Maximizing Biomass Production and Carotenoid-like Pigments Yield in Kocuria sediminis As04 Through Culture Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Kocuria Strain from Airborne Samples
2.2. Culture Optimization
Taguchi Experimental Design
2.3. Microbial Kinetics from Kocuria sediminis As04
2.3.1. Pre-Inoculum Production
2.3.2. Taguchi Experiment
2.3.3. Determination of Biomass and Total Viable Count
2.3.4. Determination of Total Carotenoid Pigments
2.4. Pigment Identification by MS/MS
2.4.1. Pigment Extraction
2.4.2. Chromatographic and Mass Spectrometry Conditions and Data Processing
2.5. Structural and Chemical Characterization by SEM and FTIR
2.6. Statistical Analysis
3. Results and Discussion
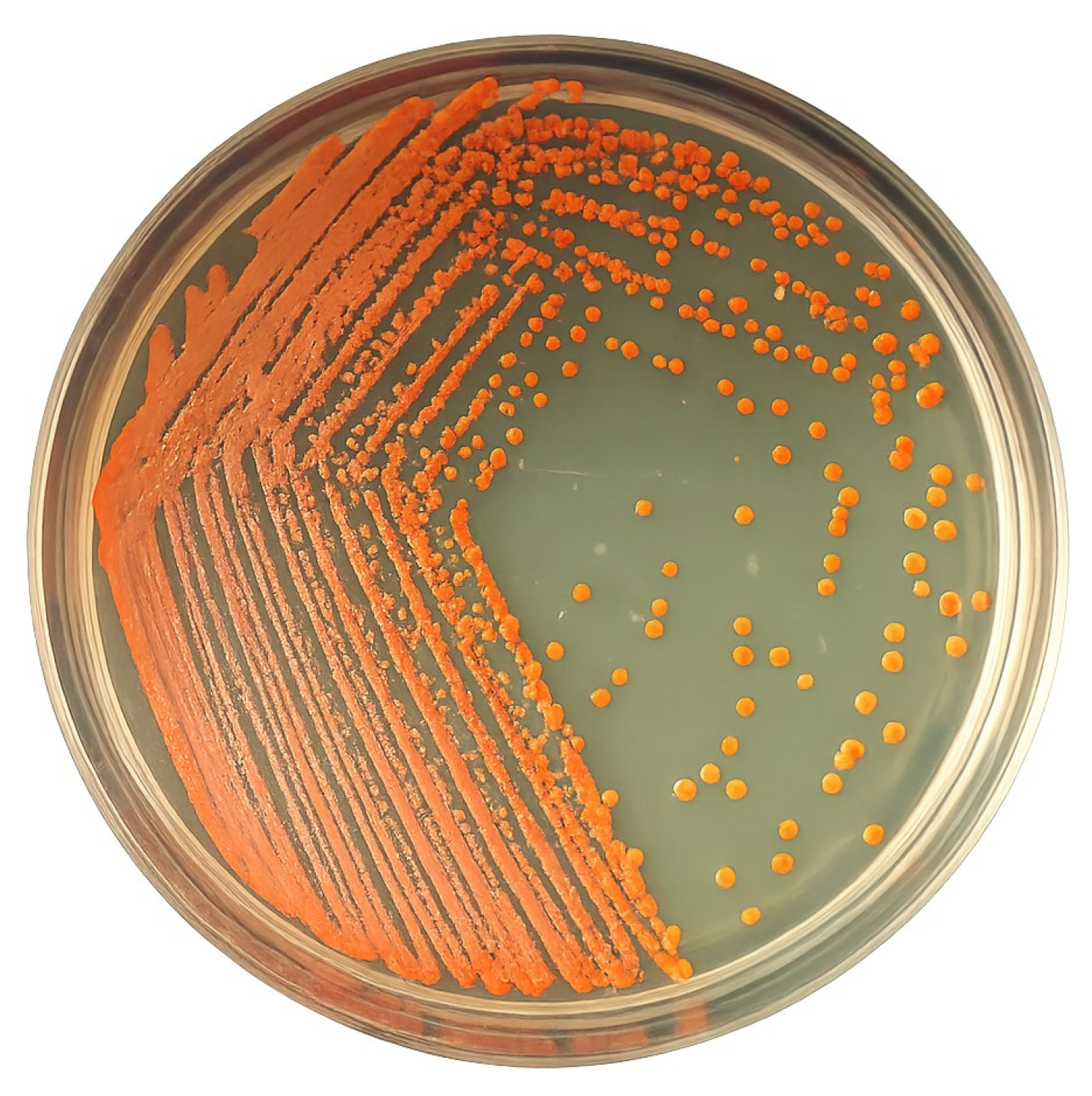
3.1. Isolation and Identification
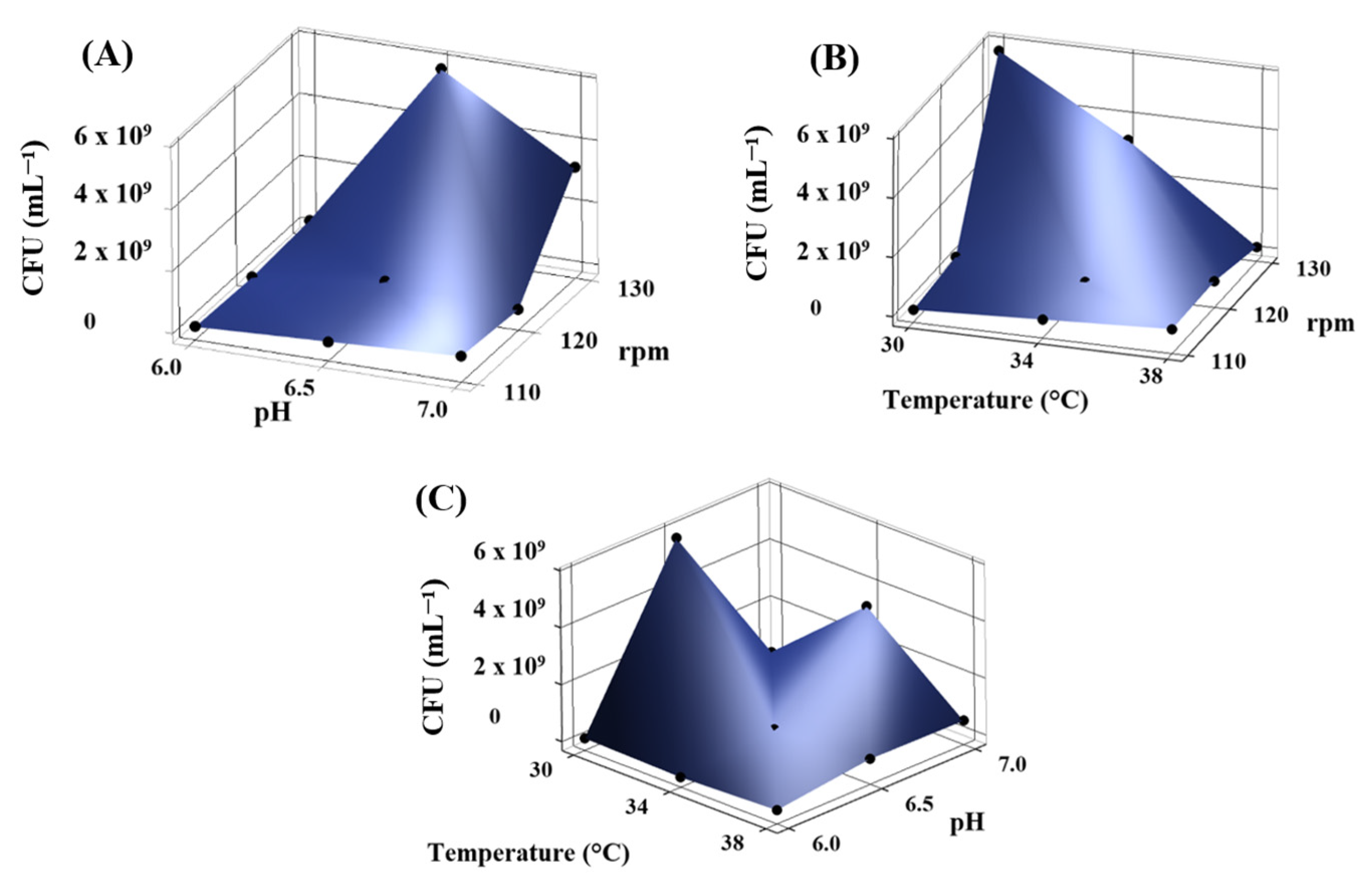
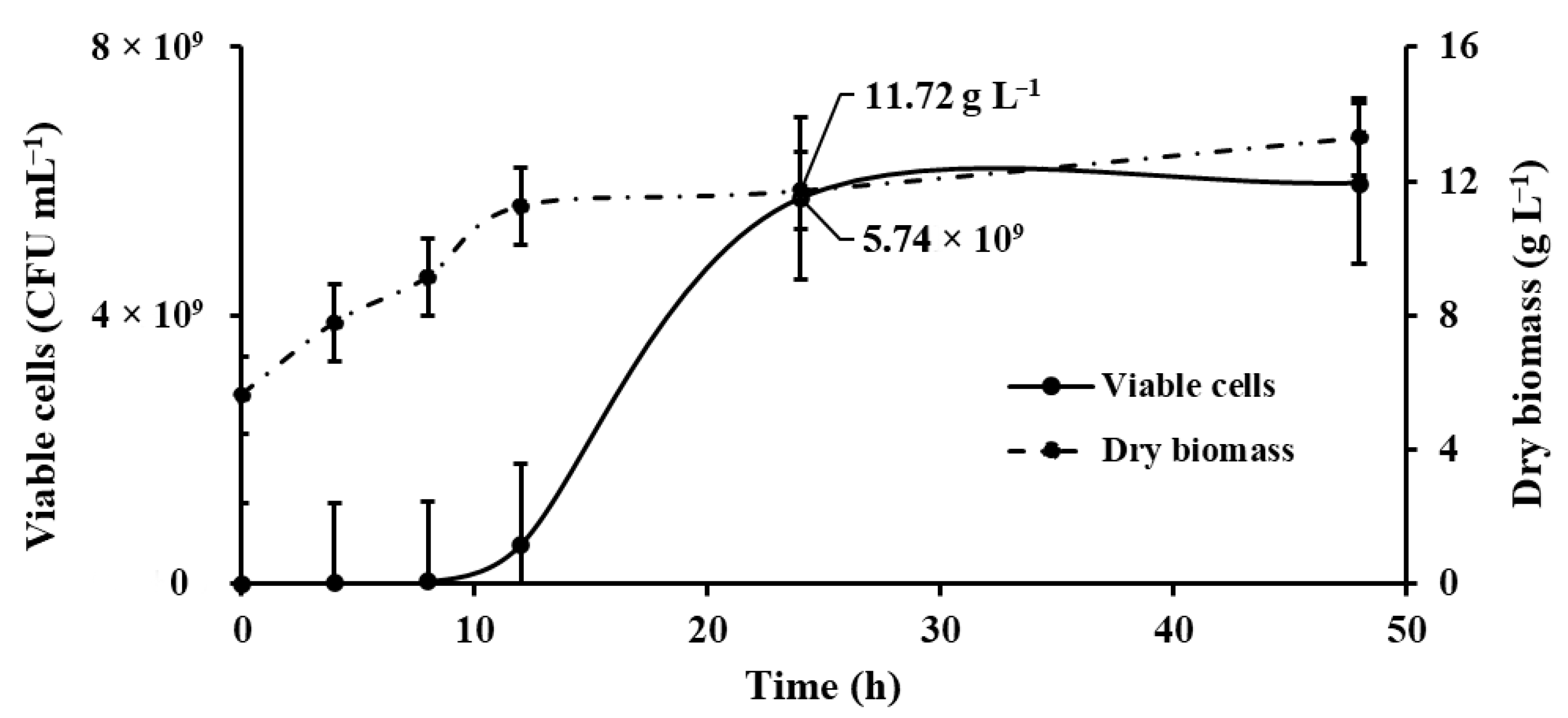
3.2. Kocuria sediminis Culture Optimization
3.3. Total Pigments
3.4. Determination of Pigments from Kocuria sediminis Biomass by MS/MS
3.5. Characterization of Kocuria sediminis AS04 Biomass by SEM and FTIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrasekaran, M.; Paramasivan, M. Plant Growth-Promoting Bacterial (PGPB) Mediated Degradation of Hazardous Pesticides: A Review. Int. Biodeterior. Biodegrad. 2024, 190, 105769. [Google Scholar] [CrossRef]
- Mauceri, A.; Puccio, G.; Faddetta, T.; Abbate, L.; Polito, G.; Caldiero, C.; Renzone, G.; Lo Pinto, M.; Alibrandi, P.; Vaccaro, E.; et al. Integrated Omics Approach Reveals the Molecular Pathways Activated in Tomato by Kocuria Rhizophila, a Soil Plant Growth-Promoting Bacterium. Plant Physiol. Biochem. 2024, 210, 108609. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Pan, X.; Zhang, D. Remediation of Copper-Contaminated Soil by Kocuria Flava CR1, Based on Microbially Induced Calcite Precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Bergey, D.H. Bergey’s Manual ® of Systematic Bacteriology; Springer: New York, NY, USA, 2012; Volume 5. [Google Scholar]
- Bala, M.; Kaur, C.; Kaur, I.; Khan, F.; Mayilraj, S. Kocuria sediminis sp. Nov., Isolated from a Marine Sediment Sample. Antonie Van Leeuwenhoek 2012, 101, 469–478. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Bacteria as Inoculants in Agricultural Soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Menaa, F.; Khan, B.A.; Mohammad, F.V.; Ahmad, V.U.; Djeribi, R.; Menaa, B. Isolation, Purification, Structural Elucidation and Antimicrobial Activities of Kocumarin, a Novel Antibiotic Isolated from Actinobacterium Kocuria Marina CMG S2 Associated with the Brown Seaweed Pelvetia Canaliculata. Microbiol. Res. 2018, 206, 186–197. [Google Scholar] [CrossRef]
- Dif, G.; Belaouni, H.A.; Goudjal, Y.; Yekkour, A.; Djemouai, N.; Zitouni, A. Potential for Plant Growth Promotion of Kocuria Arsenatis Strain ST19 on Tomato under Salt Stress Conditions. S. Afr. J. Bot. 2021, 138, 94–104. [Google Scholar] [CrossRef]
- Guardado-Fierros, B.G.; Tuesta-Popolizio, D.A.; Lorenzo-Santiago, M.A.; Rodriguez-Campos, J.; Contreras-Ramos, S.M. Comparative Study between Salkowski Reagent and Chromatographic Method for Auxins Quantification from Bacterial Production. Front. Plant Sci. 2024, 15, 1378079. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial Pigments and Their Applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Rather, L.J.; Mir, S.S.; Ganie, S.A.; Shahid-ul-Islam; Li, Q. Research Progress, Challenges, and Perspectives in Microbial Pigment Production for Industrial Applications—A Review. Dye. Pigment. 2023, 210, 110989. [Google Scholar] [CrossRef]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Advances in Bioprocess Engineering for Optimising Chlorella Vulgaris Fermentation: Biotechnological Innovations and Applications. Foods 2024, 13, 4154. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Woła̧cewicz, M.; Pyter, W.; Joshi, N.; Drewniak, L.; Pranaw, K. Colorful Treasure From Agro-Industrial Wastes: A Sustainable Chassis for Microbial Pigment Production. Front. Microbiol. 2022, 13, 832918. [Google Scholar] [CrossRef]
- Xu, S.; Gao, S.; An, Y. Research Progress of Engineering Microbial Cell Factories for Pigment Production. Biotechnol. Adv. 2023, 65, 108150. [Google Scholar] [CrossRef]
- Harshini, P.; Varghese, R.; Pachamuthu, K.; Ramamoorthy, S. Enhanced Pigment Production from Plants and Microbes: A Genome Editing Approach. 3 Biotech 2025, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta Mandal, D.; Majumdar, S. Bacteria as Biofactory of Pigments: Evolution beyond Therapeutics and Biotechnological Advancements. J. Biosci. Bioeng. 2023, 135, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.K.; Corrêa, R.C.G.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200. [Google Scholar] [CrossRef]
- Mendes-Silva, T.d.C.D.; Vidal, E.E.; de Souza, R.d.F.R.; Schmidt, K.d.C.; Mendes, P.V.D.; da Silva Andrade, R.F.; da Silva Oliveira, F.G.; de Lucena, B.T.L.; de Oliveira, M.B.M.; dos Santos Correia, M.T.; et al. Production of Carotenoid Sarcinaxanthin by Kocuria Palustris Isolated from Northeastern Brazil Caatinga Soil and Their Antioxidant and Photoprotective Activities. Electron. J. Biotechnol. 2021, 53, 44–53. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Dewanjee, S.; Riaz, M. Carotenoids: Structure and Function in the Human Body; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; ISBN 9783030464592. [Google Scholar]
- Sundararajan, P.; Ramasamy, S.P. Current Perspectives on Industrial Application of Microbial Carotenoid as an Alternative to Synthetic Pigments. Sustain. Chem. Pharm. 2024, 37, 101353. [Google Scholar] [CrossRef]
- Brahma, D.; Dutta, D. Antioxidant Property of Beta-Cryptoxanthin Produced by Kocuria Marina DAGII. Mater. Today Proc. 2022, 57, 1833–1837. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, A.; Sharma, J. Degradation Study of Lindane by Novel Strains Kocuria sp. DAB-1Y and Staphylococcus sp. DAB-1W. Bioresour. Bioprocess. 2016, 3, 53. [Google Scholar] [CrossRef]
- Kulkarni, V.M.; Dixit, A.S.; Patwardhan, A.V.; Bajwa, A.S. A Different Approach to Augment Pigment Production and Its Extraction from Kocuria Flava by Using Ultrasound Technique. J. Biol. Act. Prod. Nat. 2018, 8, 34–42. [Google Scholar] [CrossRef]
- Ashraf, Y.Z.K. Degradation of Diesel-Oil by a Newly Isolated Kocuria sediminis DDK6. Afr. J. Microbiol. Res. 2017, 11, 400–407. [Google Scholar] [CrossRef]
- Khalifa, A.Y.Z. Scanning Electron Microscopy and Antibiotic Sensitivity of the Actinobacterium, Kocuria sediminis DDK6. J. Appl. Biol. Biotechnol. 2017, 5, 018–022. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Ibrahim, G.S.; El-Shall, F.N.; Arafa, A.A.; Shalabi, A.; El Awady, M.E. Bio-Production and Characterization of Carotenoid Yellow Pigment from Kocuria sp. GMA and Exploring Its Sustainable Antioxidant, Antimicrobial and Antibiofilm Properties. Egypt. J. Chem. 2024, 67, 57–68. [Google Scholar] [CrossRef]
- Britton, G. Functions of Intact Carotenoids. In Carotenoids; Birkhäuser Basel: Basel, Switzerland; pp. 189–212.
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Guardado-Fierros, B.G.; Lorenzo-Santiago, M.A.; Kirchmayr, M.R.; Patrón-Soberano, O.A.; Rodriguez-Campos, J.; Contreras-Ramos, S.M. Biocontrol and Plant Growth-Promoting Activities of Airborne Bacteria. World J. Microbiol. Biotechnol. 2025, 41, 131. [Google Scholar] [CrossRef]
- Tuesta-Popolizio, D.A.; Velázquez-Fernández, J.B.; Rodriguez-Campos, J.; Contreras-Ramos, S.M. Isolation and Identification of Extremophilic Bacteria with Potential as Plant Growth Promoters (Pgpb) of A Geothermal Site: A Case Study. Geomicrobiol. J. 2021, 38, 436–450. [Google Scholar] [CrossRef]
- Gutiérrez-Santa Ana, A.; Carrillo-Cerda, H.A.; Rodriguez-Campos, J.; Kirchmayr, M.R.; Contreras-Ramos, S.M.; Velázquez-Fernández, J.B. Volatile Emission Compounds from Plant Growth-Promoting Bacteria Are Responsible for the Antifungal Activity against F. solani. 3 Biotech 2020, 10, 292. [Google Scholar] [CrossRef]
- ASTM D2974-20; Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils. ASTM International: West Conshohocken, PA, USA, 2020.
- Zhi, R.; Yang, A.; Zhang, G.; Zhu, Y.; Meng, F.; Li, X. Effects of Light-Dark Cycles on Photosynthetic Bacteria Wastewater Treatment and Valuable Substances Production. Bioresour. Technol. 2019, 274, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Long, H.; Su, S.; Wu, Y.; Wang, H. Metabolomics Analysis Reveals the Physiological Mechanism Underlying Growth Restriction in Maize Roots under Continuous Negative Pressure and Stable Water Supply. Agric. Water Manag. 2022, 263, 107452. [Google Scholar] [CrossRef]
- Liu, P.P.; Shan, G.S.; Zhang, F.; Chen, J.N.; Jia, T.Z. Metabolomics Analysis and Rapid Identification of Changes in Chemical Ingredients in Crude and Processed Astragali Radix by UPLC-QTOF-MS Combined with Novel Informatics UNIFI Platform. Chin. J. Nat. Med. 2018, 16, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Vannabhum, M.; Ziangchin, N.; Thepnorarat, P.; Akarasereenont, P. Metabolomic Analysis of Thai Herbal Analgesic Formula Based on Ultra-High-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. Heliyon 2023, 9, e18296. [Google Scholar] [CrossRef]
- Hou, Y.F.; Bai, L.; Guo, S.; Hu, J.B.; Zhang, S.S.; Liu, S.J.; Zhang, Y.; Li, S.; Ho, C.T.; Bai, N.S. Nontargeted Metabolomic Analysis of Four Different Parts of Actinidia Arguta by UPLC-Q-TOF-MSE. Food Res. Int. 2023, 163, 112228. [Google Scholar] [CrossRef]
- Metwally, R.A.; El-Sersy, N.A.; El Sikaily, A.; Sabry, S.A.; Ghozlan, H.A. Optimization and Multiple in Vitro Activity Potentials of Carotenoids from Marine Kocuria sp. RAM1. Sci. Rep. 2022, 12, 18203. [Google Scholar] [CrossRef]
- Alam, T.; Din, S.U.; Abdullah, M.; Ali, M.; Badshah, M.; Farman, M.; Khan, S.; Hasan, F.; Shah, A.A. Bioactive Metabolites from Radioresistant Bacterium Kocuria sp. TMM 11 and Their Role in Prevention of Ultraviolet-Induced Photodamages. Curr. Microbiol. 2025, 82, 243. [Google Scholar] [CrossRef] [PubMed]
- Gashi, N.; Szőke, Z.; Fauszt, P.; Dávid, P.; Mikolás, M.; Gál, F.; Stündl, L.; Remenyik, J.; Paholcsek, M. Bioaerosols in Agriculture: A Comprehensive Approach for Sustainable Crop Health and Environmental Balance. Agronomy 2025, 15, 1003. [Google Scholar] [CrossRef]
- Quadri, S.R. Survival Strategy, Metabolic Potential and Taxonomic Reframe of Kocuria polaris. J. Pure Appl. Microbiol. 2024, 18, 1620–1626. [Google Scholar] [CrossRef]
- Nedwell, D.B. Effect of Low Temperature on Microbial Growth: Lowered Affinity for Substrates Limits Growth at Low Temperature. FEMS Microbiol. Ecol. 1999, 30, 101–111. [Google Scholar] [CrossRef]
- Mulakhudair, A.R.; Al-Mashhadani, M.K.H.; Kokoo, R. Tracking of Dissolved Oxygen Distribution and Consumption Pattern in a Bespoke Bacterial Growth System. Chem. Eng. Technol. 2022, 45, 1683–1690. [Google Scholar] [CrossRef]
- Boer, V.M.; Crutchfield, C.A.; Bradley, P.H.; Botstein, D.; Rabinowitz, J.D. Growth-Limiting Intracellular Metabolites in Yeast Growing under Diverse Nutrient Limitations. Mol. Biol. Cell 2010, 21, 198–211. [Google Scholar] [CrossRef]
- Ortiz-Cortés, L.Y.; Ventura-Canseco, L.M.C.; Abud-Archila, M.; Ruíz-Valdiviezo, V.M.; Velázquez-Ríos, I.O.; Alvarez-Gutiérrez, P.E. Evaluation of Temperature, PH and Nutrient Conditions in Bacterial Growth and Extracellular Hydrolytic Activities of Two Alicyclobacillus spp. Strains. Arch. Microbiol. 2021, 203, 4557–4570. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, W.-J.; Ryu, J.; Yerefu, Y.; Tesfaw, A. Amylase Production by the New Strains of Kocuria rosea and Micrococcus endophyticus Isolated from Soil in the Guassa Community Conservation Area. Fermentation 2025, 11, 211. [Google Scholar] [CrossRef]
- Mousa, W.K.; Abu-Izneid, T.; Salah-Tantawy, A. High-Throughput Sequencing Reveals the Structure and Metabolic Resilience of Desert Microbiome Confronting Climate Change. Front. Plant Sci. 2024, 15, 1294173. [Google Scholar] [CrossRef]
- Vidal, L.; Christen, P.; Coello, M.N. Feather Degradation by Kocuria Rosea in Submerged Culture. World J. Microbiol. Biotechnol. 2000, 16, 551–554. [Google Scholar] [CrossRef]
- Bertsch, A.; Coello, N. A Biotechnological Process for Treatment and Recycling Poultry Feathers as a Feed Ingredient. Bioresour. Technol. 2005, 96, 1703–1708. [Google Scholar] [CrossRef]
- Mitra, R.; Chaudhuri, S.; Dutta, D. Modelling the Growth Kinetics of Kocuria marina DAGII as a Function of Single and Binary Substrate during Batch Production of Β-Cryptoxanthin. Bioprocess Biosyst. Eng. 2017, 40, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.-Y.; Seo, K.-H. Isolation and Characterization of Halophilic Kocuria salsicia Strains from Cheese Brine. Food Sci. Anim. Resour. 2022, 42, 252–265. [Google Scholar] [CrossRef]
- Timkina, E.; Drábová, L.; Palyzová, A.; Řezanka, T.; Maťátková, O.; Kolouchová, I. Kocuria Strains from Unique Radon Spring Water from Jachymov Spa. Fermentation 2022, 8, 35. [Google Scholar] [CrossRef]
- Ramesh, C.; Prasastha, V.R.; Venkatachalam, M.; Dufossé, L. Natural Substrates and Culture Conditions to Produce Pigments from Potential Microbes in Submerged Fermentation. Fermentation 2022, 8, 460. [Google Scholar] [CrossRef]
- Kochhar, N.; Kavya, I.K.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the Microorganism of Extreme Environments and Their Applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef] [PubMed]
- Rezaeeyan, Z.; Safarpour, A.; Amoozegar, M.A.; Babavalian, H.; Tebyanian, H.; Shakeri, F. High Carotenoid Production by a Halotolerant Bacterium, Kocuria sp. Strain QWT-12 and Anticancer Activity of Its Carotenoid. EXCLI J. 2017, 16, 840–851. [Google Scholar]
- Mal, S.A.; Ibrahim, G.S.; Al Khalaf, M.I.; Al-Hejin, A.M.; Bataweel, N.M.; Abu-Zaid, M. Production and Partial Characterization of Yellow Pigment Produced by Kocuria flava Isolate and Testing Its Antioxidant and Antimicrobial Activity. Int. J. Life Sci. Pharma Res. 2022, 10, L58–L66. [Google Scholar] [CrossRef]
- Feldmane, L.; Raita, S.; Berzina, I.; Geiba, Z.; Mika, T.; Kuzmika, I.; Spalvins, K. Effects of Temperature, PH, and Agitation on Growth and Butanol Production of Clostridium Acetobutylicum, Clostridium Beijerinckii, and Clostridium Saccharoperbutylacetonicum. Environ. Clim. Technol. 2024, 28, 71–83. [Google Scholar] [CrossRef]
- Coleman, M.E.; Tamplin, M.L.; Phillips, J.G.; Marmer, B.S. Influence of Agitation, Inoculum Density, PH, and Strain on the Growth Parameters of Escherichia coli O157:H7—Relevance to Risk Assessment. Int. J. Food Microbiol. 2003, 83, 147–160. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Chen, S.-P.; Nguyen, T.H.; Nguyen, M.T.; Tran, T.T.T.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.-H.; Wang, S.-L. Novel Efficient Bioprocessing of Marine Chitins into Active Anticancer Prodigiosin. Mar. Drugs 2019, 18, 15. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Wang, S.-L.; Nguyen, D.-N.; Nguyen, A.-D.; Nguyen, T.-H.; Doan, M.-D.; Ngo, V.-A.; Doan, C.-T.; Kuo, Y.-H.; Nguyen, V.-B. Bioprocessing of Marine Chitinous Wastes for the Production of Bioactive Prodigiosin. Molecules 2021, 26, 3138. [Google Scholar] [CrossRef]
- Hegazy, A.A.; Abu-Hussien, S.H.; Elsenosy, N.K.; El-Sayed, S.M.; Abo El-Naga, M.Y. Optimization, Characterization and Biosafety of Carotenoids Produced from Whey Using Micrococcus Luteus. BMC Biotechnol. 2024, 24, 74. [Google Scholar] [CrossRef]
- Gholami, M.; Etemadifar, Z.; Bouzari, M. Isolation a New Strain of Kocuria rosea Capable of Tolerating Extreme Conditions. J. Environ. Radioact. 2015, 144, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nemer, G.; Louka, N.; Vorobiev, E.; Salameh, D.; Nicaud, J.-M.; Maroun, R.G.; Koubaa, M. Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation 2021, 7, 36. [Google Scholar] [CrossRef]
- Vachali, P.; Bhosale, P.; Bernstein, P.S. Microbial Carotenoids. In Microbial Carotenoids From Fungi: Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 898, pp. 41–59. [Google Scholar] [CrossRef]
- Volk, W.A.; Pennington, S. The pigments of the photosynthetic bacterium Rhodomicrobium vannielii. J. Bacteriol. 1950, 59, 169. [Google Scholar] [CrossRef]
- Chi, S.C.; Mothersole, D.J.; Dilbeck, P.; Niedzwiedzki, D.M.; Zhang, H.; Qian, P.; Vasilev, C.; Grayson, K.J.; Jackson, P.J.; Martin, E.C.; et al. Assembly of Functional Photosystem Complexes in Rhodobacter Sphaeroides Incorporating Carotenoids from the Spirilloxanthin Pathway. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Bóna-Lovász, J.; Bóna, A.; Ederer, M.; Sawodny, O.; Ghosh, R. A Rapid Method for the Extraction and Analysis of Carotenoids and Other Hydrophobic Substances Suitable for Systems Biology Studies with Photosynthetic Bacteria. Metabolites 2013, 3, 912–930. [Google Scholar] [CrossRef]
- Autenrieth, C.; Ghosh, R. The Methoxylated, Highly Conjugated C40 Carotenoids, Spirilloxanthin and Anhydrorhodovibrin, Can Be Separated Using High Performance Liquid Chromatography with Safe and Environmentally Friendly Solvents. Metabolites 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; García-Martín, J.M.; Álvarez, R.; Palmero, A.; Esteban, J.; Pérez-Jorge, C.; Arcos, D.; Vallet-Regí, M. Nanocolumnar Coatings with Selective Behavior towards Osteoblast and Staphylococcus Aureus Proliferation. Acta Biomater. 2015, 15, 20–28. [Google Scholar] [CrossRef]
- Shanmuga Leela, A.; Jaya Lakshmi, S.S.; Leela, K.V.; Tanuj, M.L.; George, M.G.; Jayaprakash, V. Kocuria rosea Sepsis in an Immunocompromised Patient: A Case Report. Cureus 2024, 16, e68418. [Google Scholar] [CrossRef]
- Guardado-Fierros, B.G.; Lorenzo-Santiago, M.A.; Gumiere, T.; Aid, L.; Rodriguez-Campos, J.; Contreras-Ramos, S.M. Glyphosate Biodegradation by Airborne Plant Growth-Promoting Bacteria: Influence on Soil Microbiome Dynamics. Agriculture 2025, 15, 362. [Google Scholar] [CrossRef]
- Es-haghi, A.; Amiri, M.S.; Taghavizadeh Yazdi, M.E. Ferula Latisecta Gels for Synthesis of Zinc/Silver Binary Nanoparticles: Antibacterial Effects against Gram-Negative and Gram-Positive Bacteria and Physicochemical Characteristics. BMC Biotechnol. 2024, 24, 51. [Google Scholar] [CrossRef]
- Li, C.-J.; Jiang, Z.-M.; Zhi, X.-Y.; Chen, H.-H.; Yu, L.-Y.; Li, G.-F.; Zhang, Y.-Q. Genomic Insights into Kocuria: Taxonomic Revision and Identification of Five IAA-Producing Extremophiles. Front. Microbiol. 2025, 16, 1547983. [Google Scholar] [CrossRef] [PubMed]
- Udensi, J.; Loskutova, E.; Loughman, J.; Byrne, H.J. Quantitative Raman Analysis of Carotenoid Protein Complexes in Aqueous Solution. Molecules 2022, 27, 4724. [Google Scholar] [CrossRef] [PubMed]
- Pallath, N.; Francis, B.; Devanesan, S.; Farhat, K.; Balakrishnan, M. Isolation and Characterization of Novel Carotenoid Pigment from Marine Planococcus maritimus MBP-2 and Their Biological Applications. J. King Saud Univ. Sci. 2023, 35, 102872. [Google Scholar] [CrossRef]
- Lestari, S.W.; Hertika, A.M.S.; Yona, D.; Buwono, N.R. Functional Groups in Microalgal Extracellular Polymeric Substances: A Promising Biopolymer for Microplastic Mitigation in Marine Ecosystems. Ecol. Eng. Environ. Technol. 2025, 26, 171–182. [Google Scholar] [CrossRef]
- Karacaoğlu, B.; Koçer, A.T.; İnan, B.; Bütün, İ.; Mercimek, R.; Ghorbani, M.; Koşar, A.; Balkanlı, D. Microfluidic Chip-Assisted Separation Process and Post-Chip Microalgae Cultivation for Carotenoid Production. J. Appl. Phycol. 2025, 37, 35–53. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, J.; Verma, N. Production, Characterization and Environmental Applications of Biosurfactants from Bacillus amyloliquefaciens and Bacillus subtilis. Biocatal. Agric. Biotechnol. 2018, 16, 132–139. [Google Scholar] [CrossRef]
- Hendawy, S.H.M.; Alzan, H.F.; Abdel-Ghany, H.S.M.; Suarez, C.E.; Kamel, G. Biochemical Analysis of Hyalomma dromedarii Salivary Glands and Gut Tissues Using SR-FTIR Micro-Spectroscopy. Sci. Rep. 2024, 14, 8515. [Google Scholar] [CrossRef]
- Kassem, A.; Abbas, L.; Coutinho, O.; Opara, S.; Najaf, H.; Kasperek, D.; Pokhrel, K.; Li, X.; Tiquia-Arashiro, S. Applications of Fourier Transform-Infrared Spectroscopy in Microbial Cell Biology and Environmental Microbiology: Advances, Challenges, and Future Perspectives. Front. Microbiol. 2023, 14, 1304081. [Google Scholar] [CrossRef]



| Treatment Code | Temperature (°C) | Stirring Speed (rpm) | pH |
|---|---|---|---|
| T30:110:6.0 | 30 | 110 | 6.0 |
| T34:110:6.5 | 34 | 110 | 6.5 |
| T38:110:7.0 | 38 | 110 | 7.0 |
| T34:120:6.0 | 34 | 120 | 6.0 |
| T38:120:6.5 | 38 | 120 | 6.5 |
| T30:120:7.0 | 30 | 120 | 7.0 |
| T38:130:6.0 | 38 | 130 | 6.0 |
| T30:130:6.5 | 30 | 130 | 6.5 |
| T34:130:7.0 | 34 | 130 | 7.0 |
| Treatments | Dry Biomass (g L−1) * | |||||
|---|---|---|---|---|---|---|
| T0 | T4 | T8 | T12 | T24 | T48 | |
| NC | 5.14 ± 0.25 Db | 6.24 ± 0.07 Ca | 6.59 ± 0.16 BCa | 7.1 ± 0.24 Bc | 8.64 ± 0.41 Ad | 8.71 ± 0.32 Ac |
| T30:110:6.0 | 7.53 ± 0.19 Ba | 8.22 ± 0.8 Aa | 8.45 ± 1.06 Aa | 9.39 ± 0.68 Aabc | 9.54 ± 0.16 Acd | 10.13 ± 1.02 Abc |
| T34:110:6.5 | 7.15 ± 0.38 Baab | 9.03 ± 2.1 Aa | 9.3 ± 1.2 Aa | 9.5 ± 0.32 Aabc | 9.58 ± 0.12 Acd | 10.37 ± 0.93 Abc |
| T38:110:7.0 | 6.42 ± 1.02 Cab | 6.73 ± 0.39 Ba | 7.06 ± 0.93 Ba | 9.73 ± 0.67 Aabc | 10.02 ± 0.17 Abc | 10.75 ± 0.7 Aabc |
| T34:120:6.0 | 5.74 ± 0.19 Dab | 6.933 ± 0.7 Ca | 8.83 ± 1.2 BCa | 9.91 ± 0.79 ABab | 11.35 ± 0.4 Aa | 11.57 ± 1.0 Aabc |
| T38:120:6.5 | 6.61 ± 0.24 Bab | 6.9 ± 0.81 Aa | 8.48 ± 1.04 Aa | 8.74 ± 2.0 Aabc | 9.11 ± 0.13 Acd | 9.57 ± 0.82 Ac |
| T30:120:7.0 | 5.79 ± 0.1.2 Dab | 6.72 ± 0.7 Ca | 7.63 ± 0.26 BCa | 8.29 ± 1.38 ABbc | 9.34 ± 0.27 ABcd | 9.78 ± 0.59 Ac |
| T38:130:6.0 | 5.95 ± 0.65 Dab | 6.71 ± 1.5 Ca | 7.3 ± 0.58 Ca | 9.84 ± 0.53 Babc | 11.27 ± 0.20 ABab | 12.52 ± 0.5 Aab |
| T30:130:6.5 | 5.64 ± 1.03 Dab | 7.8 ± 0.16 Ca | 9.15 ± 1.54 BCa | 11.27 ± 0.6 ABa | 11.72 ± 1.18 Aa | 13.3 ± 0.74 Aa |
| T34:130:7.0 | 6.13 ± 1.53 Bab | 8.0 ± 1.2 Ba | 8.05 ± 1.8 Ba | 8.5 ± 0.41 ABbc | 9.3 ± 0.15 ABcd | 11.44 ± 1.8 Aabc |
| Factors and Interactions | Dry Biomass | Viable Cells | Total Pigments |
|---|---|---|---|
| p-Value | |||
| Temperature | 0.002 | 0.477 | 0.912 |
| Agitation | <0.0001 | 0.208 | 0.023 |
| pH | 0.002 | 0.598 | 0.272 |
| Temperature × Agitation | 0.005 | 0.281 | 0.166 |
| Temperature × pH | 0.03 | 0.713 | 0.502 |
| Agitation × pH | 0.138 | 0.257 | 0.003 |
| Temperature × Agitation × pH | 0.084 | 0.479 | 0.05 |
| Peak | Putative Identification | Retention Time (min) | Exact Mass (Da) | Experimental Mass (m/z) |
|---|---|---|---|---|
| 1 | Phytoene | 4.05 | 544.500 | 545.293 |
| 2 | Rhodovibrin | 4.62 | 584.459 | 585.327 |
| 3 | β-cryptoxanthin | 4.69 | 552.433 | 553.337 |
| 4 | 3,4-Didehydrorhodopin | 5.09 | 552.433 | 553.321 |
| 5 | Keto-anhydro-rhodovibrin | 5.31 | 580.428 | 581.368 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Mora, D.J.; Flores-Dávalos, A.G.; Lorenzo-Santiago, M.A.; Guardado-Fierros, B.G.; Rodriguez-Campos, J.; Contreras-Ramos, S.M. Maximizing Biomass Production and Carotenoid-like Pigments Yield in Kocuria sediminis As04 Through Culture Optimization. Microorganisms 2025, 13, 1555. https://doi.org/10.3390/microorganisms13071555
López-Mora DJ, Flores-Dávalos AG, Lorenzo-Santiago MA, Guardado-Fierros BG, Rodriguez-Campos J, Contreras-Ramos SM. Maximizing Biomass Production and Carotenoid-like Pigments Yield in Kocuria sediminis As04 Through Culture Optimization. Microorganisms. 2025; 13(7):1555. https://doi.org/10.3390/microorganisms13071555
Chicago/Turabian StyleLópez-Mora, Daniela Jakeline, Andrea Goreti Flores-Dávalos, Miguel Angel Lorenzo-Santiago, Beatriz Genoveva Guardado-Fierros, Jacobo Rodriguez-Campos, and Silvia Maribel Contreras-Ramos. 2025. "Maximizing Biomass Production and Carotenoid-like Pigments Yield in Kocuria sediminis As04 Through Culture Optimization" Microorganisms 13, no. 7: 1555. https://doi.org/10.3390/microorganisms13071555
APA StyleLópez-Mora, D. J., Flores-Dávalos, A. G., Lorenzo-Santiago, M. A., Guardado-Fierros, B. G., Rodriguez-Campos, J., & Contreras-Ramos, S. M. (2025). Maximizing Biomass Production and Carotenoid-like Pigments Yield in Kocuria sediminis As04 Through Culture Optimization. Microorganisms, 13(7), 1555. https://doi.org/10.3390/microorganisms13071555






