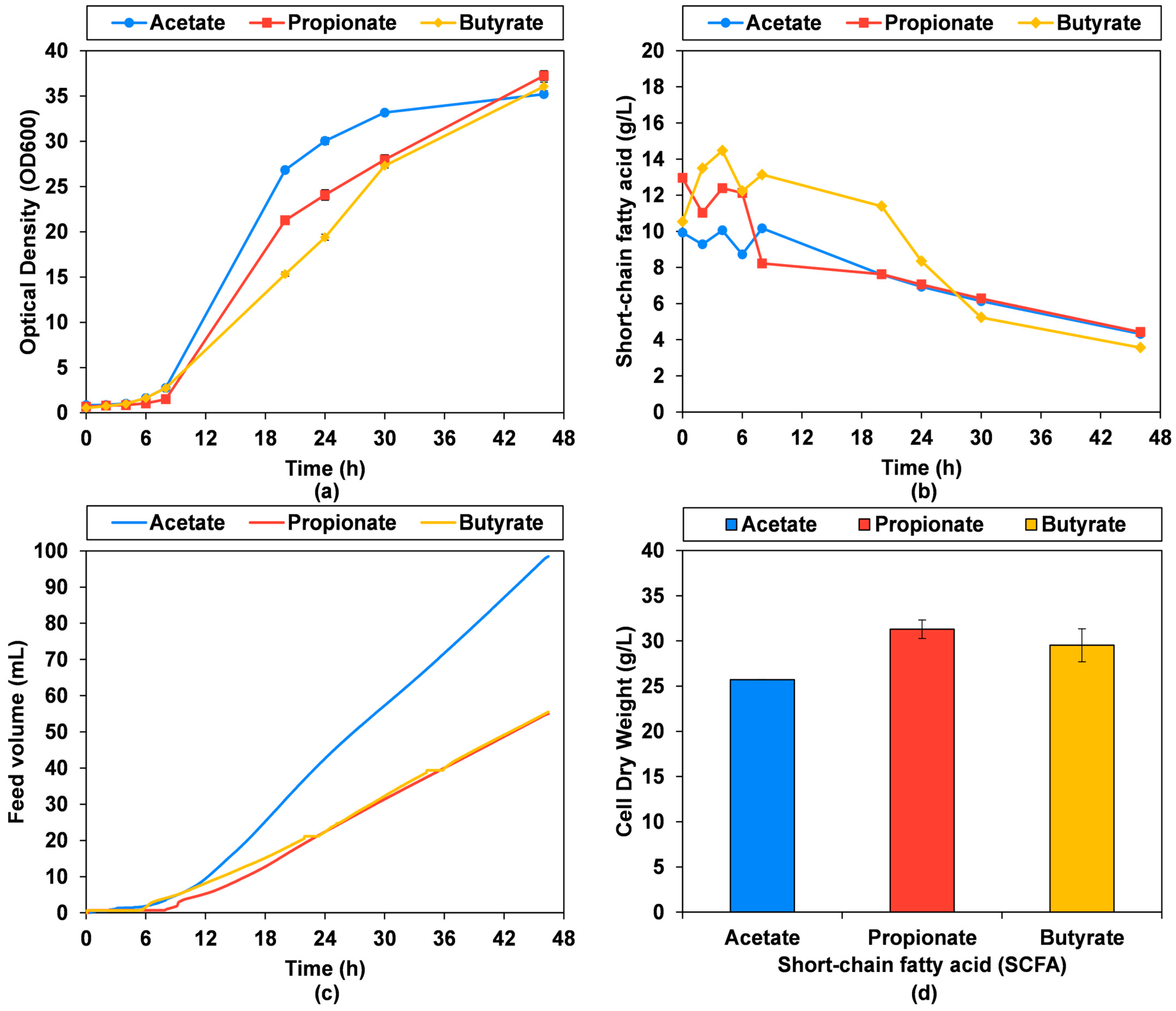

| Fed-Batch pH-Stat Fermentation 20 g/L Sodium SCFA, 5 M SCFA Feed | |||
|---|---|---|---|
| pH-stat parameters (dwb) | Acetate | Propionate | Butyrate |
| pH set-point | 6.0 ± 0.1 | 6.0 ± 0.1 | 6.0 ± 0.1 |
| Time (t) | 46.5 h | 46.5 h | 46.5 h |
| Batch volume (VBatch) | 200 mL | 200 mL | 200 mL |
| Feed volume (VFeed) | 98.5 mL | 55.0 mL | 55.5 mL |
| Batch concentration (SCFABatch) | 14.6 g/L | 15.4 g/L | 16.0 g/L |
| Feed concentration (SCFAFeed) | 300 g/L | 370 g/L | 441 g/L |
| Final concentration (SCFAFinal) | 4.32 g/L | 4.42 g/L | 3.56 g/L |
| pH-stat TRY (dwb) | Acetate | Propionate | Butyrate |
| Biomass titer (X) | 25.7 ± 0.0 g/L | 31.3 ± 1.0 g/L | 29.5 ± 1.8 g/L |
| Biomass rate (RX) | 0.54 ± 0.00 g/L/h | 0.67 ± 0.02 g/L/h | 0.64 g/L/h ± 0.04 |
| Biomass yield (YX/S) | 0.25 ± 0.00 g/g | 0.36 ± 0.01 g/g | 0.28 ± 0.02 g/g |
| Fed-Batch pH-Stat Fermentation 20 g/L Sodium SCFA, 5 M SCFA Feed | |||
|---|---|---|---|
| C. jadinii composition (dwb) | Acetate | Propionate | Butyrate |
| Crude protein | 36.6% ± 2.8% | 41.3% ± 0.0% | 38.9% ± 2.4% |
| Crude lipid | 17.3% | 15.1% | 13.7% |
| C. jadinii fatty acids (dwb) | Acetate | Propionate | Butyrate |
| Pentadecanoic acid (C15:0) | 0% | 1% | 0% |
| Palmitic acid (C16:0) | 15% | 9% | 10% |
| Palmitoleic acid (C16:1) | 2% | 2% | 1% |
| Margaric acid (C17:0) | 0% | 9% | 0% |
| Margaroleic acid (C17:1) | 0% | 27% | 0% |
| Stearic acid (C18:0) | 2% | 1% | 3% |
| Oleic acid (C18:1) | 41% | 26% | 31% |
| Linoleic acid (C18:2) | 29% | 20% | 51% |
| Linolenic acid (C18:3) | 11% | 6% | 4% |
| Total saturated | 16% | 19% | 14% |
| Total unsaturated | 84% | 81% | 86% |
| Total odd-chain | 0% | 37% | 0% |
| Total even-chain | 100% | 63% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermansen, C.; Siao, R.; Chua, G.G.; Lee, M.R.X.; Thong, A.; Weingarten, M.; Lindley, N.; Peterson, E.C. Short-Chain Fatty Acid Utilization in Cyberlindnera jadinii for Single-Cell Protein and Odd-Chain Fatty Acid Production. Microorganisms 2025, 13, 1558. https://doi.org/10.3390/microorganisms13071558
Hermansen C, Siao R, Chua GG, Lee MRX, Thong A, Weingarten M, Lindley N, Peterson EC. Short-Chain Fatty Acid Utilization in Cyberlindnera jadinii for Single-Cell Protein and Odd-Chain Fatty Acid Production. Microorganisms. 2025; 13(7):1558. https://doi.org/10.3390/microorganisms13071558
Chicago/Turabian StyleHermansen, Christian, Rowanne Siao, Gi Gi Chua, Mikko Ru Xuan Lee, Aaron Thong, Melanie Weingarten, Nic Lindley, and Eric Charles Peterson. 2025. "Short-Chain Fatty Acid Utilization in Cyberlindnera jadinii for Single-Cell Protein and Odd-Chain Fatty Acid Production" Microorganisms 13, no. 7: 1558. https://doi.org/10.3390/microorganisms13071558
APA StyleHermansen, C., Siao, R., Chua, G. G., Lee, M. R. X., Thong, A., Weingarten, M., Lindley, N., & Peterson, E. C. (2025). Short-Chain Fatty Acid Utilization in Cyberlindnera jadinii for Single-Cell Protein and Odd-Chain Fatty Acid Production. Microorganisms, 13(7), 1558. https://doi.org/10.3390/microorganisms13071558







