Waterborne Transmission Driving the Prevalence of Blastocystis sp. in Los Ríos Region, Southern Chile
Abstract
1. Introduction
2. Material and Method
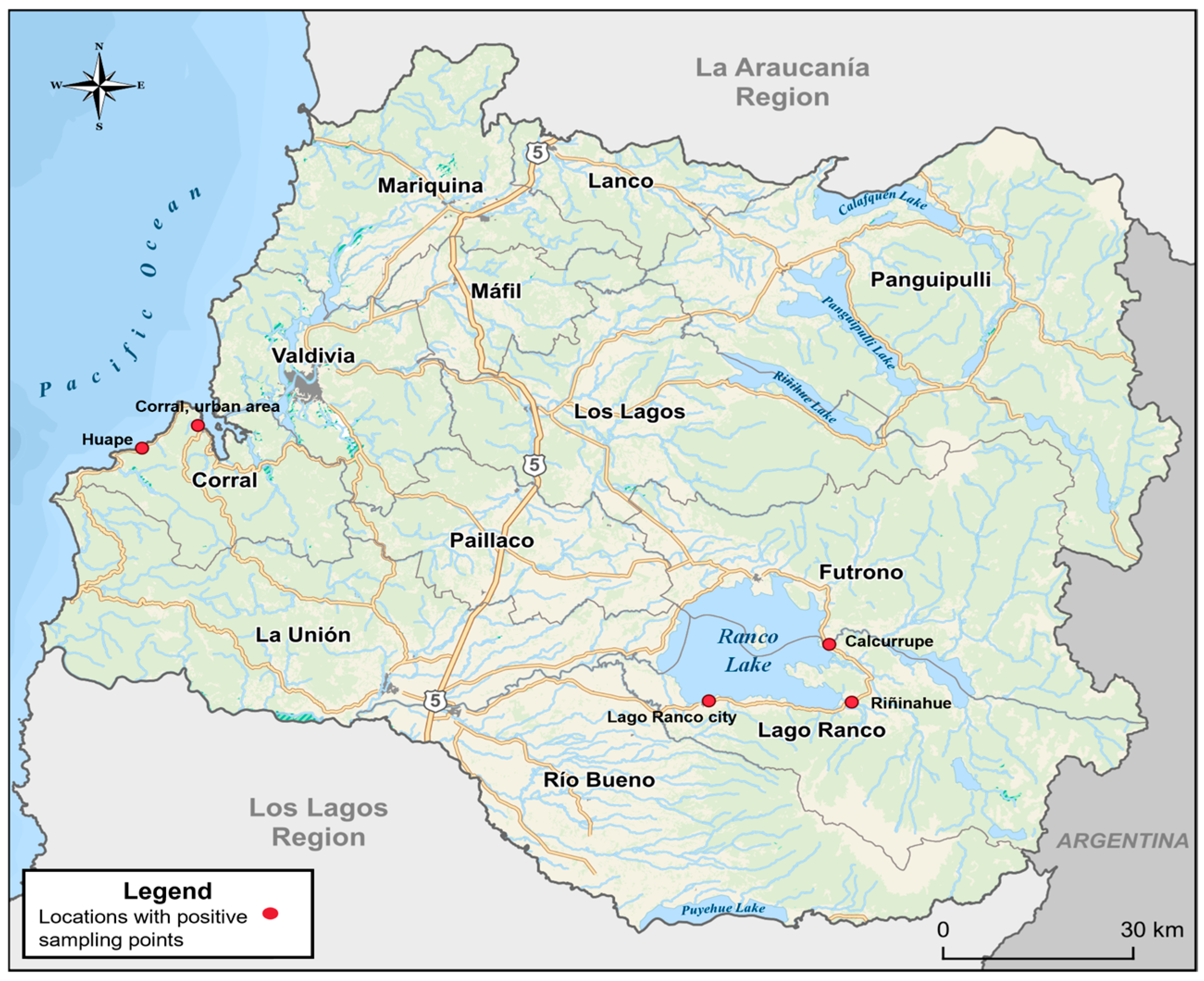
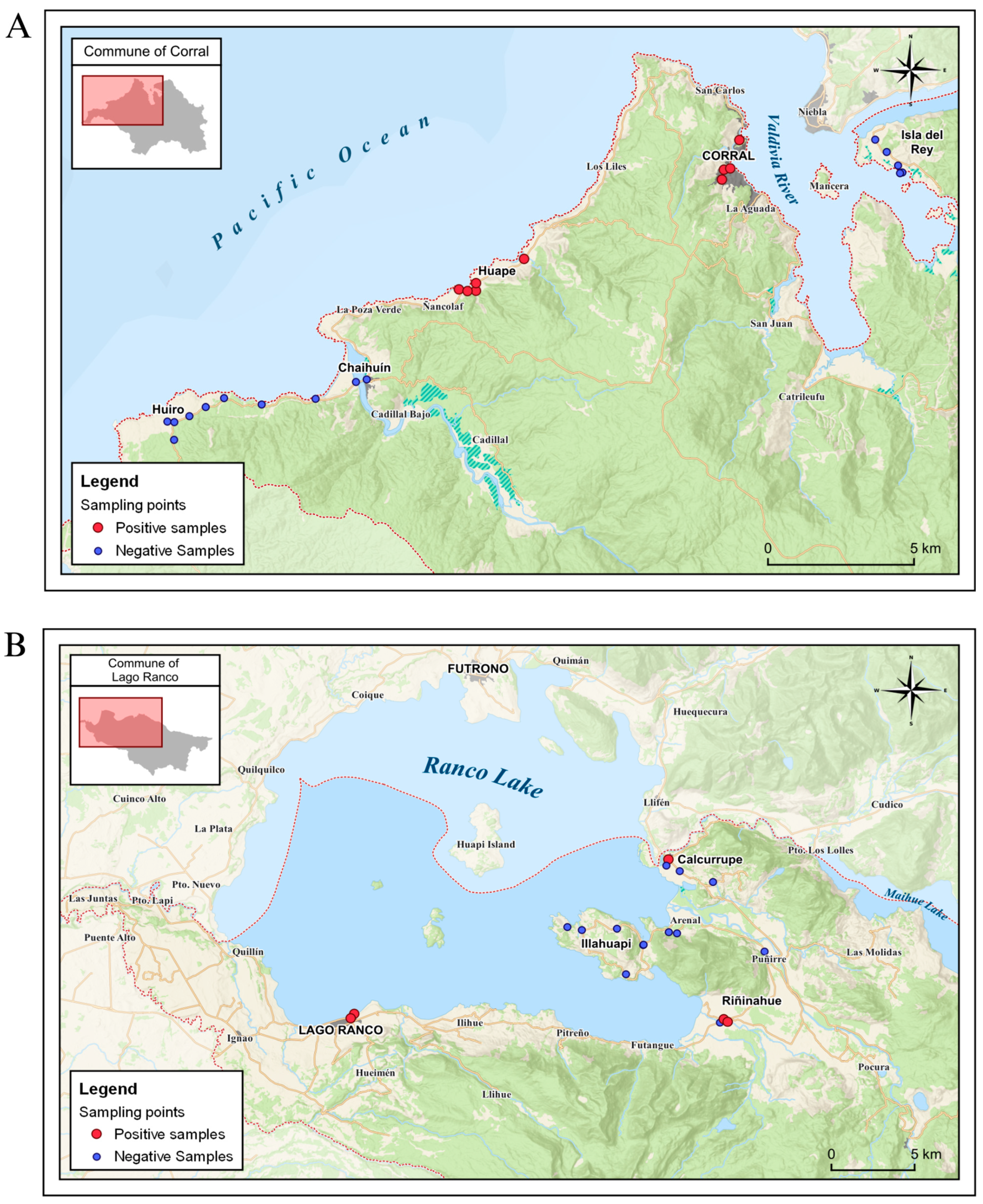
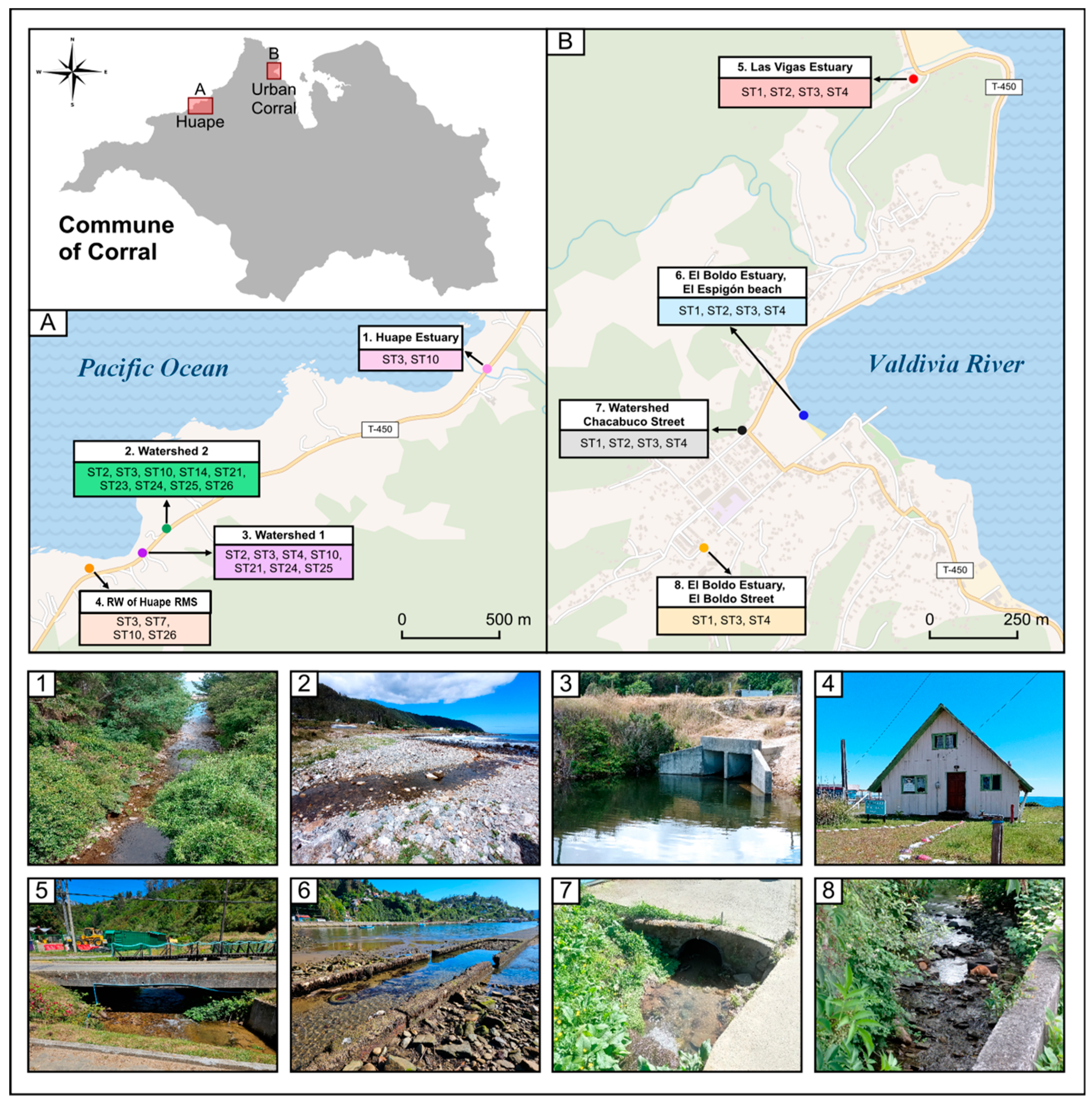
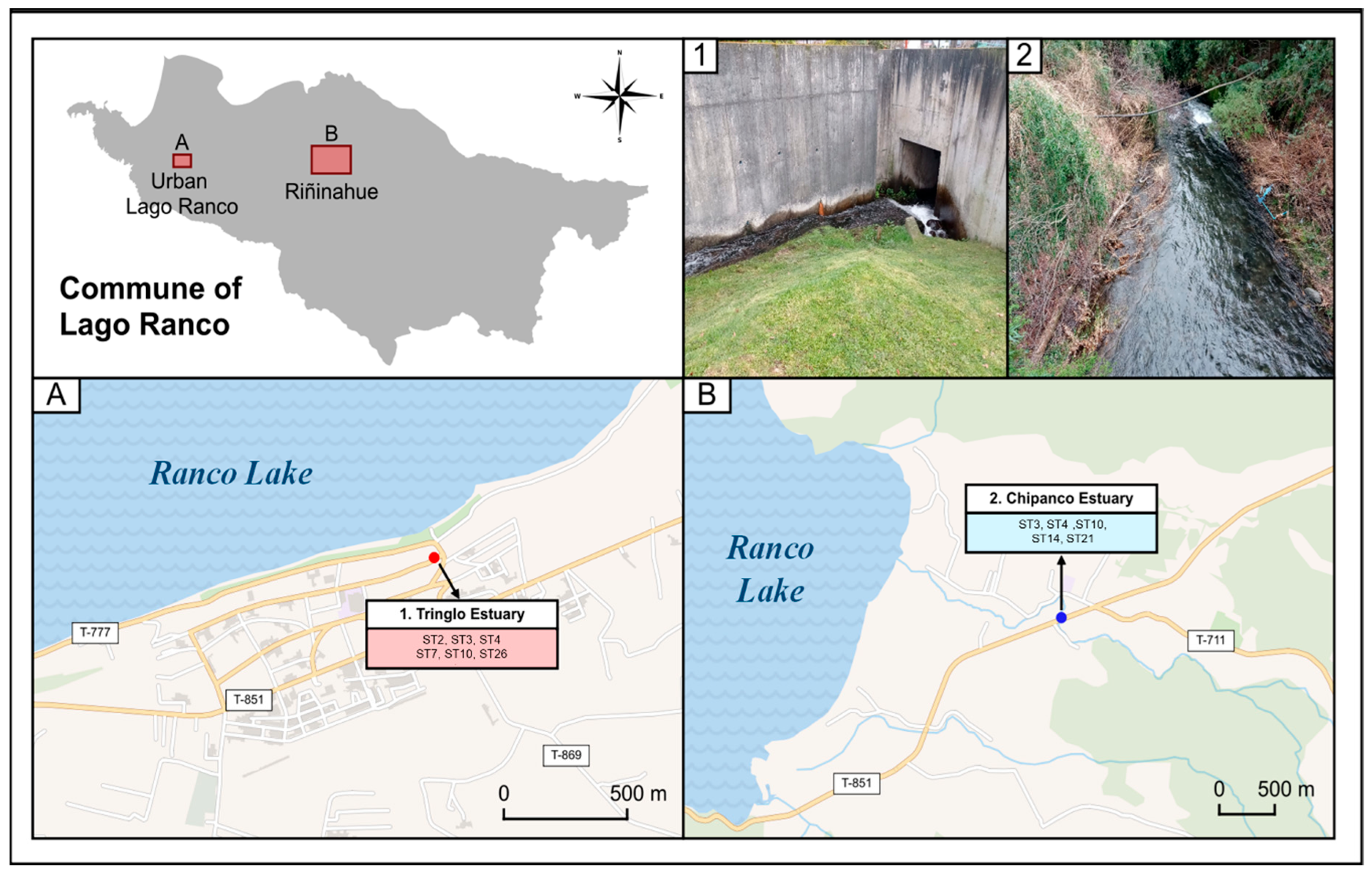
2.1. Characterization of the Study Area
2.2. Sampling
2.3. Analysis of Abiotic Parameters in Water Samples
2.4. Water Sample Processing
2.5. Analysis of Water Samples by Microscopy
2.6. Extracting DNA from Water Samples
2.7. PCR
2.8. Analysis of Subtypes of Blastocystis sp. by Using Next-Generation Sequencing
2.9. Statistical Analyses
3. Results
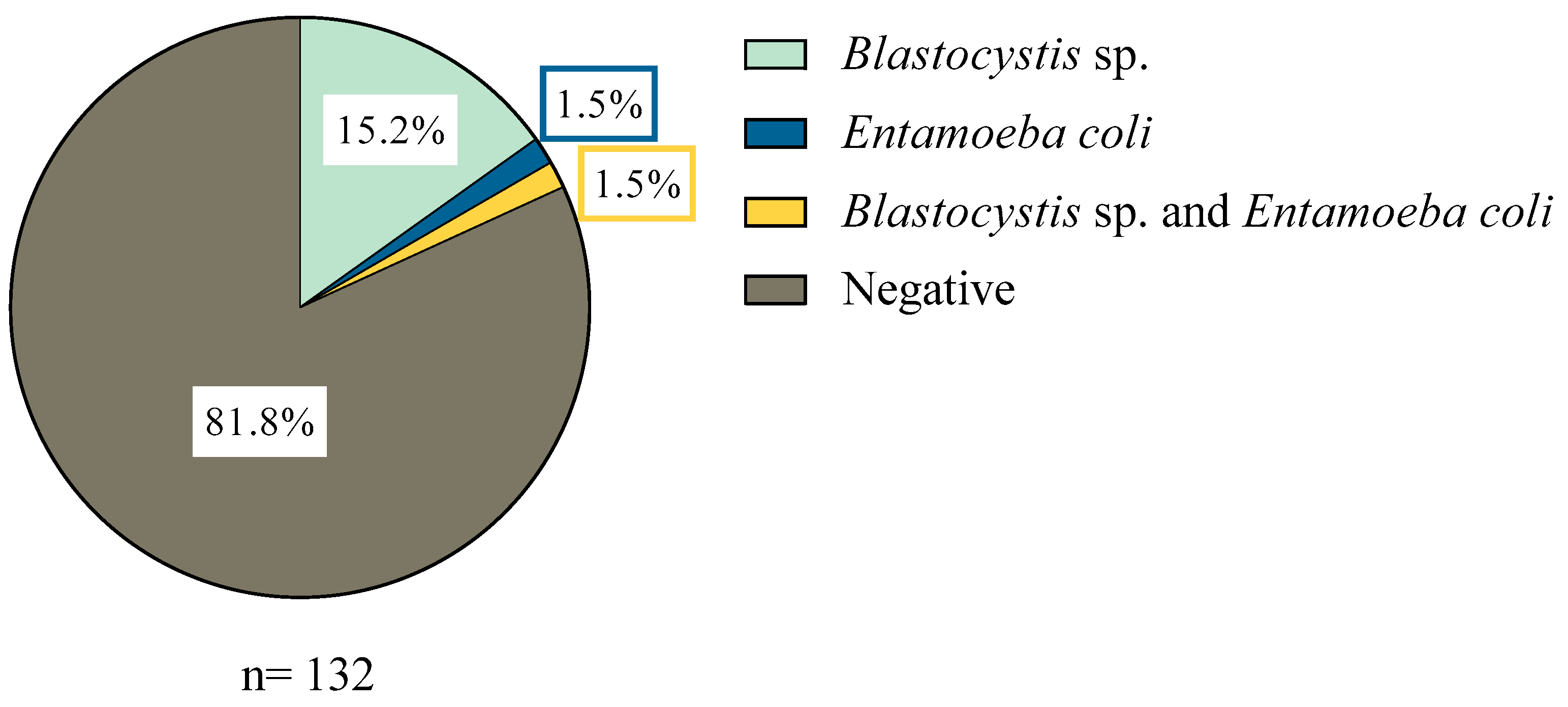
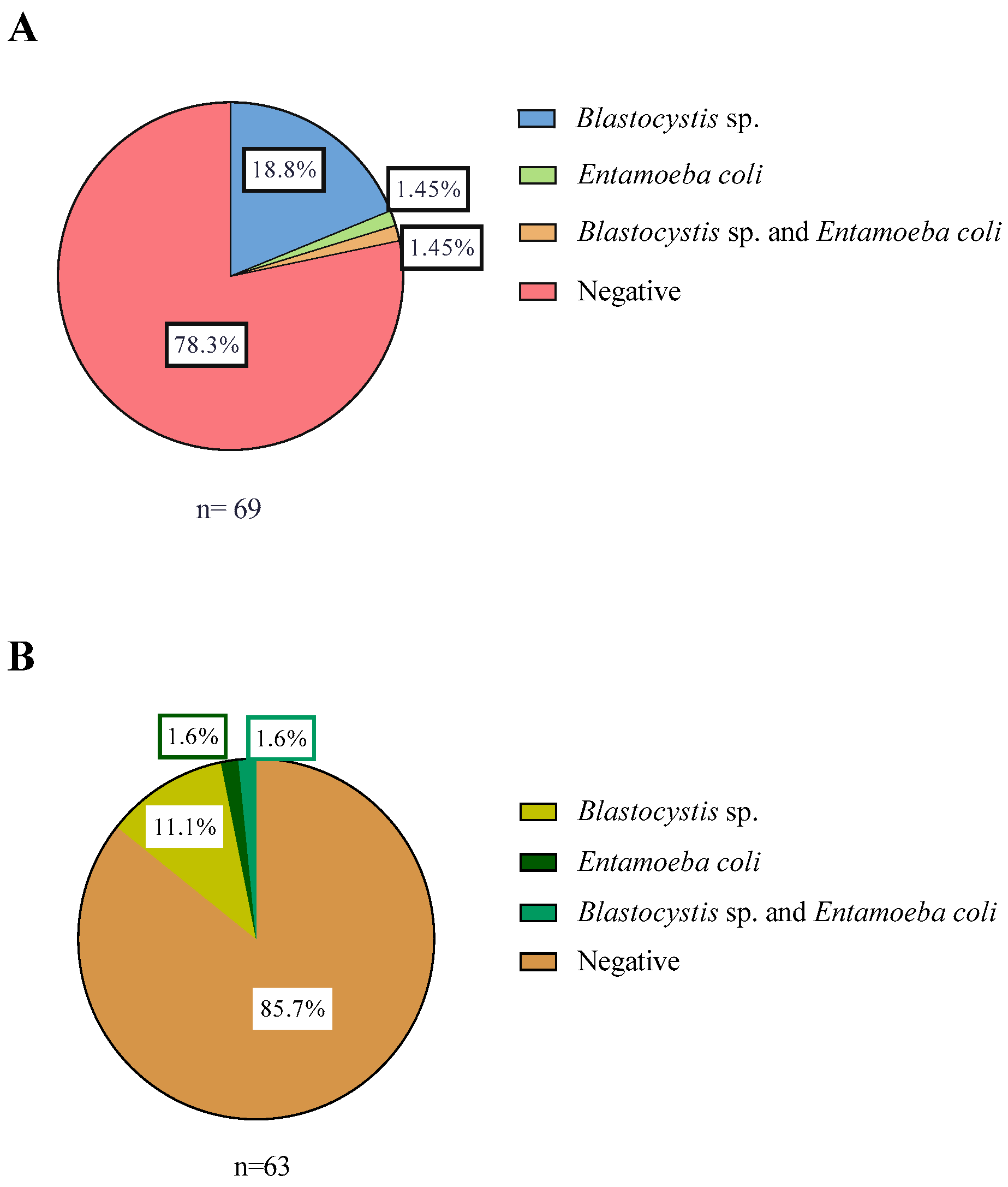
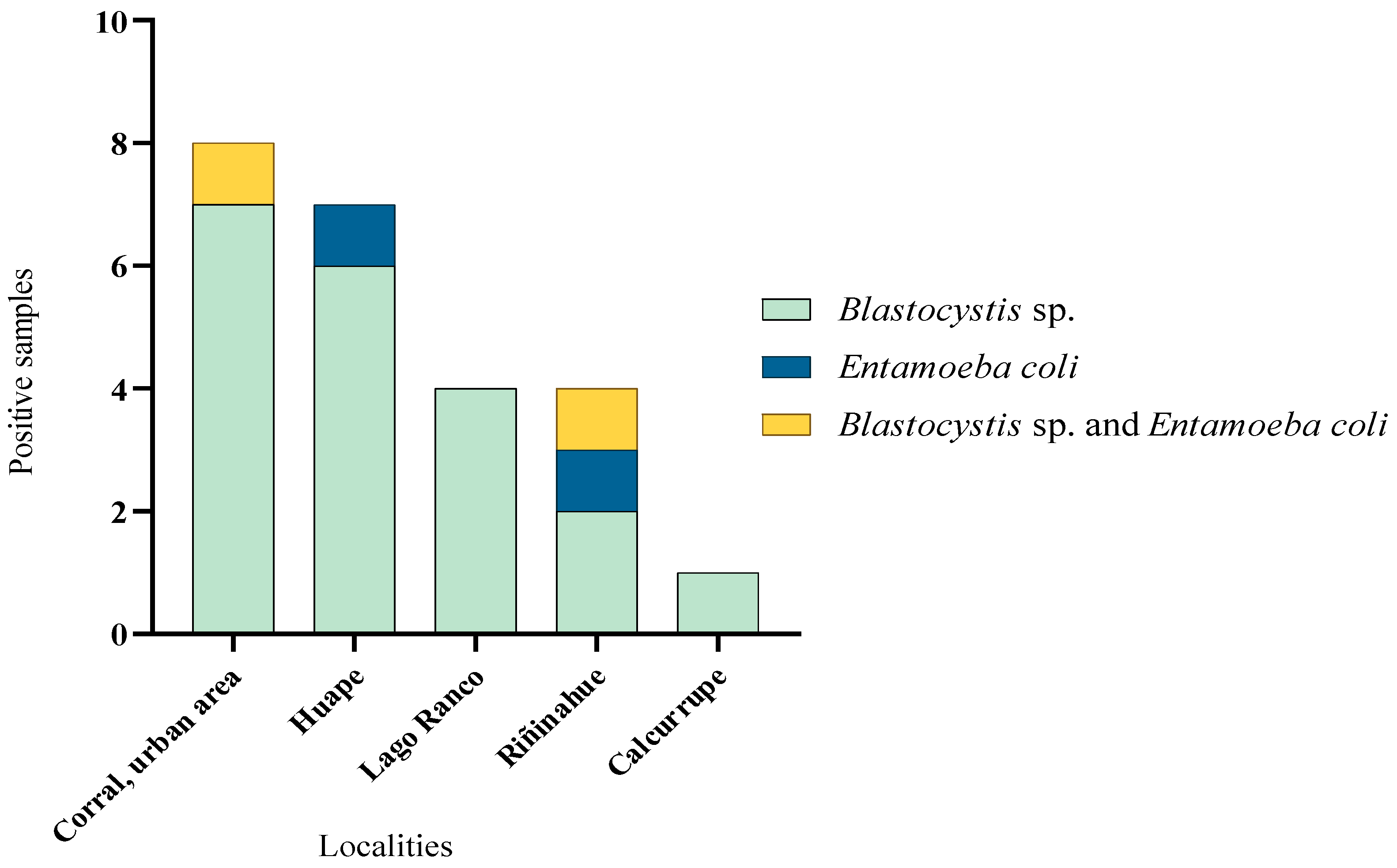
3.1. Distribution of Samples with Parasites
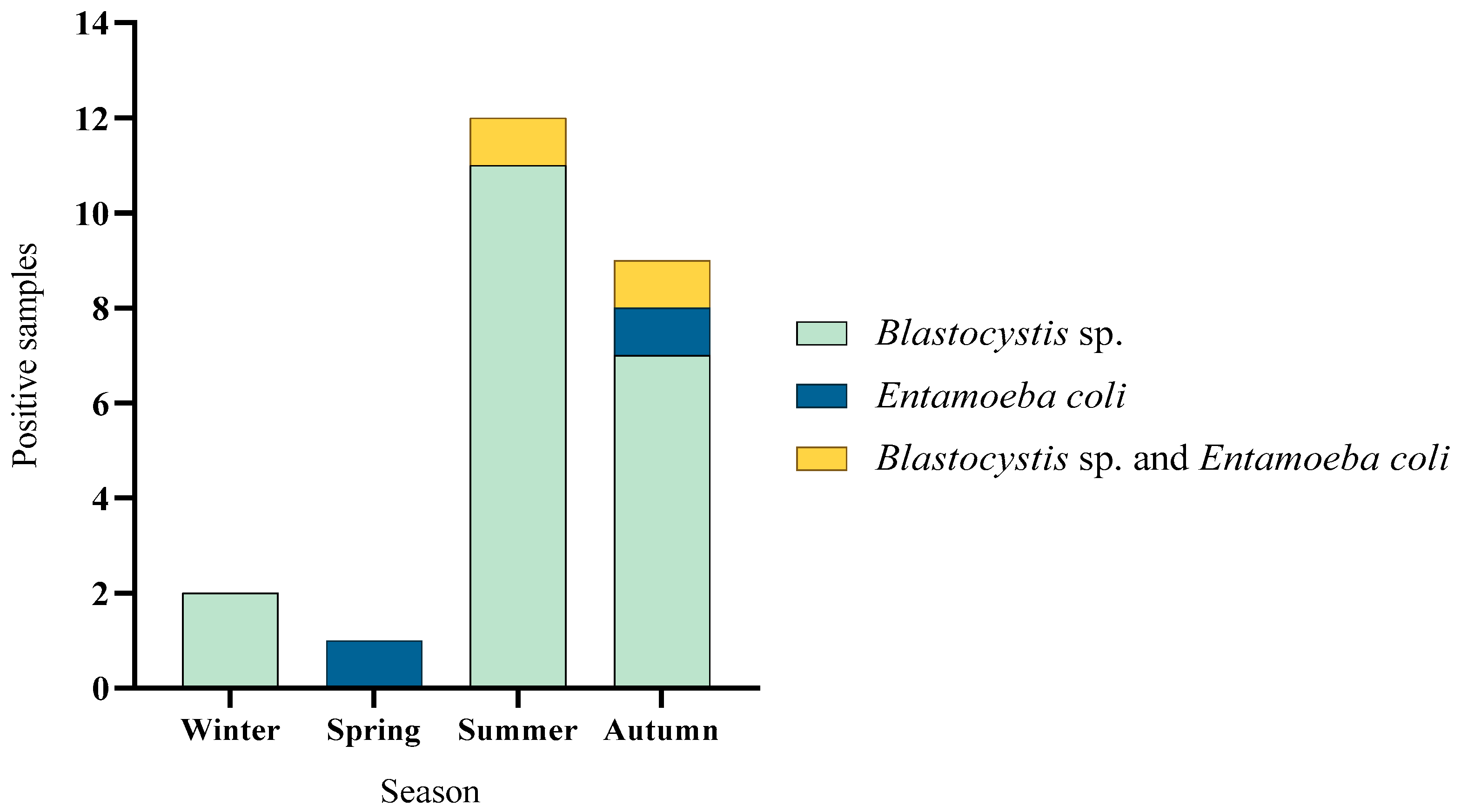
3.2. Seasonal Variation in Parasite Occurrence
3.3. Subtypes of Blastocystis sp.
3.4. Environmental Factors Associated with Blastocystis sp. Presence
4. Discussion
Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurice, A.P.; Jenkin, A.; Norton, R.E.; Hamilton, A.; Ho, Y.H. Epidemiology of Parasitic Diseases. In The Surgical Management of Parasitic Diseases; Tsoulfas, G., Hoballah, J.J., Velmahos, G.C., Ho, Y.H., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–21. ISBN 978-3-030-47948-0. [Google Scholar]
- Bourli, P.; Eslahi, A.V.; Tzoraki, O.; Karanis, P. Waterborne transmission of protozoan parasites: A review of worldwide outbreaks–An update 2017–2022. J. Water Health 2023, 21, 1421–1447. [Google Scholar] [CrossRef]
- Rojo, G.; Cuadros, J. Malaria y Protozoos Intestinales. Enferm. Infecc. Microbiol. Clin. 2016, 34, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Pritt, B.S. Parasites. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Ahmed, M. Intestinal Parasitic Infections in 2023. Gastroenterol. Res. 2023, 16, 127–140. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Leung, A.A.M.; Wong, A.H.C.; Sergi, C.M.; Kam, J.K.M. Giardiasis: An Overview. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Lalle, M.; Cacciò, S.M. Giardiasis from a One Health Perspective. In Zoonoses: Infections Affecting Humans and Animals; Sing, A., Ed.; Springer: Cham, Switzerland, 2023; pp. 1285–1311. ISBN 978-3-031-27164-9. [Google Scholar] [CrossRef]
- Instituto de Salud Pública de Chile Boletines de Vigilancia de Laboratorio. Vigilancia de Diarreas por Agentes Parasitarios en Menores de 5 Años, Chile 2008–2012. 2012. Available online: https://www.ispch.gob.cl/boletin/page/13/ (accessed on 20 May 2025).
- Barra, M.; Bustos, L.; Ossa, X. Desigualdad en la prevalencia de parasitosis intestinales en escolares de una escuela urbana y dos rurales de la comuna de Puerto Monnt. Rev. Med. Chile 2016, 144, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Toloza, L.; Cancino, B. Evolución de la prevalencia de enteroparasitosis en la ciudad de Talca, Región del Maule, Chile. Rev. Chil. Infectología 2010, 27, 336–340. [Google Scholar] [CrossRef]
- Apt, W.; Zulantay, I.; Canals, M.; Fredes, F. Visión de las parasitosis humanas en Chile. Parasitol. Latinoam. 2021, 70, 47–67. [Google Scholar]
- Torres, P.; Otth, L.; Montefusco, A.; Wilson, G.; Ramírez, C.; Acuña, M. Infección por protozoos y helmintos intestinales en escolares de sectores ribereños, con distintos niveles de contaminación fecal, del río Valdivia, Chile. Bol. Chil. Parasitol. 1997, 52, 3–11. [Google Scholar]
- Sanhueza-Teneo, D.; Cerna, O.; Chesnais, C.B.; Cárdenas, D.; Camus, P. High parasite prevalence driven by the human-animal-environment interface: A One Health study in an urban area in southern of Chile. Front. Vet. Sci. 2025, 12, 1536861. [Google Scholar] [CrossRef]
- Sanhueza-Teneo, D.; Venegas, T.; Videla, F.; Chesnais, C.B.; Loncoman, C.; Valenzuela-Nieto, G. Prevalence and Genetic Diversity of Parasites in Humans and Pet Dogs in Rural Areas of Los Ríos Region, Southern Chile. Pathogens 2025, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.V. Detection of parasites in the environment. Parasitology 1998, 117 (Suppl), S113–S141. [Google Scholar] [CrossRef]
- Ajeagah, G.; Njine, T.; Bilong Bilong, C.; Foto, S.; Wouafo, M.; Nola, M. Seasonal Distribution of Enteric Opportunistic Cryptosporidium spp. Oocystis and Giardia spp. Cysts in a Tropical Water Basin, Cameroon. Water 2010, 2, 44–57. [Google Scholar]
- Betancourt, W.; Querales, L. Parásitos Protozoarios Entéricos en Ambientes Acuáticos: Métodos de concentración y detección. Interciencia 2008, 33, 418–423. [Google Scholar]
- Del Coco, V.F.; Molina, N.B.; Basualdo, J.A.; Córdoba, M.A. Blastocystis spp.: Avances, controversias y desafíos futuros. Rev. Argent. Microbio 2017, 49, 110–118. [Google Scholar] [CrossRef]
- Koehler, A.V.; Herath, H.M.P.D.; Hall, R.S.; Wilcox, S.; Gasser, R.B. Marked genetic diversity within Blastocystis in Australian wildlife revealed using a next generation sequencing–phylogenetic approach. Int. J. Parasitol. Parasites Wildl. 2024, 23, 100902. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Karimi-Pour-Saryazdi, A.; Rahmanian, V.; Bahadory, S.; Abdoli, A.; Rezanezhad, H. Global prevalence and subtype distribution of Blastocystis sp. in rodents, birds, and water supplies: A systematic review and meta-analysis. Prev. Vet. Med. 2022, 208, 105770. [Google Scholar] [CrossRef]
- Adao, D.E.V.; Rivera, W.L. Subtype–host patterns and genetic differentiation of Blastocystis sp. in the Philippines. Heliyon 2024, 10, e29019. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.I.; Chye, T.T.; Karmacharya, B.M.; Govind, S.K. Blastocystis sp.: Waterborne zoonotic organism, a possibility? Parasit. Vectors 2012, 5, 130. [Google Scholar] [CrossRef]
- Suarez, P.; Vallejos-Almirall, A.; Fernández, I.; Gonzalez-Chavarria, I.; Alonso, J.L.; Vidal, G. Identification of Cryptosporidium parvum and Blastocystis hominis subtype ST3 in Cholga mussel and treated sewage: Preliminary evidence of fecal contamination in harvesting area. Food Waterborne Parasitol. 2024, 34, e00214. [Google Scholar] [CrossRef]
- Aguilera, N.; Villagra, P. Territorial and multidimensional contrasts between urban and rural community resilience to disaster in the Corral Commune. Rev. De Urban. 2023, 49, 66–92. [Google Scholar] [CrossRef]
- Biblioteca del Congreso Nacional de Chile (BCN). Corral Reporte Comunal. 2023. Available online: https://www.bcn.cl/siit/reportescomunales/comunas_v.html?anno=2023&idcom=14102 (accessed on 20 March 2025).
- Instituto de Investigaciones Agropecuarias (INIA). 2024. Available online: https://agrometeorologia.cl/PP_TOTAL# (accessed on 15 May 2025).
- Biblioteca del Congreso Nacional de Chile (BCN). Lago Ranco Reporte Comunal. 2023. Available online: https://www.bcn.cl/siit/reportescomunales/comunas_v.html?anno=2023&idcom=14203 (accessed on 20 March 2025).
- Oyarzo, C.; Efectos del Cambio Climático en la Evolución de los Niveles de Agua de Lago Ranco. Universidad Andrés Bello. 2022. Available online: https://repositorio.unab.cl/xmlui/handle/ria/50860 (accessed on 20 May 2025).
- Amoah, I.D.; Singh, G.; Stenström, T.A.; Reddy, P. Detection and quantification of soil-transmitted helminths in environmental samples: A review of current state-of-the-art and future perspectives. Acta Trop. 2017, 169, 187–201. [Google Scholar] [CrossRef]
- Kumar, T.; Onichandran, S.; Lim, Y.A.L.; Sawangjaroen, N.; Ithoi, I.; Andiappan, H. Comparative Study on Waterborne Parasites between Malaysia and Thailand: A New Insight. Am. Soc. Trop. Med. Hyg. 2014, 90, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; López, M.C.; Galeano, L.A.; Qvarnstrom, Y.; Houghton, K.; Ramírez, J.D. Molecular detection and genotyping of pathogenic protozoan parasites in raw and treated water samples from southwest Colombia. Parasit. Vectors 2018, 11, 563. [Google Scholar] [CrossRef]
- García, L. Diagnostic Medical Parasitoly, 5th ed.; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Hinlo, R.; Gleeson, D.; Lintermans, M.; Furlan, E. Methods to maximise recovery of environmental DNA from water samples. PLoS ONE 2017, 12, e0179251. [Google Scholar] [CrossRef] [PubMed]
- Santín, M.; Gómez-Muñoz, M.T.; Solano-Aguilar, G.; Fayer, R. Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol. Res. 2011, 109, 205–212. [Google Scholar] [CrossRef]
- Maloney, J.G.; Molokin, A.; Santin, M. Assessment of next generation amplicon sequencing of the beta-giardin gene for the detection of Giardia duodenalis assemblages and mixed infections. Food Waterborne Parasitol. 2020, 21, e00098. [Google Scholar] [CrossRef]
- Rattaprasert, P.; Nitatsukprasert, C.; Thima, K.; Chavalishewinkoon-Permitr, P. Development of nested PCR for identification of Entamoeba coli in stools samples. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Khalifa, R.M.A.; Ahmad, A.K.; Abdel-Hafeez, E.H. Present Status of Protozoan Pathogens Causing Water—Borne Disease in Northern Part of El-Minia Governorate, Egypt. J. Egypt. Soc. Parasitol. 2014, 44, 559–566. [Google Scholar] [CrossRef]
- Richard, R.L.; Ithoi, I.; Abd- Majid, M.A.; Wan-Sulaiman, W.Y.; Tan, T.C.; Nissapatorn, V. Monitoring of Waterborne Parasites in Two Drinking Water Treatment Plants: A Study in Sarawak, Malaysia. Int. J. Environ. Res. Public. Health 2016, 13, 641. [Google Scholar] [CrossRef] [PubMed]
- Karaman, Ü.; Kolören, Z.; Seferoğlu, O.; Ayaz, E.; Demirel, E. Presence of Parasites in Environmental Waters in Samsun and Its Districts. Turk. Parazitol. Derg. 2017, 41, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Falcone, A.C.; Sanguino-Jorquera, D.G.; Unzaga, J.M. Review of the concentration procedures used to determine the parasitological water quality in Latin America (2000–2022). Water Pract. Technol. 2024, 19, 3578. [Google Scholar] [CrossRef]
- Attah, A.O.; Sanggari, A.; Li, L.I.; Nik-Him, N.A.I.I.; Ismail, A.H.; Meor-Termizi, F.H. Blastocystis occurrence in water sources worldwide from 2005 to 2022: A review. Parasitol. Res. 2023, 122, 1–10. [Google Scholar] [CrossRef]
- Moreno-Mesonero, L.; Amorós, I.; Moreno, Y.; Alonso, J.L. Simultaneous detection of less frequent waterborne parasitic protozoa in reused wastewater using amplicon sequencing and qPCR techniques. J. Environ. Manag. 2022, 314, 115029. [Google Scholar] [CrossRef]
- Hatam-Nahavandi, K.; Mahvi, A.H.; Mohebali, M.; Keshavarz, H.; Mobedi, I.; Rezaeian, M. Detection of parasitic particles in domestic and urban wastewaters and assessment of removal efficiency of treatment plants in Tehran, Iran. J. Environ. Health Sci. Eng. 2015, 13, 4. [Google Scholar] [CrossRef]
- Basualdo, J.; Pezzani, B.; Luca, M.; Córdoba, A.; Apezteguía, M. Screening of the municipal water system of La Plata, Argentina, for human intestinal parasites. Int. J. Hyg. Environ. Health 2000, 203, 177–182. [Google Scholar] [CrossRef]
- Kumar, T.; Majid, M.A.A.; Onichandran, S.; Jaturas, N.; Andiappan, H.; Salibay, C.C. Presence of Cryptosporidium parvum and Giardia lamblia in water samples from Southeast Asia: Towards an integrated water detection system. Infect. Dis. Poverty 2016, 5, 3. [Google Scholar] [CrossRef]
- Ramos, M.R.; Magtibay, C.M. Detection and Identification of Waterborne Parasites in Taguibo Watershed Forest Reserve, Butuan City. Asia Pac. J. Allied Health Sci. 2020, 3, 21–28. [Google Scholar]
- Adamska, M.; Sawczuk, M.; Kolodziejczyk, L.; Skotarczak, B. Assessment of molecular methods as a tool for detecting pathogenic protozoa isolated from water bodies. J. Water Health 2015, 13, 953–959. [Google Scholar] [CrossRef]
- Guilavogui, T.; Gantois, N.; Even, G.; Desramaut, J.; Dautel, E.; Denoyelle, C. Detection, Molecular Identification and Transmission of the Intestinal Protozoa Blastocystis sp. in Guinea from a Large-Scale Epidemiological Study Conducted in the Conakry Area. Microorganisms 2022, 10, 446. [Google Scholar] [CrossRef]
- Jinatham, V.; Maxamhud, S.; Popluechai, S.; Tsaousis, A.D.; Gentekaki, E. Blastocystis One Health Approach in a Rural Community of Northern Thailand: Prevalence, Subtypes and Novel Transmission Routes. Front. Microbiol. 2021, 12, 746340. [Google Scholar] [CrossRef]
- Fusaro, C.; Bernal, J.E.; Baldiris-Ávila, R.; González-Cuello, R.; Cisneros-Lorduy, J.; Reales-Ruiz, A. Molecular Prevalence and Subtypes Distribution of Blastocystis spp. in Humans of Latin America: A Systematic Review. Trop. Med. Infect. Dis. 2024, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Abarca, N.; Santín, M.; Ortega, S.; Maloney, J.G.; George, N.S.; Molokin, A. Molecular Detection and Characterization of Blastocystis sp. and Enterocytozoon bieneusi in Cattle in Northern Spain. Vet. Sci. 2021, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- McCain, A.; Gruneck, L.; Popluechai, S.; Tsaousis, A.D.; Gentekaki, E. Circulation and colonisation of Blastocystis subtypes in schoolchildren of various ethnicities in rural northern Thailand. Epidemiol. Infect. 2023, 151, e77. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gou, J.; Yang, B.; Du, J.; Yao, H.; Ren, M. Prevalence and Subtype Distribution of Blastocystis in Tibetan Sheep in Qinghai Province, Northwestern China. Protist 2023, 174, 125948. [Google Scholar] [CrossRef]
- Peña, S.; Carrasco, G.; Rojas, P.; Castillo, D.; Ozaki, L.S.; Mercado, R. Determination of subtypes of Blastocystis sp. in Chilean patients with and without inflammatory bowel syndrome, A preliminary report. Parasite Epidemiol. Control 2020, 8, e00125. [Google Scholar] [CrossRef]
- Cable, J.; Barber, I.; Boag, B.; Ellison, A.R.; Morgan, E.R.; Murray, K. Global change, parasite transmission and disease control: Lessons from ecology. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372, 20160088. [Google Scholar] [CrossRef]
- Simon-Oke, I.A.; Afolabi, O.J.; Obimakinde, E.T. Parasitic contamination of water sources in Akure, Ondo State, Nigeria. J. Basic Appl. Zool. 2020, 81, 50. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]

| Localities | Winter | Spring | Summer | Autumn |
|---|---|---|---|---|
| Corral, urban area | 2 | 4 | 4 | 4 |
| Huape | 4 | 5 | 5 | 5 |
| Chaihuín | 3 | 3 | 2 | 2 |
| Huiro | 2 | 7 | 6 | 6 |
| Isla del Rey | - | 5 | - | - |
| Total samples | 11 | 24 | 17 | 17 |
| Total samples in Corral commune | 69 | |||
| Localities | Winter | Spring | Summer | Autumn |
|---|---|---|---|---|
| Lago Ranco, city | 2 | 2 | 2 | 2 |
| Illahuapi | 4 | 9 | 3 | 7 |
| Riñinahue | 4 | 4 | 4 | 4 |
| Calcurrupe | 4 | 4 | 4 | 4 |
| Total samples | 14 | 19 | 13 | 17 |
| Total samples in Lago Ranco commune | 63 | |||
| Parasite | Gen | GenBank Code | Oligonucleotide Sequence 5′ to 3′ | PCR Product Size | Reference |
|---|---|---|---|---|---|
| Blastocystis sp. | 18s | XM_013038637.1 | FS-GGAGGTAGTGACAATAAATC AS-GCTTTCGCACTTGTTCATC | 538 bp | Santín et al., 2011 [34] |
| Giardia duodenalis | β-giardin | XM_001705373.1 | FS-GAACGAGATCGAGGTCCG AS-CTCGACGAGCTTCGTGTT | 455 bp | Maloney et al., 2020 [35] |
| Entamoeba coli | SSU Rrna | AF149915.1 | FS-CTAAGCACAAAGTCCTAGTATGATG AS-CCTCATCGATTACACTCCCAG-AC | 166 bp | Rattaprasert et al., 2021 [36] |
| Variable | Total Mean ± SD | Blastocystis − Mean ± SD | Blastocystis + Mean ± SD | p-Value (Mann–Whitney) |
|---|---|---|---|---|
| Temperature (°C) | 12.89 ± 2.55 | 12.68 ± 2.49 | 13.97 ± 2.65 | 0.021 |
| pH | 6.94 ± 0.58 | 6.95 ± 0.60 | 6.91 ± 0.48 | 0.831 |
| TDS (ppm) | 163.18 ± 479.52 | 186.18 ± 522.53 | 48.18 ± 22.81 | 0.120 |
| EC (mS/cm) | 0.34 ± 0.96 | 0.39 ± 1.04 | 0.11 ± 0.05 | 0.096 |
| Precipitation (mm) | 30.61 ± 33.52 | 28.44 ± 33.12 | 41.48 ± 34.17 | 0.002 |
| Relative Humidity (%) | 84.85 ± 7.32 | 85.36 ± 7.22 | 82.59 ± 7.48 | 0.095 |
| Ambient Temperature (°C) | 10.03 ± 3.90 | 9.50 ± 3.38 | 11.97 ± 4.99 | 0.034 |
| Farmhouses distance (km) | 0.09 ± 0.19 | 0.10 ± 0.21 | 0.05 ± 0.04 | 0.707 |
| Variable | aOR (IC 95%) | p-Value |
|---|---|---|
| Lago Ranco region (Ref. Corral) | 0.24 [0.02–2.39] | 0.222 |
| Type of water (Ref. standing water) | ||
| No standing water/river | 0.48 [0.04–6.20] | 0.572 |
| Potable water | 0.04 [0.00–0.87] | 0.041 |
| TDS | 1.02 [0.86–1.21] | 0.831 |
| Water temperature (°C) | 1.15 [0.76–1.73] | 0.510 |
| Ph | 0.35 [0.03–3.62] | 0.379 |
| EC (mS/cm) | 8.91 × 10−9 [1.29 × 10−47–6.16 × 1030] | 0.685 |
| Precipitations (mm) | 1.03 [1.00–1.06] | 0.036 |
| Relative humidity (%) | 0.74 [0.52–1.04] | 0.079 |
| Ambient temperature (°C) | 0.69 [0.39–1.23] | 0.205 |
| Farmhouses distance (km) | 5.81 × 10−9 [7.14 × 10−16–0.0472] | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhueza Teneo, D.; Chesnais, C.B.; Manzano, J.; Moll, M.P.; Téllez, A.; Valenzuela-Nieto, G. Waterborne Transmission Driving the Prevalence of Blastocystis sp. in Los Ríos Region, Southern Chile. Microorganisms 2025, 13, 1549. https://doi.org/10.3390/microorganisms13071549
Sanhueza Teneo D, Chesnais CB, Manzano J, Moll MP, Téllez A, Valenzuela-Nieto G. Waterborne Transmission Driving the Prevalence of Blastocystis sp. in Los Ríos Region, Southern Chile. Microorganisms. 2025; 13(7):1549. https://doi.org/10.3390/microorganisms13071549
Chicago/Turabian StyleSanhueza Teneo, Daniel, Cedric B. Chesnais, Javiera Manzano, María Paz Moll, Analía Téllez, and Guillermo Valenzuela-Nieto. 2025. "Waterborne Transmission Driving the Prevalence of Blastocystis sp. in Los Ríos Region, Southern Chile" Microorganisms 13, no. 7: 1549. https://doi.org/10.3390/microorganisms13071549
APA StyleSanhueza Teneo, D., Chesnais, C. B., Manzano, J., Moll, M. P., Téllez, A., & Valenzuela-Nieto, G. (2025). Waterborne Transmission Driving the Prevalence of Blastocystis sp. in Los Ríos Region, Southern Chile. Microorganisms, 13(7), 1549. https://doi.org/10.3390/microorganisms13071549





