Low Tenacity of Toxoplasma gondii Tachyzoites In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Tachyzoites
2.2. Tachyzoite Treatment
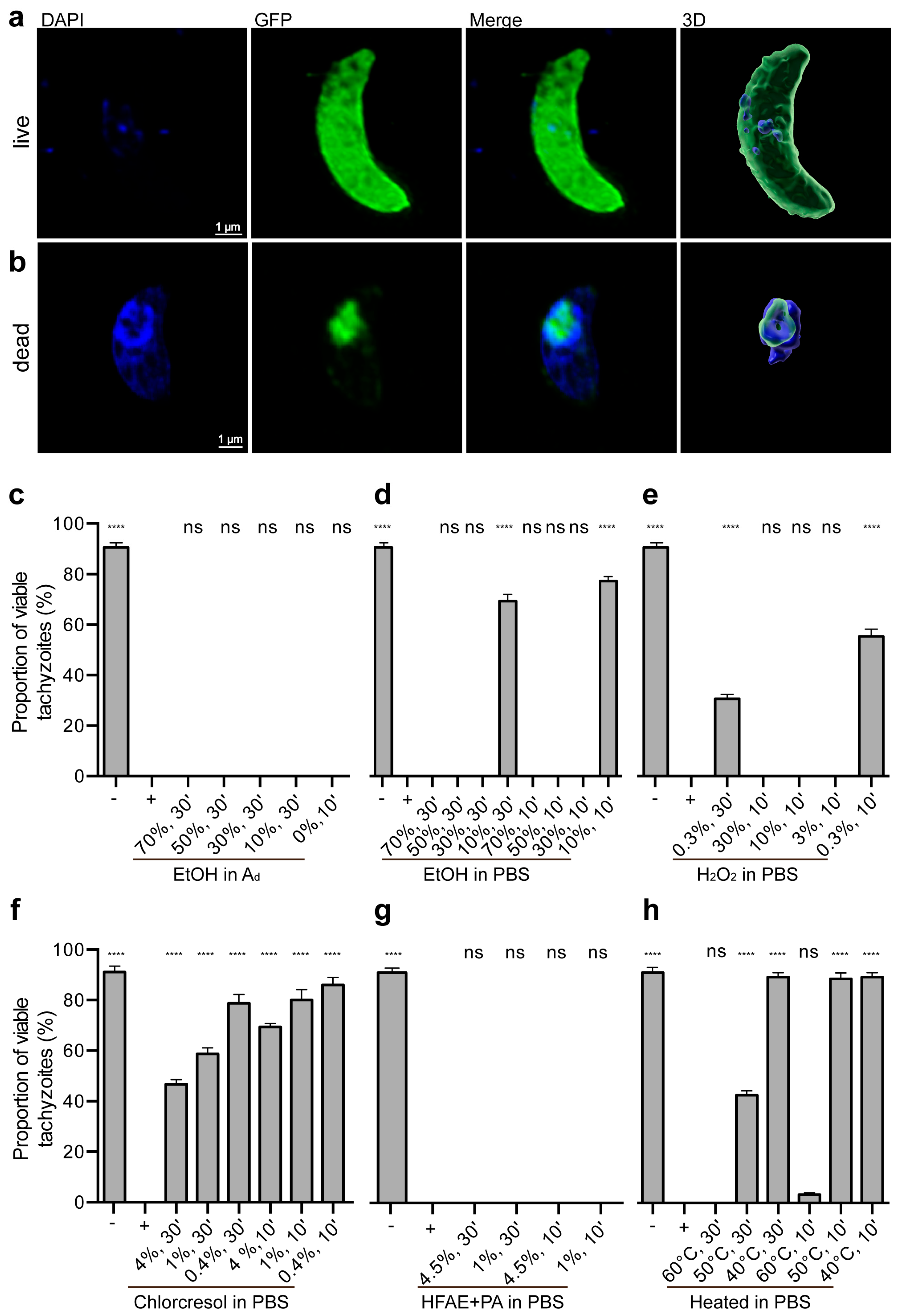
2.3. Live/Dead Staining
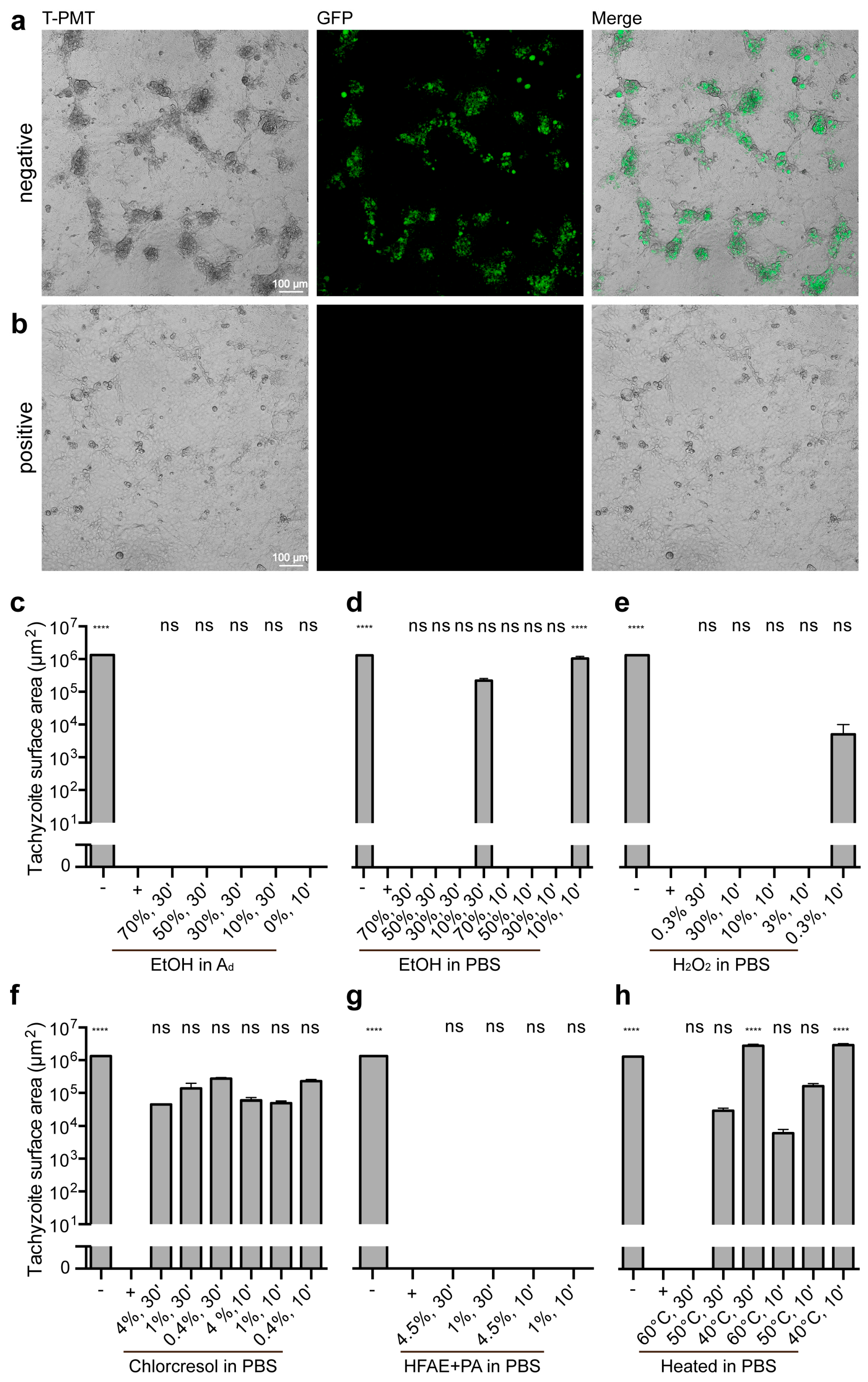
2.4. Ability to Reproduce
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Inactivation Methods on Tachyzoite Viability and Proliferation
3.2. Impact of Treatments on Tachyzoite Survival
3.2.1. Controls
3.2.2. Ethanol Treatments
3.2.3. Hydrogen Peroxide Treatments
3.2.4. p-chloro-m-cresol Treatments
3.2.5. o-Hydroxydiphenyl Fatty Acid Eutectic + Peracetic Acid Treatments
3.2.6. Heat Treatments
3.3. Impact of Treatments on Tachyzoite Proliferation
3.3.1. Controls
3.3.2. Ethanol Treatments
3.3.3. Hydrogen Peroxide Treatments
3.3.4. p-chloro-m-cresol Treatments
3.3.5. o-Hydroxydiphenyl Fatty Acid Eutectic + Peracetic Acid Treatments
3.3.6. Heat Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Chlorcresol | p-chloro-m-cresol |
| chlorcresol in PBS | p-chloro-m-cresol diluted in PBS |
| cLSM | Confocal laser scanning microscopy |
| DAPI | 4′,6-diamidino-2-phenylindole |
| dH2O | Double-distilled water |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EtOH in Ad | Ethanol diluted in double-distilled water |
| EtOH in PBS | Ethanol diluted in PBS |
| FBS | Fetal bovine serum |
| GFP | Green fluorescent protein |
| H2O2 | Hydrogen peroxide |
| H2O2 in PBS | Hydrogen peroxide diluted in PBS |
| HFAE + PA | o-hydroxydiphenyl fatty acid eutectic + peracetic acid |
| HFAE + PA in PBS | o-hydroxydiphenyl fatty acid eutectic component + peracetic acid diluted in PBS |
| MOI | Multiplicity of infection |
| PBS | Phosphate-buffered saline |
| SEM | Standard error of the mean |
| T. gondii | Toxoplasma gondii |
References
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed]
- Blader, I.; Coleman, B.; Chen, C.-T.; Gubbels, M.-J. The lytic cycle of Toxoplasma gondii: 15 years later. Annu. Rev. Microbiol. 2015, 69, 463–485. [Google Scholar] [CrossRef]
- Dubey, J.P.; Miller, N.L.; Frenkel, J.K. The Toxoplasma gondii oocyst from cat feces. J. Exp. Med. 1970, 132, 636–662. [Google Scholar] [CrossRef]
- Miller, N.L.; Frenkel, J.K.; Dubey, J.P. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. J. Parasitol. 1972, 58, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, EL, USA, 2010; ISBN 9781420092363. [Google Scholar]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Bigna, J.J.; Tochie, J.N.; Tounouga, D.N.; Bekolo, A.O.; Ymele, N.S.; Youda, E.L.; Sime, P.S.; Nansseu, J.R. Global, regional, and country seroprevalence of Toxoplasma gondii in pregnant women: A systematic review, modelling and meta-analysis. Sci. Rep. 2020, 10, 12102. [Google Scholar] [CrossRef]
- McConkey, G.A.; Martin, H.L.; Bristow, G.C.; Webster, J.P. Toxoplasma gondii infection and behaviour—location, location, location? J. Exp. Biol. 2013, 216, 113–119. [Google Scholar] [CrossRef]
- Parlog, A.; Schlüter, D.; Dunay, I.R. Toxoplasma gondii induced neuronal alterations. Parasite Immunol. 2015, 37, 159–170. [Google Scholar] [CrossRef]
- Salimi, M.; Shojaee, S.; Keshavarz, H.; Mohebali, M. Cyst formation from virulent RH strain of Toxoplasma gondii tachyzoite: In vitro cultivation. Iran. J. Parasitol. 2016, 11, 81–85. [Google Scholar]
- Jabari, S.; Keshavarz, H.; Salimi, M.; Morovati, H.; Mohebali, M.; Shojaee, S. In vitro culture of Toxoplasma gondii in HeLa, Vero, RBK and A549 cell lines. Infez. Med. 2018, 26, 145–147. [Google Scholar] [PubMed]
- Pinto-Ferreira, F.; Paschoal, A.T.P.; Pasquali, A.K.S.; Bernardes, J.C.; Caldart, E.T.; Freire, R.L.; Mitsuka-Breganó, R.; Navarro, I.T. Techniques for inactivating Toxoplasma gondii oocysts: A systematic review. Rev. Bras. Parasitol. Vet. 2021, 30, e026420. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Veterinärmedizinische Gesellschaft, e.V. DVG-Desinfektionsmittelliste für den Tierhaltungsbereich. Available online: https://www.desinfektion-dvg.de/dvg-desinfektionsmittellisten/tierhaltung (accessed on 31 January 2025).
- Alizadeh, M.A.; Jazaeri, S.; Shemshadi, B.; Hashempour-Baltork, F.; Sarlak, Z.; Pilevar, Z.; Hosseini, H. A review on inactivation methods of Toxoplasma gondii in foods. Pathog. Glob. Health. 2018, 112, 306–319. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.-Q.; Lin, X.-J.; Xiang, S.-P.; Jiang, L.-Y.; Cai, J.-H.; Sun, J.-M.; Tan, F.; Mou, Y.-N. Two small-molecule inhibitors of Toxoplasma gondii proliferation in vitro. Front. Cell. Infect. Microbiol. 2023, 13, 1145824. [Google Scholar] [CrossRef]
- Xing, M.; Yang, N.; Jiang, N.; Wang, D.; Sang, X.; Feng, Y.; Chen, R.; Wang, X.; Chen, Q. A sialic acid-binding protein SABP1 of Toxoplasma gondii mediates host cell attachment and invasion. J. Infect. Dis. 2020, 222, 126–135. [Google Scholar] [CrossRef]
- Räisänen, S.; Saari, M. The survival of Toxoplasma gondii trophozoites in changes in osmotic pressure. Med. Biol. 1976, 54, 152–155. [Google Scholar]
- Wang, X.; Jobe, M.; Tyler, K.M.; Steverding, D. Efficacy of common laboratory disinfectants and heat on killing trypanosomatid parasites. Parasit. Vectors. 2008, 1, 35. [Google Scholar] [CrossRef]
- Alves, E.; Benns, H.J.; Magnus, L.; Dominicus, C.; Dobai, T.; Blight, J.; Wincott, C.J.; Child, M.A. An extracellular redox signal triggers calcium release and impacts the asexual development of Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2021, 11, 728425. [Google Scholar] [CrossRef]
- Delling, C.; Lendner, M.; Müller, U.; Daugschies, A. Improvement of in vitro evaluation of chemical disinfectants for efficacy on Cryptosporidium parvum oocysts. Vet. Parasitol. 2017, 245, 5–13. [Google Scholar] [CrossRef]
- Da Marchiori, N.C.; Silva, F.M.; Martins, M.L.; Amaral Junior, H.; Da Silva, B.C. Hydrogen peroxide and chlorine dioxide against parasite Ichthyophthirius multifiliis (Protozoa, Ciliophora) in jundiá fingerlings. Cienc. Rural 2017, 47, e20170257. [Google Scholar] [CrossRef][Green Version]
- Lee, M.; Lee, E. The effectiveness of hydrogen peroxide liquid or gas plasma on protozoan oocysts. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 265. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Kotula, A.W.; Sharar, A.; Andrews, C.D.; Lindsay, D.S. Effect of high temperature on infectivity of Toxoplasma gondii tissue cysts in pork. J. Parasitol. 1990, 76, 201–204. [Google Scholar] [CrossRef] [PubMed]
- El-Nawawi, F.A.; Tawfik, M.A.; Shaapan, R.M. Methods for inactivation of Toxoplasma gondii cysts in meat and tissues of experimentally infected sheep. Foodborne Pathog. Dis. 2008, 5, 687–690. [Google Scholar] [CrossRef]
- Gamble, H.R. Parasites associated with pork and pork products. Rev. Sci. Tech. 1997, 16, 496–506. [Google Scholar] [CrossRef]
| Reagent | Concentration (%) | Incubation Medium | Incubation Time (m) |
|---|---|---|---|
| Negative control/− | - | PBS | 30 |
| Positive control/+ (Paraformaldehyde) | 4 | PBS | 30 |
| Ethanol | 70 | dH2O | 30 |
| Ethanol | 50 | dH2O | 30 |
| Ethanol | 30 | dH2O | 30 |
| Ethanol | 10 | dH2O | 30 |
| Ethanol | 0 | dH2O | 10 |
| Ethanol | 70 | PBS | 30 |
| Ethanol | 50 | PBS | 30 |
| Ethanol | 30 | PBS | 30 |
| Ethanol | 10 | PBS | 30 |
| Ethanol | 70 | PBS | 10 |
| Ethanol | 50 | PBS | 10 |
| Ethanol | 30 | PBS | 10 |
| Ethanol | 10 | PBS | 10 |
| H2O2 | 0.3 | PBS | 30 |
| H2O2 | 30 | PBS | 10 |
| H2O2 | 10 | PBS | 10 |
| H2O2 | 3 | PBS | 10 |
| H2O2 | 0.3 | PBS | 10 |
| Chlorcresol | 4 | PBS | 30 |
| Chlorcresol | 1 | PBS | 30 |
| Chlorcresol | 0.4 | PBS | 30 |
| Chlorcresol | 4 | PBS | 10 |
| Chlorcresol | 1 | PBS | 10 |
| Chlorcresol | 0.4 | PBS | 10 |
| HFAE + PA | 4.5 | PBS | 30 |
| HFAE + PA | 1 | PBS | 30 |
| HFAE + PA | 4.5 | PBS | 10 |
| HFAE + PA | 1 | PBS | 10 |
| Agent | Temperature (°C) | Incubation medium | Incubation time (m) |
| Heating | 60 | PBS | 30 |
| Heating | 50 | PBS | 30 |
| Heating | 40 | PBS | 30 |
| Heating | 60 | PBS | 10 |
| Heating | 50 | PBS | 10 |
| Heating | 40 | PBS | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochow, T.; Kalusa, M.; Tonndorf-Martini, P.; Röhrmann, N.; Fietz, S.A.; Murnik, L.-C. Low Tenacity of Toxoplasma gondii Tachyzoites In Vitro. Microorganisms 2025, 13, 1517. https://doi.org/10.3390/microorganisms13071517
Grochow T, Kalusa M, Tonndorf-Martini P, Röhrmann N, Fietz SA, Murnik L-C. Low Tenacity of Toxoplasma gondii Tachyzoites In Vitro. Microorganisms. 2025; 13(7):1517. https://doi.org/10.3390/microorganisms13071517
Chicago/Turabian StyleGrochow, Thomas, Mirjam Kalusa, Pauline Tonndorf-Martini, Nicole Röhrmann, Simone A. Fietz, and Lea-Christina Murnik. 2025. "Low Tenacity of Toxoplasma gondii Tachyzoites In Vitro" Microorganisms 13, no. 7: 1517. https://doi.org/10.3390/microorganisms13071517
APA StyleGrochow, T., Kalusa, M., Tonndorf-Martini, P., Röhrmann, N., Fietz, S. A., & Murnik, L.-C. (2025). Low Tenacity of Toxoplasma gondii Tachyzoites In Vitro. Microorganisms, 13(7), 1517. https://doi.org/10.3390/microorganisms13071517






