Adaptive Evolution of Sporosarcina pasteurii Enhances Saline–Alkali Resistance for High-Performance Concrete Crack Repair via MICP
Abstract
1. Introduction
2. Material and Method
2.1. Strain and Culture Method
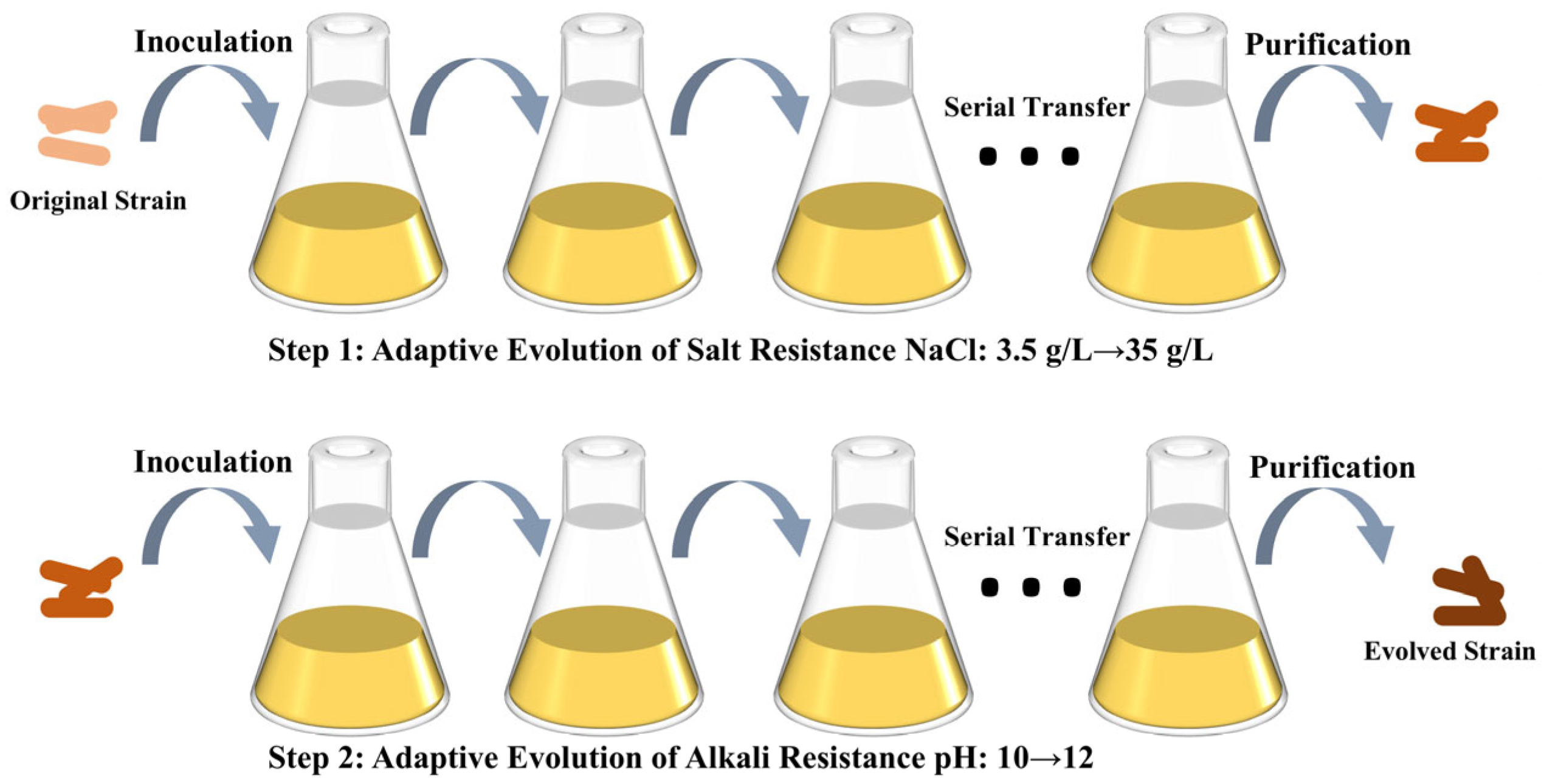
2.2. Laboratory Adaptive Evolutionary Approaches
2.3. Measurement of Urease Activity
2.4. Measurement of Mineralization Product
2.5. Particle Size Analysis of Mineralized Sediment
2.6. X-Ray Diffraction (XRD) Analysis
2.7. Scanning Electron Microscope Test (SEM) Analysis
2.8. Genome Resequencing
2.9. Transcriptome Sequencing
2.10. Concrete Crack Repair Test
2.11. Evaluation of Restoration Effect
2.12. Statistical Analysis
3. Results and Discussion
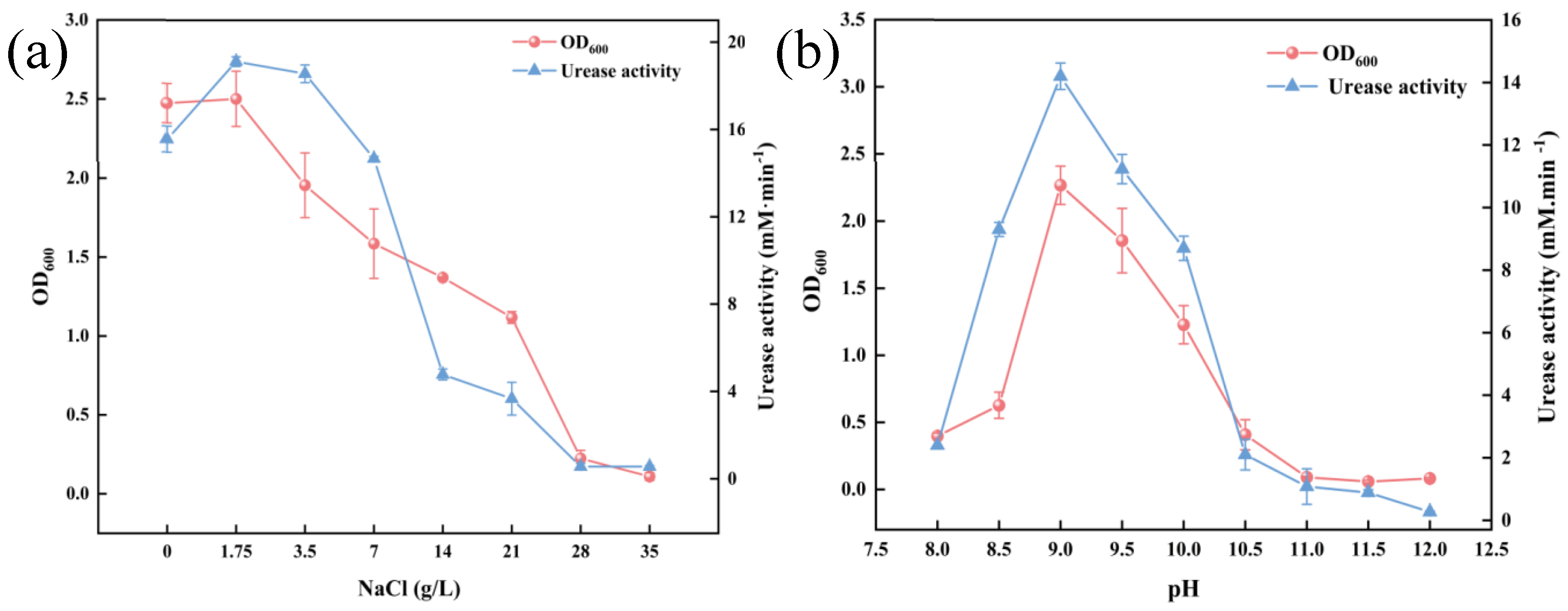
3.1. Salt and Alkali Stress Tolerance of S. pasteurii
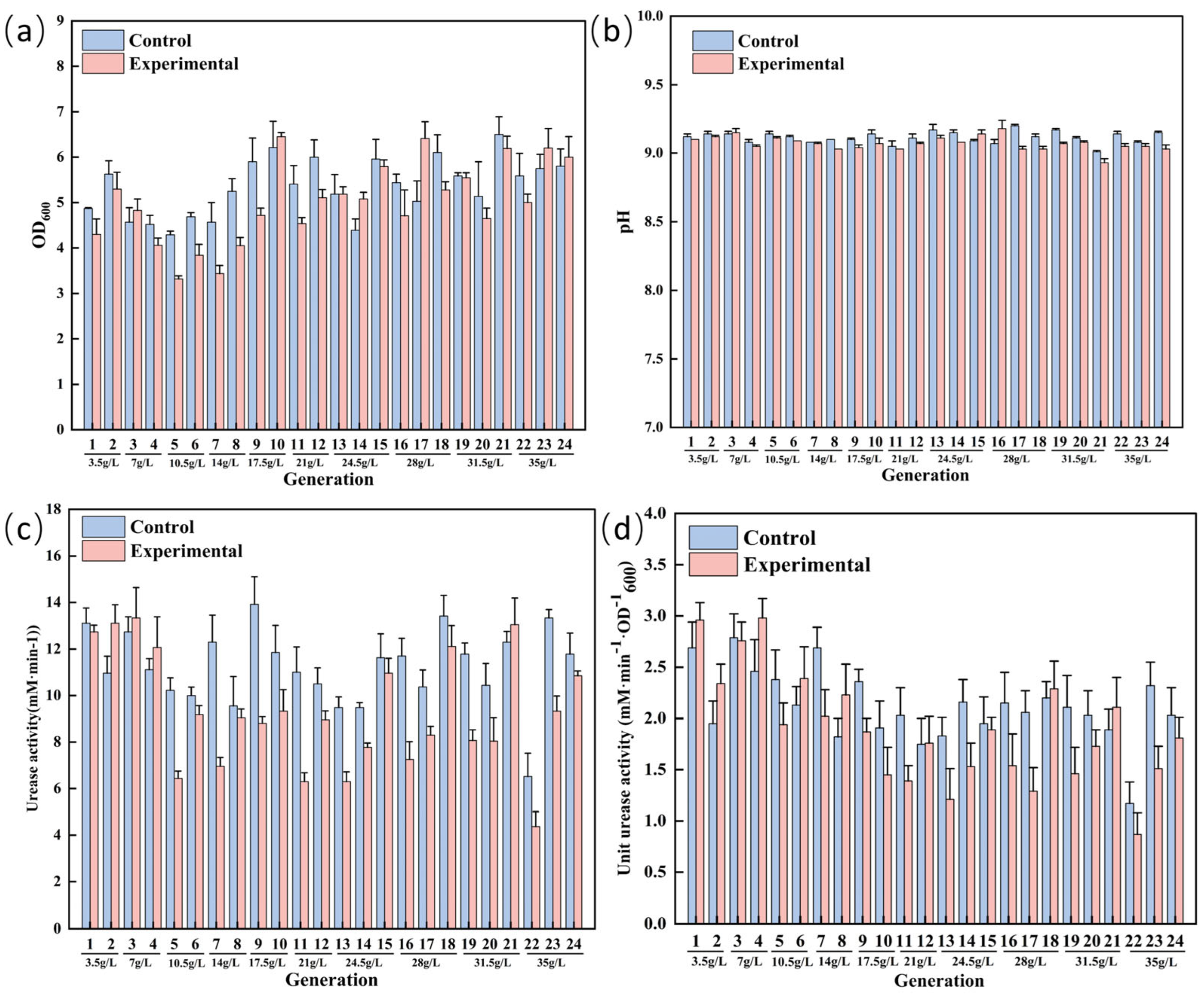
3.2. ALE of S. pasteurii for Enhanced Saline Tolerance
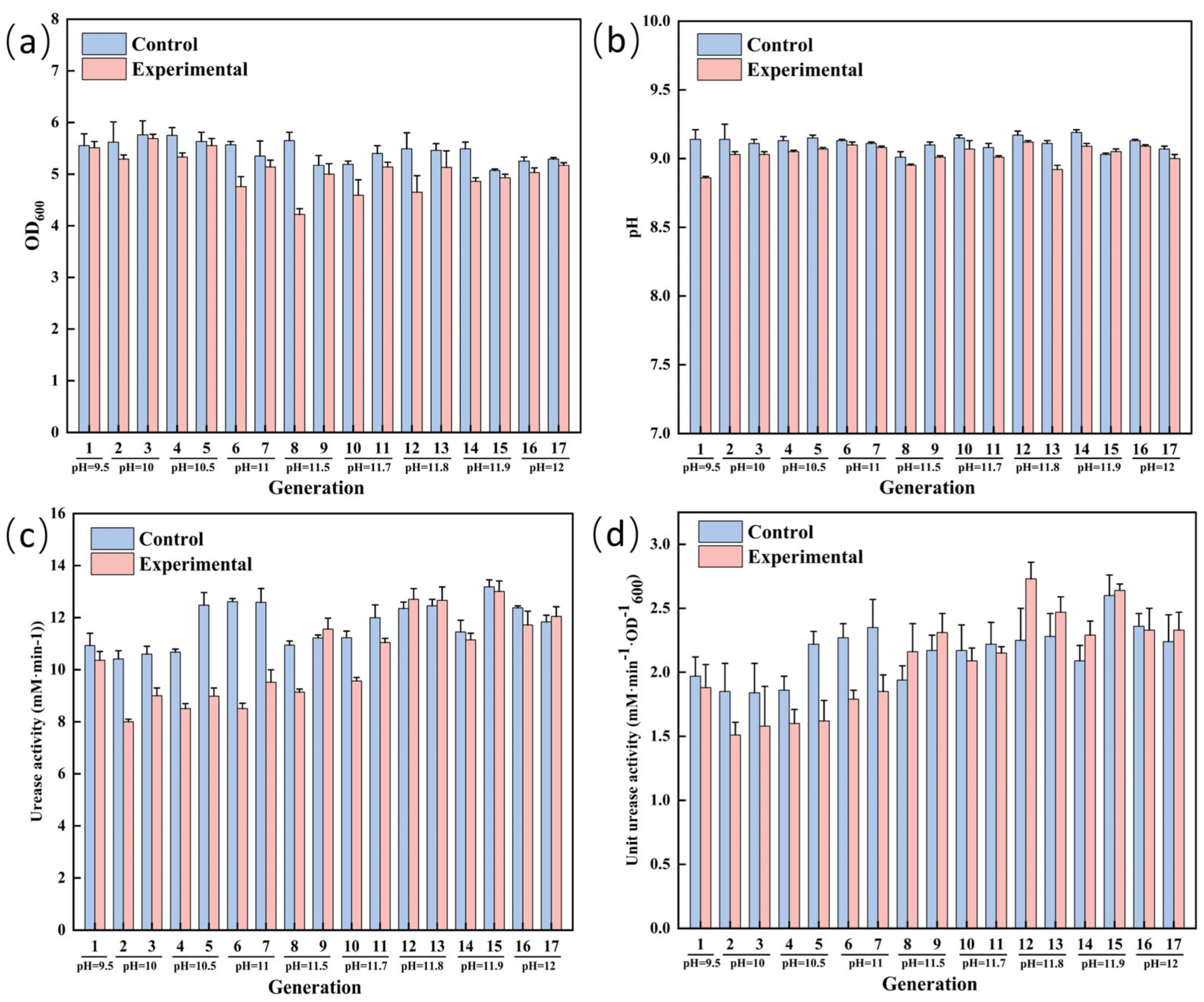
3.3. ALE of S. pasteurii for Enhanced Alkaline Tolerance
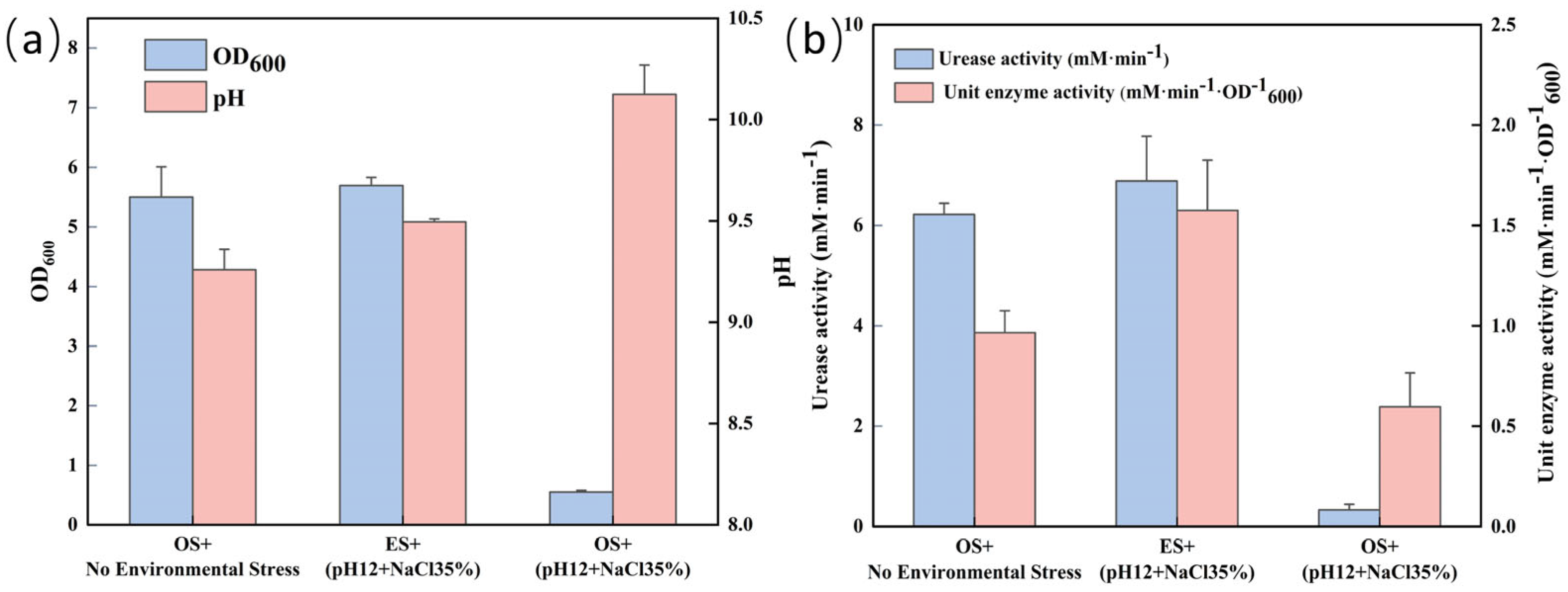
3.4. Comparison of Physiological Indexes of S. pasteurii
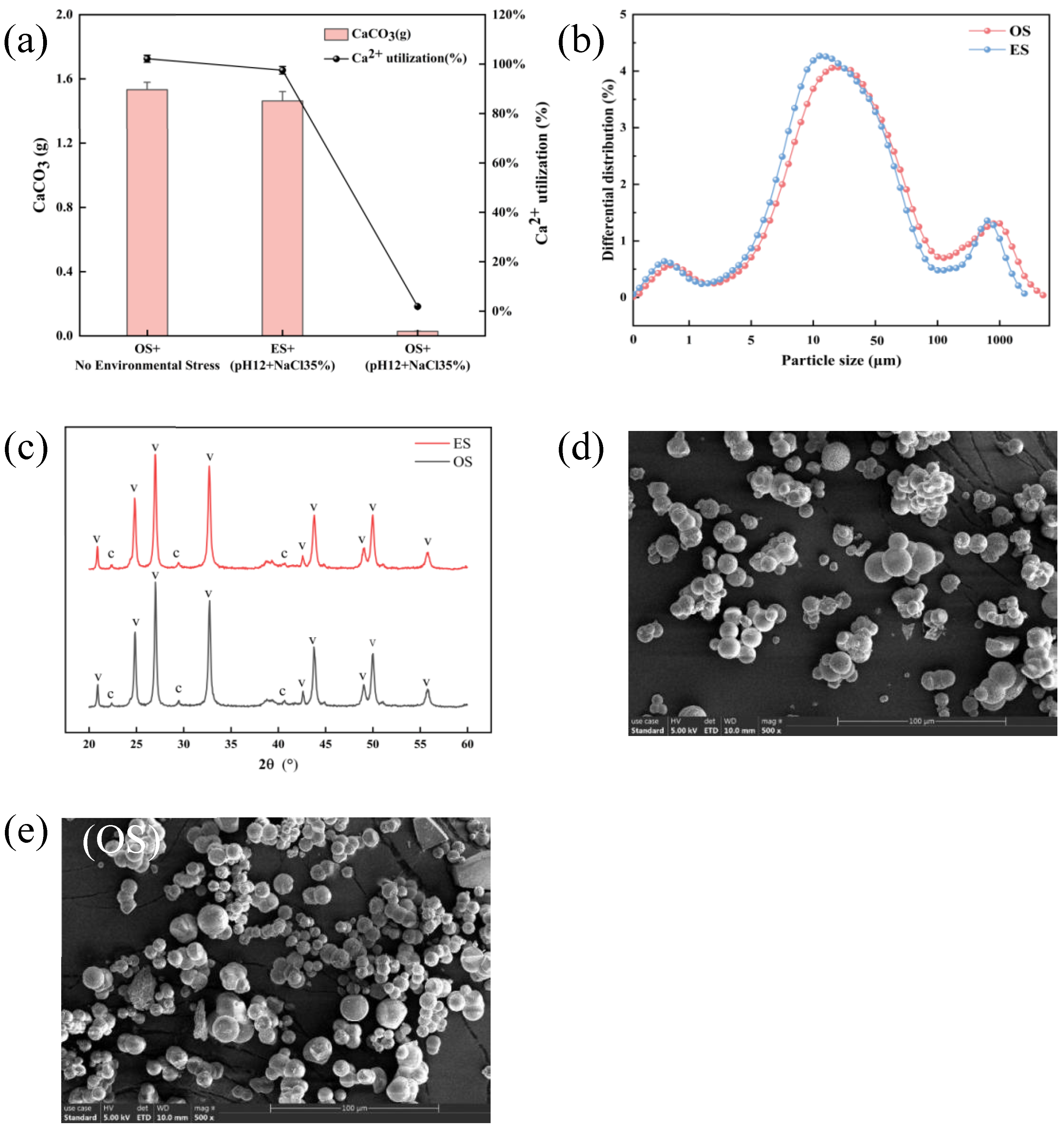
3.5. Comparison of Biomineralization Performance and Microstructural Characterization Analysis of S. pasteurii
3.6. Genome Sequencing Analysis
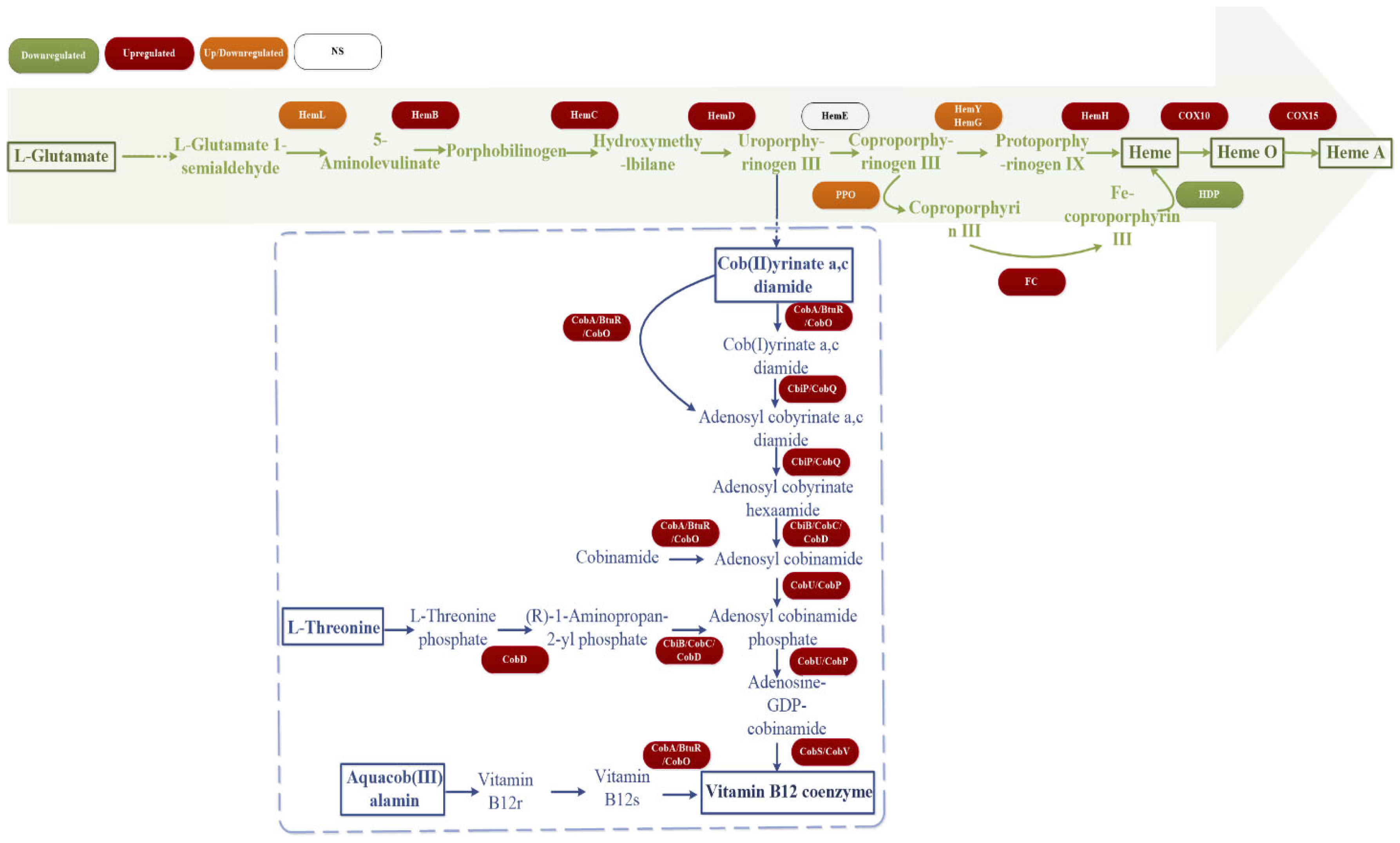
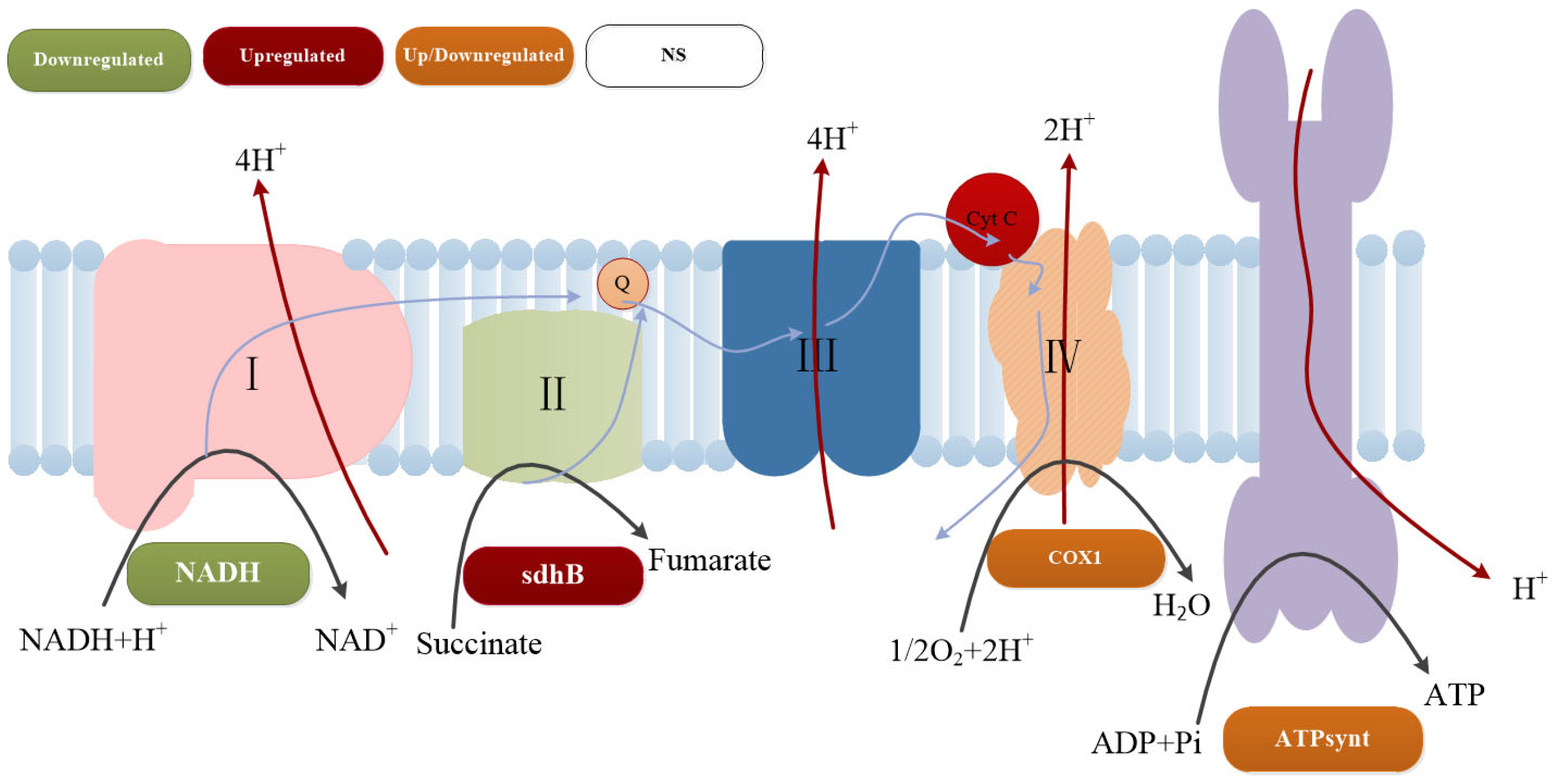
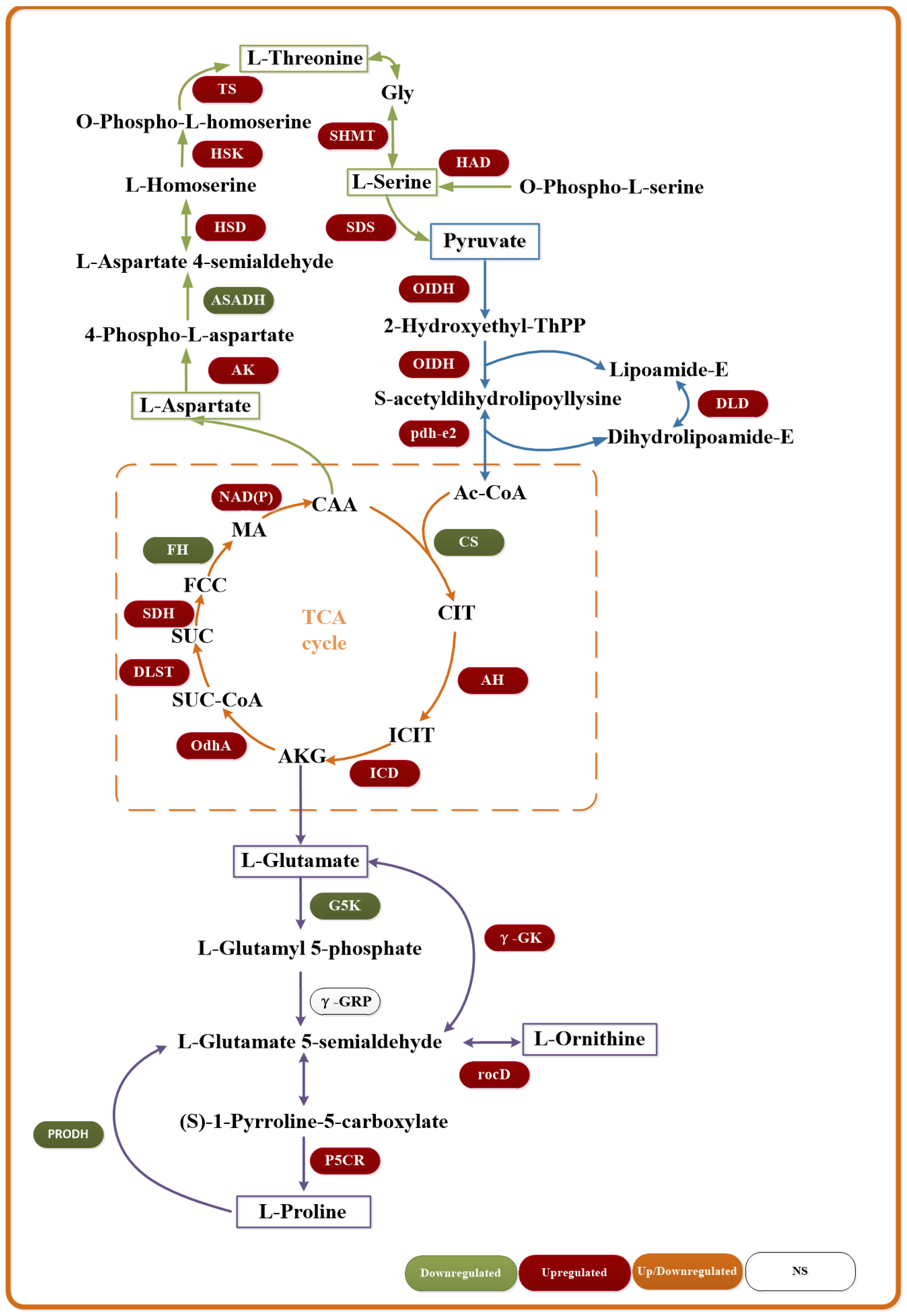
3.7. Transcriptome Sequencing Analysis
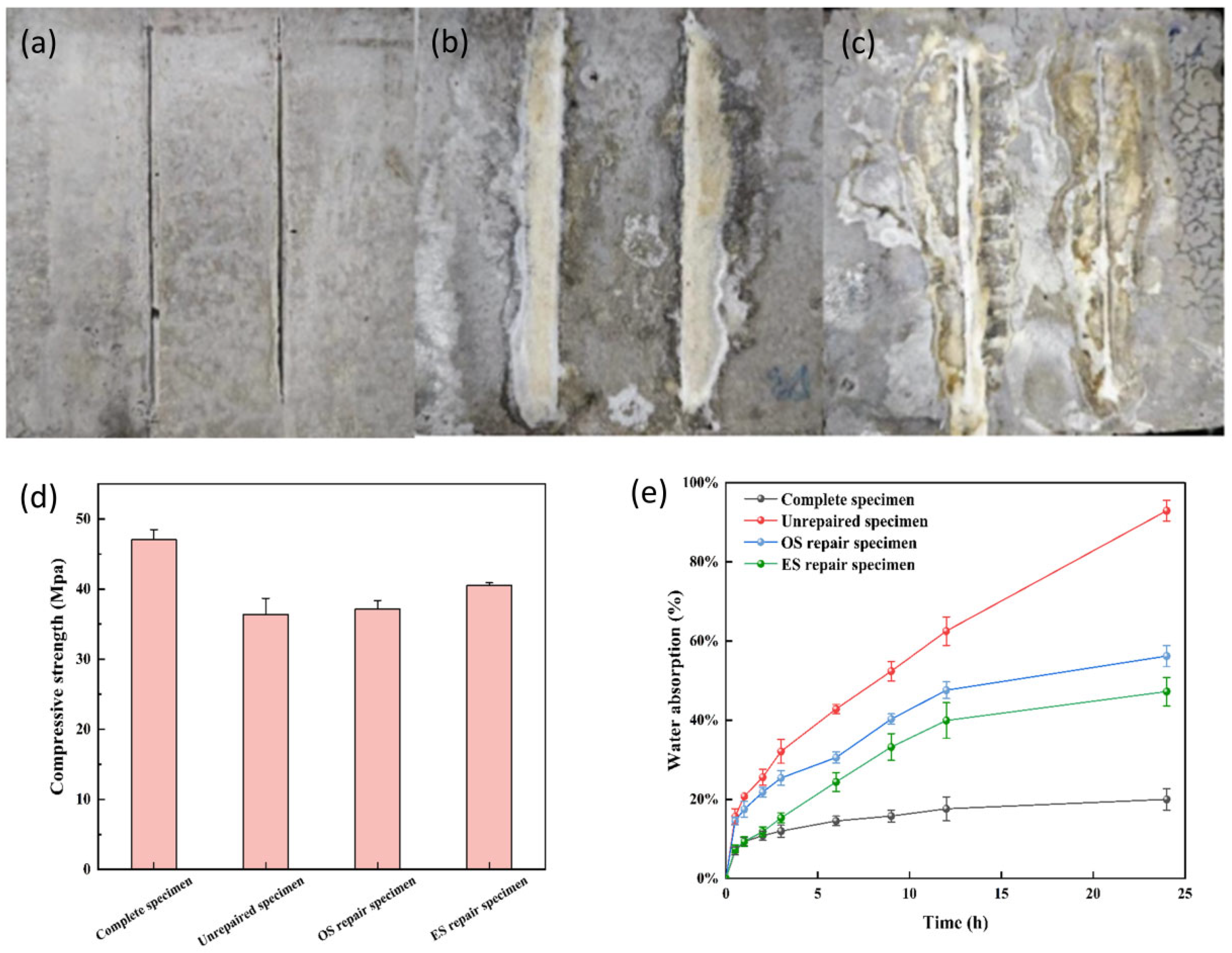
3.8. Comparison of Compressive Strength Repair Effect of S. pasteurii
3.9. Comparison of the Anti Permeability Repair Effect of S. pasteurii
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragab, A.M.; Elgammal, M.A.; Hodhod, O.A.; Ahmed, T.E. Evaluation of Field Concrete Deterioration under Real Conditions of Seawater Attack. Constr. Build. Mater. 2016, 119, 130–144. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Zeng, X.; Luo, Z.; Wang, K.; Sheng, D. Effect of Microlimestone on Properties of Self-Consolidating Concrete with Manufactured Sand and Mineral Admixture. Front. Struct. Civ. Eng. 2020, 14, 1545–1560. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Dong, W.; Tam, V.W.Y.; Yu, T. Durability Deterioration of Concrete under Marine Environment from Material to Structure: A Critical Review. J. Build. Eng. 2021, 35, 102074. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, R.; Liu, K.; Sun, S. Research Progress on Durability of Marine Concrete under the Combined Action of Cl− Erosion, Carbonation, and Dry–Wet Cycles. Rev. Adv. Mater. Sci. 2022, 61, 622–637. [Google Scholar] [CrossRef]
- Fan, Q.; Fan, L.; Quach, W.-M.; Zhang, R.; Duan, J.; Sand, W. Application of Microbial Mineralization Technology for Marine Concrete Crack Repair: A Review. J. Build. Eng. 2023, 69, 106299. [Google Scholar] [CrossRef]
- Zheng, T.; Qian, C.; Su, Y. Influences of Different Calcium Sources on the Early Age Cracks of Self-Healing Cementitious Mortar. Biochem. Eng. J. 2021, 166, 107849. [Google Scholar] [CrossRef]
- Zheng, X.; Bai, W.; Wei, Y.; Wang, Z.; Wang, J.; Zhang, Y.; Yu, J. Jadeite Original Stone Inspired PBA Core-Shell Architecture Endowed Fire-Safe and Mechanic-Robust EP Composites with Low Toxicity. Ceram. Int. 2023, 49, 10839–10851. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological Precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of Calcite (Calcium Carbonate) Crystals by Soil Bacteria Is a General Phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wu, L.; Wang, C.; Chen, R. The Method of Repairing Microcracks Based on Microbiologically Induced Calcium Carbonate Precipitation. Adv. Cem. Res. 2020, 32, 262–272. [Google Scholar] [CrossRef]
- Qian, C.; Ren, L.; Xue, B.; Cao, T. Bio-Mineralization on Cement-Based Materials Consuming CO2 from Atmosphere. Constr. Build. Mater. 2016, 106, 126–132. [Google Scholar] [CrossRef]
- Williams, S.L.; Kirisits, M.J.; Ferron, R.D. Influence of Concrete-Related Environmental Stressors on Biomineralizing Bacteria Used in Self-Healing Concrete. Constr. Build. Mater. 2017, 139, 611–618. [Google Scholar] [CrossRef]
- Khoshtinat, S. Advancements in Exploiting Sporosarcina Pasteurii as Sustainable Construction Material: A Review. Sustainability 2023, 15, 13869. [Google Scholar] [CrossRef]
- van Paassen, L.A.; Ghose, R.; van der Linden, T.J.M.; van der Star, W.R.L.; van Loosdrecht, M.C.M. Quantifying Biomediated Ground Improvement by Ureolysis: Large-Scale Biogrout Experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Zhang, G.-Z.; Liu, C.; Zhang, J.; Wang, H.; Liu, J. Enhanced MICP Efficacy for Cracks Repair of Marine Mortar with Coral Sand: Coupled Effects of Seawater and Basalt Fiber on Products Morphology. Constr. Build. Mater. 2025, 470, 140647. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Jia, C.; Li, D. A Comparative Study on Chloride Diffusion in Concrete Exposed to Different Marine Environment Conditions. J. Build. Eng. 2024, 94, 109845. [Google Scholar] [CrossRef]
- Shi, A.; Fan, F.; Broach, J.R. Microbial Adaptive Evolution. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab076. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Tong, Z.; Wu, W.; Chen, X.; Deng, X.; Xie, Y. Research on the Effect of Natural Seawater in Domesticating Bacillus Pasteurii and Reinforcing Calcareous Sand. JMSE 2024, 12, 542. [Google Scholar] [CrossRef]
- Nemati, M.; Greene, E.A.; Voordouw, G. Permeability Profile Modification Using Bacterially Formed Calcium Carbonate: Comparison with Enzymic Option. Process Biochem. 2005, 40, 925–933. [Google Scholar] [CrossRef]
- D’Agostino, N.; Li, W.; Wang, D. High-Throughput Transcriptomics. Sci. Rep. 2022, 12, 20313. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics Technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Sah, N.; Kuehu, D.L.; Khadka, V.S.; Deng, Y.; Peplowska, K.; Jha, R.; Mishra, B. RNA Sequencing-Based Analysis of the Laying Hen Uterus Revealed the Novel Genes and Biological Pathways Involved in the Eggshell Biomineralization. Sci. Rep. 2018, 8, 16853. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Ren, D.; Herrera, S.; Pan, S.; Tamura, T.; Inagaki, K.; Kisailus, D. Integrated Transcriptomic and Proteomic Analyses of a Molecular Mechanism of Radular Teeth Biomineralization in Cryptochiton Stelleri. Sci. Rep. 2019, 9, 856. [Google Scholar] [CrossRef]
- Gupta, J.A.; Thapa, S.; Verma, M.; Som, R.; Mukherjee, K.J. Genomics and Transcriptomics Analysis Reveals the Mechanism of Isobutanol Tolerance of a Laboratory Evolved Lactococcus Lactis Strain. Sci. Rep. 2020, 10, 10850. [Google Scholar] [CrossRef]
- Cole, S.T.; Girons, I.S. Bacterial Genomics. FEMS Microbiol. Rev. 1994, 14, 139–160. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Vinay, N.; Wang, D.; Mo, F.; Liao, Y.; Wen, X. Microbial Functional Genes within Soil Aggregates Drive Organic Carbon Mineralization under Contrasting Tillage Practices. Land Degrad. Dev. 2023, 34, 3618–3635. [Google Scholar] [CrossRef]
- Xu, N.; Yang, X.; Yang, Q.; Guo, M. Comparative Genomic and Transcriptomic Analysis of Phenol Degradation and Tolerance in Acinetobacter Lwoffii through Adaptive Evolution. Int. J. Mol. Sci. 2023, 24, 16529. [Google Scholar] [CrossRef]
- Adaptive Laboratory Evolution of Pseudomonas Putida KT2440 Improves P-Coumaric and Ferulic Acid Catabolism and Tolerance. Metab. Eng. Commun. 2020, 11, e00143. [CrossRef]
- Zhang, X.; Luo, Z.; Ji, J.; Sun, Y.; Tang, H.; Li, Y. Intelligent Surface Cracks Detection in Bridges Using Deep Neural Network. Int. J. Str. Stab. Dyn. 2024, 24, 2450046. [Google Scholar] [CrossRef]
- Snoeck, D.; Steuperaert, S.; Van Tittelboom, K.; Dubruel, P.; De Belie, N. Visualization of Water Penetration in Cementitious Materials with Superabsorbent Polymers by Means of Neutron Radiography. Cem. Concr. Res. 2012, 42, 1113–1121. [Google Scholar] [CrossRef]
- Han, P.; Geng, W.; Li, M.; Jia, S.; Yin, J.; Xue, R. Improvement of Biomineralization of Sporosarcina Pasteurii as Biocementing Material for Concrete Repair by Atmospheric and Room Temperature Plasma Mutagenesis and Response Surface Methodology. J. Microbiol. Biotechnol. 2021, 31, 1311–1322. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Paassen, L.A.V.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Ministry of Transport of the People’s Republic of China (MOT). Technical Specification for Concrete Test and Inspection of Water Transport Engineering (JTS/T 236-2019); China Communications Press: Beijing, China, 2019. (In Chinese) [Google Scholar]
- Almajed, A.; Lateef, M.A.; Moghal, A.A.B.; Lemboye, K. State-of-the-Art Review of the Applicability and Challenges of Microbial-Induced Calcite Precipitation (MICP) and Enzyme-Induced Calcite Precipitation (EICP) Techniques for Geotechnical and Geoenvironmental Applications. Crystals 2021, 11, 370. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 6281. [Google Scholar] [CrossRef]
- Fuchino, K.; Bruheim, P. Increased Salt Tolerance in Zymomonas Mobilis Strain Generated by Adaptative Evolution. Microb. Cell Fact. 2020, 19, 147. [Google Scholar] [CrossRef]
- Li, Y.; Rao, J.; Meng, F.; Wang, Z.; Liu, D.; Yu, H. Combination of Mutagenesis and Adaptive Evolution to Engineer Salt-tolerant and Aroma-producing Yeast for Soy Sauce Fermentation. J. Sci. Food Agric. 2021, 101, 4288–4297. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Zhang, B.; Bao, J. pH Shifting Adaptive Evolution Stimulates the Low pH Tolerance of Pediococcus Acidilactici and High L-Lactic Acid Fermentation Efficiency. Bioresour. Technol. 2025, 416, 131813. [Google Scholar] [CrossRef]
- da Silva Mazareli, R.C.; Montoya, A.C.V.; Delforno, T.P.; Centurion, V.B.; de Oliveira, V.M.; Silva, E.L.; Varesche, M.B.A. Enzymatic Routes to Hydrogen and Organic Acids Production from Banana Waste Fermentation by Autochthonous Bacteria: Optimization of pH and Temperature. Int. J. Hydrogen Energy 2021, 46, 8454–8468. [Google Scholar] [CrossRef]
- Koga, T.; Ishizu, M.; Watanabe, K.; Miyamoto, H.; Oshiro, M.; Sakai, K.; Tashiro, Y. Dilution Rates and Their Transition Modes Influence Organic Acid Productivity and Bacterial Community Structure on Continuous Meta-Fermentation Using Complex Microorganisms. J. Biosci. Bioeng. 2023, 136, 391–399. [Google Scholar] [CrossRef]
- Khoshtinat, S.; Marano, C.; Kioumarsi, M. Computational Modeling of Biocementation by S. Pasteurii: Effect of Initial pH. Discov. Mater. 2025, 5, 65. [Google Scholar] [CrossRef]
- Ng, W.S.; Lee, M.L.; Hii, S.L. An Overview of the Factors Affecting Microbial-Induced Calcite Precipitation and Its Potential Application in Soil Improvement. World Acad. Sci. 2012, 6, 188–194. [Google Scholar] [CrossRef]
- Harnpicharnchai, P.; Mayteeworakoon, S.; Kitikhun, S.; Chunhametha, S.; Likhitrattanapisal, S.; Eurwilaichitr, L.; Ingsriswang, S. High Level of Calcium Carbonate Precipitation Achieved by Mixed Culture Containing Ureolytic and Nonureolytic Bacterial Strains. Lett. Appl. Microbiol. 2022, 75, 888–898. [Google Scholar] [CrossRef]
- Henze, J.; Randall, D.G. Microbial Induced Calcium Carbonate Precipitation at Elevated pH Values (>11) Using Sporosarcina Pasteurii. J. Environ. Chem. Eng. 2018, 6, 5008–5013. [Google Scholar] [CrossRef]
- Lv, C.; Tang, C.-S.; Zhang, J.-Z.; Pan, X.-H.; Liu, H. Effects of Calcium Sources and Magnesium Ions on the Mechanical Behavior of MICP-Treated Calcareous Sand: Experimental Evidence and Precipitated Crystal Insights. Acta Geotech. 2023, 18, 2703–2717. [Google Scholar] [CrossRef]
- Jiang, C.; Hu, L.; He, N.; Liu, Y.; Zhao, H.; Jiang, Z. Different Calcium Sources Affect the Products and Sites of Mineralized Cr(VI) by Microbially Induced Carbonate Precipitation. Chemosphere 2024, 363, 142977. [Google Scholar] [CrossRef]
- Pickerill, E.S.; Kurtz, R.P.; Tharp, A.; Sanz, P.G.; Begum, M.; Bernstein, D.A. Pseudouridine Synthase 7 Impacts Candida Albicans rRNA Processing and Morphological Plasticity. Yeast 2019, 36, 669–677. [Google Scholar] [CrossRef]
- Margus, L.; Aivar, L.; Jaanus, R. Random Pseuoduridylation in Vivo Reveals Critical Region of Escherichia Coli 23S rRNA for Ribosome Assembly. Nucleic Acids Res. 2017, 45, 6098–6108. [Google Scholar] [CrossRef]
- Bates Utz, C.; Nguyen, A.B.; Smalley, D.J.; Anderson, A.B.; Conway, T. GntP Is the Escherichia Coli Fructuronic Acid Transporter and Belongs to the UxuR Regulon. J. Bacteriol. 2004, 186, 7690–7696. [Google Scholar] [CrossRef]
- Muthiah, A.S.; Aruni, W.; Robles, A.G.; Dou, Y.; Roy, F.; Fletcher, H.M. In Porphyromonas Gingivalis VimF Is Involved in Gingipain Maturation through the Transfer of Galactose. PLoS ONE 2013, 8, e63367. [Google Scholar] [CrossRef]
- El Qaidi, S.; Chen, K.; Halim, A.; Siukstaite, L.; Rueter, C.; Hurtado-Guerrero, R.; Clausen, H.; Hardwidge, P.R. NleB/SseK Effectors from Citrobacter rodentium, Escherichia coli, and Salmonella enterica display Distinct Differences in Host Substrate Specificity. J. Biol. Chem. 2017, 292, 11423–11430. [Google Scholar] [CrossRef]
- Breisch, J.; Averhoff, B. Identification of Osmo-dependent and Osmo-independent Betaine-choline-carnitine Transporters in Acinetobacter Baumannii: Role in Osmostress Protection and Metabolic Adaptation. Environ. Microbiol. 2020, 22, 2724–2735. [Google Scholar] [CrossRef]
- Yang, T.; Nian, Y.; Lin, H.; Li, J.; Lin, X.; Li, T.; Wang, R.; Wang, L.; Beattie, G.A.; Zhang, J.; et al. Structure and Mechanism of the Osmoregulated Choline Transporter BetT. Sci. Adv. 2024, 10, eado6229. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhou, X.; Zhang, W.; Pei, F.; Ge, J. Transcriptomic Analysis of Bacteriocin Synthesis and Stress Response in Lactobacillus Paracasei HD1.7 under Acetic Acid Stress. LWT 2022, 154, 112897. [Google Scholar] [CrossRef]
- Imai, S.I.; Armstrong, C.M. Transcriptional Silencing and Longevity Protein Sir2 Is an NAD-Dependent Histone Deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Guarente, L. Sir2 Links Chromatin Silencing, Metabolism, and Aging. Genes Dev. 2000, 14, 1021–1026. [Google Scholar] [CrossRef]
- Blander, G.; Guarente, L. The Sir2 Family of Protein Deacetylases. Annu. Rev. Biochem. 2004, 73, 417–435. [Google Scholar] [CrossRef]
- Dalal, K.; Duong, F. The SecY Complex Forms a Channel Capable of Ionic Discrimination. EMBO Rep. 2010, 10, 762–768. [Google Scholar] [CrossRef]
- Croitoru, V.; Bucheli-Witschel, M.; Hägg, P.; Abdulkarim, F.; Isaksson, L.A. Generation and Characterization of Functional Mutants in the Translation Initiation Factor IF1 of Escherichia coli. Eur. J. Biochem. 2010, 271, 534–544. [Google Scholar] [CrossRef]
- Brandi, A.; Piersimoni, L.; Feto, N.A.; Spurio, R.; Alix, J.H.; Schmidt, F.; Gualerzi, C.O. Translation Initiation Factor IF2 Contributes to Ribosome Assembly and Maturation during Cold Adaptation. Nucleic Acids Res. 2019, 47, 4652–4662. [Google Scholar] [CrossRef]
- Džupponová, V.; Tomášková, N.; Antošová, A.; Sedlák, E.; Žoldák, G. Salt-Specific Suppression of the Cold Denaturation of Thermophilic Multidomain Initiation Factor 2. Int. J. Mol. Sci. 2023, 24, 6787. [Google Scholar] [CrossRef]
- Shi, X.; Joseph, S. Mechanism of Translation Termination: RF1 Dissociation Follows RF3 Dissociation from the Ribosome. Biochemistry 2016, 55, 6344–6354. [Google Scholar] [CrossRef] [PubMed]
- Beale, S.I. The Biosynthesis of δ-Aminolevulinic Acid in Chlorella1. Plant Physiol. 1970, 45, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Elfsson, B.; Wallin, I.; Eksborg, S.; Rudaeus, K.; Ros, A.M.; Ehrsson, H. Stability of 5-Aminolevulinic Acid in Aqueous Solution. Eur. J. Pharm. Sci. 1999, 7, 87–91. [Google Scholar] [CrossRef]
- Pisanti, S.; Rimondi, E.; Pozza, E.; Melloni, E.; Zauli, E.; Bifulco, M.; Martinelli, R.; Marcuzzi, A. Prenylation Defects and Oxidative Stress Trigger the Main Consequences of Neuroinflammation Linked to Mevalonate Pathway Deregulation. Int. J. Environ. Res. Public Health 2022, 19, 9061. [Google Scholar] [CrossRef]
- Warren, M.J.; Raux, E.; Schubert, H.L.; Escalantesemerena, J.C. The Biosynthesis of Adenosylcobalamin (Vitamin B12). Nat. Prod. Rep. 2002, 19, 390–412. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae Acquire Vitamin B12 through a Symbiotic Relationship with Bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Vaidyanathan, P.P.; Deutscher, M.P.; Malhotra, A. RluD, a Highly Conserved Pseudouridine Synthase, Modifies 50S Subunits More Specifically and Efficiently than Free 23S rRNA. RNA 2007, 13, 1868–1876. [Google Scholar] [CrossRef]
- Sekiya, M.; Ikeda, K.; Yonai, A.; Ishikawa, T.; Shimoyama, Y.; Kodama, Y.; Sasaki, M.; Nakanishi-Matsui, M. F-Type Proton-Pumping ATPase Mediates Acid Tolerance in Streptococcus mutans. J. Appl. Microbiol. 2023, 134, lxad073. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Q.F.; Qi, S.L.; Feng, X.J.; Han, H.L.; Xu, T.; Hua, X.J. The F-Box Protein EST1 Modulates Salt Tolerance in Arabidopsis by Regulating Plasma Membrane Na /H Antiport Activity. J. Plant Physiol. 2020, 251, 153217. [Google Scholar] [CrossRef]
- Salway, J.G. The Krebs Uric Acid Cycle: A Forgotten Krebs Cycle. Trends Biochem. Sci. 2018, 43, 847–849. [Google Scholar] [CrossRef]
- Hu, J.; Yan, J.; Wu, L.; Bao, Y.; Yu, D.; Li, J. Insight into Halotolerance of a Robust Heterotrophic Nitrifying and Aerobic Denitrifying Bacterium Halomonas Salifodinae. Bioresour. Technol. 2022, 351, 126925. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Hidese, R.; Hasunuma, T.; Kondo, A. Increased Flux in Acetyl-CoA Synthetic Pathway and TCA Cycle of Kluyveromyces Marxianus under Respiratory Conditions. Sci. Rep. 2019, 9, 5319. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Mouriès, L.; Almeida, M.; Ribeiro, C.; Peduzzi, J.; Barthélemy, M.; Milet, C.; Lopez, E. Soluble Silk-like Organic Matrix in the Nacreous Layer of the Bivalve Pinctada Maxima. Eur. J. Biochem. 2002, 269, 4994–5003. [Google Scholar] [CrossRef]
- Görgen, S.; Benzerara, K.; Skouri-Panet, F.; Gugger, M.; Chauvat, F.; Cassier-Chauvat, C. The Diversity of Molecular Mechanisms of Carbonate Biomineralization by Bacteria. Discov. Mater. 2021, 1, 2. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Bio-Cementation of Sandy Soil Using Microbially Induced Carbonate Precipitation for Marine Environments. Géotechnique 2014, 64, 1010–1013. [Google Scholar] [CrossRef]
| Gene Name | Sequence |
|---|---|
| 16s rRNA | F: AATCATTCTTGGTTCATCAAAATCACGT R: TACAATTGCTGTTTACGATGGACAC |
| E2C16_RS14315 | F: GGCTAAAAAATCAATGGTAGCCAA R: TTACCAGCTTGCTTTTTTAACGCC |
| E2C16_RS11510 | F: GAGCATTAAAGCTGAAGAAATCAGCGT R: GTTTTGAGCCATACCCATAACACCG |
| E2C16_RS9230 | F: GTGAGTTCAGTTGCTCAAAAGAAAGGC R: GTTAAATAGCCCAGCACTAATTAGATCGC |
| E2C16_RS04705 | F: TTGGATGTATCCCACCACGTGTT R: TCACGCAATTTTCCTATGCGCG |
| E2C16_RS14345 | F: GAAATTACACGAAATGAAACCAGCTG R: CCACGTTTAGGAAGTCGTTGGAAA |
| Categorization | ANNO_REGION | ANNOTATION | POS | REF | ALT | QUAL |
|---|---|---|---|---|---|---|
| non-synonymous mutation | ncRNA_exonic | E2C16_02500 | 484,733 | C | T | 2302.8 |
| ncRNA_exonic | E2C16_10645 | 2,200,942 | G | A | 2251.8 | |
| ncRNA_exonic | E2C16_11325 | 2,356,286 | C | A | 1431.8 | |
| ncRNA_exonic | E2C16_12620 | 2,635,046 | T | G | 1941.8 | |
| upstream; downstream | E2C16_03190 E2C16_03185 | 635,957 | T | A | 1338.8 | |
| tautological mutation | ncRNA_exonic | E2C16_00275 | 62,007 | C | T | 2176.8 |
| ncRNA_exonic | E2C16_06875 | 1,401,963 | T | G | 3897.8 | |
| ncRNA_exonic | E2C16_09620 | 1,965,081 | G | C | 1379.8 | |
| ncRNA_exonic | E2C16_10645 | 2,200,938 | C | A | 2281.8 | |
| ncRNA_exonic | E2C16_15700 | 3,242,631 | G | A | 1531.8 | |
| upstream; downstream | E2C16_05300 E2C16_05305 E2C16_05295 | 1,095,678 | A | T | 1021.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, H.; Dong, M.; Cheng, Z.; Mi, C.; Sun, S.; Zhu, R.; Han, P. Adaptive Evolution of Sporosarcina pasteurii Enhances Saline–Alkali Resistance for High-Performance Concrete Crack Repair via MICP. Microorganisms 2025, 13, 1526. https://doi.org/10.3390/microorganisms13071526
Liu J, Xu H, Dong M, Cheng Z, Mi C, Sun S, Zhu R, Han P. Adaptive Evolution of Sporosarcina pasteurii Enhances Saline–Alkali Resistance for High-Performance Concrete Crack Repair via MICP. Microorganisms. 2025; 13(7):1526. https://doi.org/10.3390/microorganisms13071526
Chicago/Turabian StyleLiu, Jieyu, Huaihua Xu, Min Dong, Zilin Cheng, Chenkai Mi, Shuai Sun, Ruiying Zhu, and Peipei Han. 2025. "Adaptive Evolution of Sporosarcina pasteurii Enhances Saline–Alkali Resistance for High-Performance Concrete Crack Repair via MICP" Microorganisms 13, no. 7: 1526. https://doi.org/10.3390/microorganisms13071526
APA StyleLiu, J., Xu, H., Dong, M., Cheng, Z., Mi, C., Sun, S., Zhu, R., & Han, P. (2025). Adaptive Evolution of Sporosarcina pasteurii Enhances Saline–Alkali Resistance for High-Performance Concrete Crack Repair via MICP. Microorganisms, 13(7), 1526. https://doi.org/10.3390/microorganisms13071526






