Exploration of a Postbiotic Derived from Enterococcus faecium HDRsEf1 and Its Probiotic Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line
2.2. Reagents
2.3. Bacterial Strain and Culture
2.4. Cell Culture
2.5. Preparation of Postbiotic Components
2.5.1. Preparation of Crude EPS-Ef1
2.5.2. Preparation of Proteins
2.6. Induction of Inflammatory Cytokines in MODE-K Cells by LPS at Different Concentrations
2.7. Effects of Heat-Inactivated HDRsEf1 and Its Supernatant on MODE-K Cells
2.8. Effects of Crude Heat-Inactivated Proteins (HP-Ef1) and Crude EPS-Ef1 on MODE-K Cells
2.9. EdU Assay for Evaluating Crude EPS-Ef1 Effects on MODE-K Cell Proliferation
2.10. Single-Factor Optimization of Crude EPS-Ef1 Production Conditions
2.11. Animal Husbandry and Experimental Design
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Hematoxylin and Eosin (H&E) Staining
2.14. Compositional Analysis of Crude EPS-Ef1
2.15. Characterization of Molecular Weight and Conformation of Crude EPS-Ef1
2.16. Statistical Analysis
3. Results
3.1. Cell Experiments
3.1.1. LPS Upregulates CXCL-1 mRNA Levels in MODE-K Cells
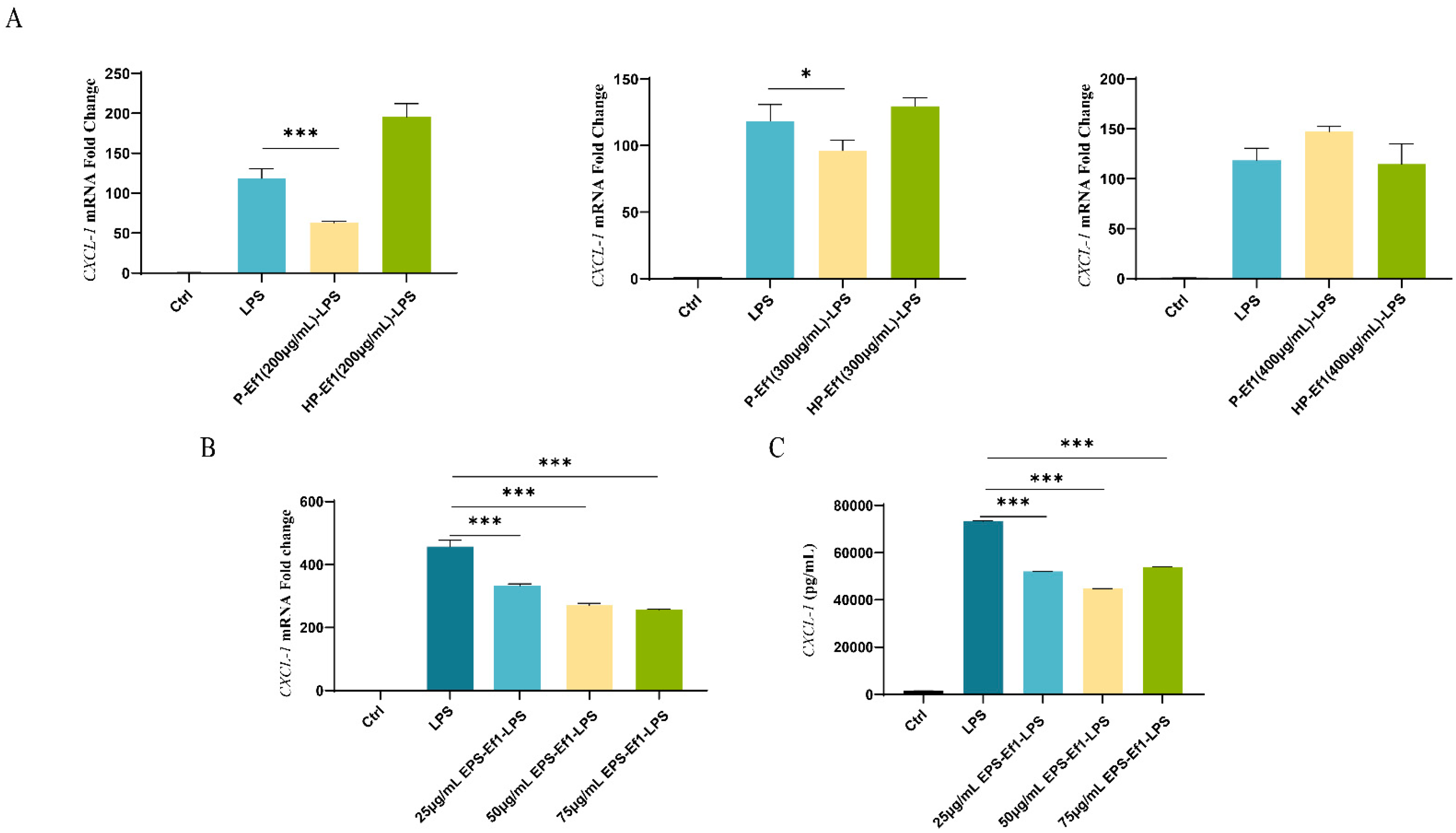
3.1.2. D-Ef1 Fails to Suppress CXCL-1 Overexpression in MODE-K Cells
3.1.3. HDRsEf1-Derived Culture Supernatants Inhibit CXCL-1 Expression in MODE-K Cells
3.1.4. Crude HP-Ef1 Fail to Suppress CXCL-1 Overexpression in MODE-K Cells
3.1.5. Crude EPS-Ef1 Suppress CXCL-1 Overexpression in MODE-K Cells
3.2. Crude EPS-Ef1 Enhances MODE-K Cell Proliferation
3.2.1. EdU Assay for Cell Proliferation
3.2.2. Crude EPS-Ef1 Upregulates PCNA mRNA Expression
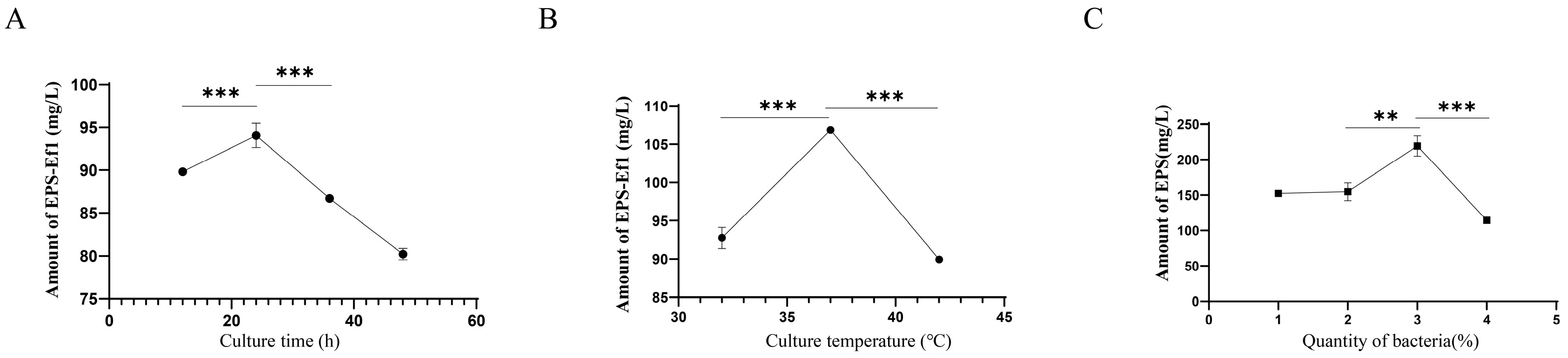
3.3. Optimization of Crude EPS-Ef1 Yield Through Cultivation Condition Modifications
3.4. Animal Experiments
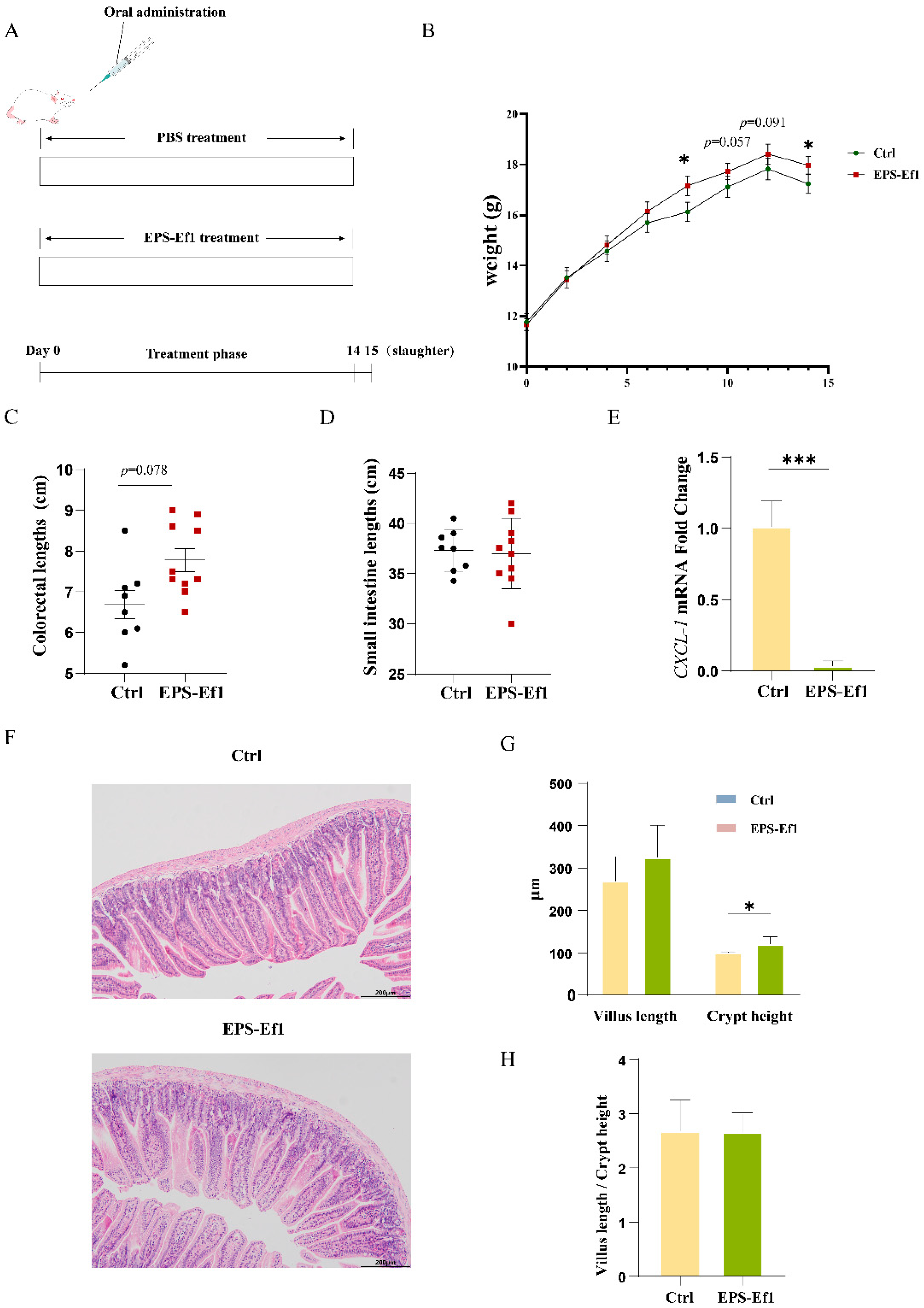
3.4.1. Crude EPS-Ef1 Promotes Body Weight Gain in Mice
3.4.2. Crude EPS-Ef1 Enhances Intestinal Crypt Depth in Mice
3.4.3. Crude EPS-Ef1 Reduces CXCL-1 Expression in Mouse Jejunum
3.5. Analysis of Monosaccharide Composition and Structure of Crude EPS-Ef1
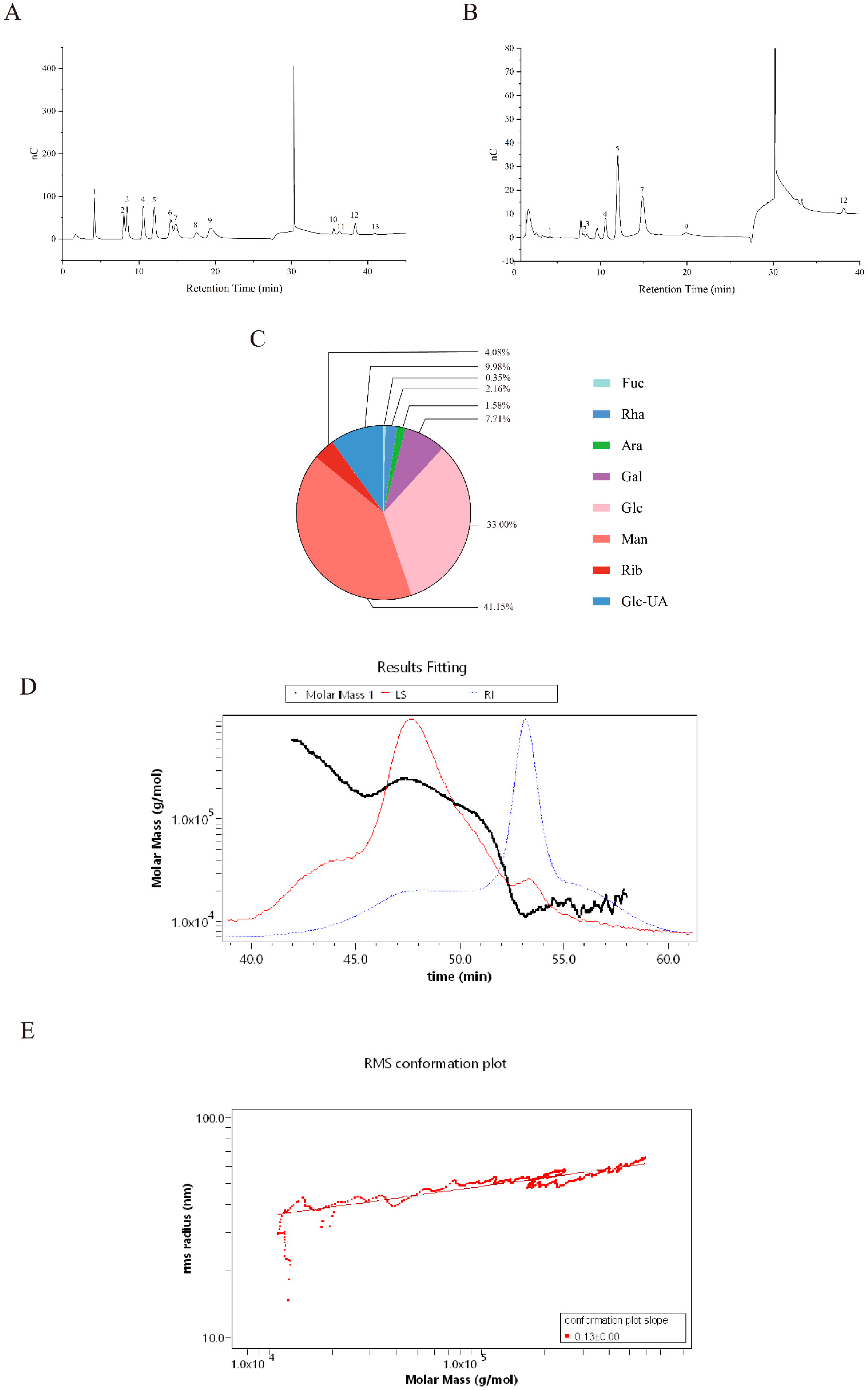
3.5.1. Monosaccharide Composition Analysis
3.5.2. Molecular Structural Characteristics of Crude EPS-Ef1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the Gut Microbiome and Mucosal Immune System. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Li, Y.; Shi, P.; Yao, K.; Lin, Q.; Wang, M.; Hou, Z.; Tang, W.; Diao, H. Diarrhea Induced by Insufficient Fat Absorption in Weaned Piglets: Causes and Nutrition Regulation. Anim. Nutr. 2024, 16, 299–305. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Tang, L.; Wang, Y.; Zhou, Y.; Li, X.; Jin, M.; Fu, A.; Li, W. Bacillus amyloliquefaciens SC06 Alleviated Intestinal Damage Induced by Inflammatory via Modulating Intestinal Microbiota and Intestinal Stem Cell Proliferation and Differentiation. Int. Immunopharmacol. 2024, 130, 111675. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Ye, L.; Liu, H.; Huang, L.; Yang, Q.; Turner, J.R.; Yu, Q. Lactobacillus Accelerates ISCs Regeneration to Protect the Integrity of Intestinal Mucosa through Activation of STAT3 Signaling Pathway Induced by LPLs Secretion of IL-22. Cell Death Differ. 2018, 25, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri Maintains Intestinal Epithelial Regeneration and Repairs Damaged Intestinal Mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Luo, J.; Yang, S.; Xiao, Q.; Wang, X.; Zhou, Z.; Xiao, Y.; Shi, D. Different Responses of Microbiota across Intestinal Tract to Enterococcus faecium HDRsEf1 and Their Correlation with Inflammation in Weaned Piglets. Microorganisms 2021, 9, 1767. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; He, Y.; Wang, Z.; Zhao, H.; Jin, X.; Shi, D.; Wang, X. Enterococcus faecium HDRsEf1 Inhibits Lipopolysaccharide-Induced Downregulation of Zona Occludens -1 Expression via Toll-like Receptor 2/4-Mediated c-Jun N-Terminal Kinase/Activator Protein-1 Signalling Pathways. J. Appl. Microbiol. 2022, 132, 605–617. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, Y.; Xiao, Y.; Wang, X.; Bi, D.; Shi, D. Probiotic characteristics of Enterococcus faecium HDRsEf1 from swine feces. Biot. Resour. 2017, 39, 339–344. (In Chinese) [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Yang, L.; Wu, G.; Wu, Q.; Peng, L.; Yuan, L. METTL3 Overexpression Aggravates LPS-Induced Cellular Inflammation in Mouse Intestinal Epithelial Cells and DSS-Induced IBD in Mice. Cell Death Discov. 2022, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shan, C.; Ning, D. Walnut Oil Alleviates LPS-Induced Intestinal Epithelial Cells Injury by Inhibiting TLR4/MyD88/NF-κB Pathway Activation. J. Food Biochem. 2021, 45, e13955. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Li, R.; He, J.; Yang, L.; Chen, J.; Xu, Z.; Zheng, B.; Yang, Y.; Xia, Z.; Tian, Y. Extraction, Structural Analysis, and Biofunctional Properties of Exopolysaccharide from Lactiplantibacillus pentosus B8 Isolated from Sichuan Pickle. Foods 2022, 11, 2327. [Google Scholar] [CrossRef]
- Diao, H.; Xing, P.; Tian, J.; Han, Z.; Wang, D.; Xiang, H.; Liu, T.; Ma, R. Toxicity of Crude Toxin Protein Produced by Cordyceps fumosorosea IF-1106 Against Myzus persicae (Sulze). J. Invertebr. Pathol. 2022, 194, 107825. [Google Scholar] [CrossRef]
- Troth, E.V.; Kyle, D.E. EdU Incorporation to Assess Cell Proliferation and Drug Susceptibility in Naegleria Fowleri. Antimicrob. Agents Chemother. 2021, 65, e0001721. [Google Scholar] [CrossRef]
- Du, B.; Fu, Y.; Wang, X.; Jiang, H.; Lv, Q.; Du, R.; Yang, Y.; Rong, R. Isolation, Purification, Structural Analysis and Biological Activities of Water-Soluble Polysaccharide from Glehniae radix. Int. J. Biol. Macromol. 2019, 128, 724–731. [Google Scholar] [CrossRef]
- Mao, N.; Yu, Y.; Lu, X.; Yang, Y.; Liu, Z.; Wang, D. Preventive Effects of Matrine on LPS-Induced Inflammation in RAW 264.7 Cells and Intestinal Damage in Mice through the TLR4/NF-κB/MAPK Pathway. Int. Immunopharmacol. 2024, 143, 113432. [Google Scholar] [CrossRef] [PubMed]
- Jack, R.S.; Fan, X.; Bernheiden, M.; Rune, G.; Ehlers, M.; Weber, A.; Kirsch, G.; Mentel, R.; Fürll, B.; Freudenberg, M.; et al. Lipopolysaccharide-Binding Protein Is Required to Combat a Murine Gram-Negative Bacterial Infection. Nature 1997, 389, 742–745. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, X.; Dai, R.; Xiao, Y.; Wang, X.; Bi, D.; Shi, D. Enterococcus faecium HDRsEf1 Protects the Intestinal Epithelium and Attenuates ETEC-Induced IL-8 Secretion in Enterocytes. Mediat. Inflamm. 2016, 2016, 7474306. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Stanton, C.; Ross, R.P.; Zhao, J.; Chen, W.; Yang, B. Exopolysaccharides Produced by Bifidobacterium longum subsp. longum YS108R Ameliorates DSS-Induced Ulcerative Colitis in Mice by Improving the Gut Barrier and Regulating the Gut Microbiota. J. Agric. Food Chem. 2024, 72, 7055–7073. [Google Scholar] [CrossRef]
- Kurki, P.; Vanderlaan, M.; Dolbeare, F.; Gray, J.; Tan, E.M. Expression of Proliferating Cell Nuclear Antigen (PCNA)/Cyclin during the Cell Cycle. Exp. Cell Res. 1986, 166, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, Q.; Zhou, T. Statistical Optimization of Novel Medium to Maximize the Yield of Exopolysaccharide From Lacticaseibacillus rhamnosus ZFM216 and Its Immunomodulatory Activity. Front. Nutr. 2022, 9, 924495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, S.; Zhao, N.; Liu, C.; Zhang, F.; Guo, Y.; Liu, H. CXCL8 Chemokine in Ulcerative Colitis. Biomed. Pharmacother. 2021, 138, 111427. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.B.; Carpenter, E.S.; Steele, N.G.; Donahue, K.L.; Nwosu, Z.C.; Pacheco, A.; Velez-Delgado, A.; Menjivar, R.E.; Lima, F.; The, S.; et al. Apolipoprotein E Promotes Immune Suppression in Pancreatic Cancer Through NF-κB-Mediated Production of CXCL1. Cancer Res. 2021, 81, 4305–4318. [Google Scholar] [CrossRef]
- Murofushi, Y.; Villena, J.; Morie, K.; Kanmani, P.; Tohno, M.; Shimazu, T.; Aso, H.; Suda, Y.; Hashiguchi, K.; Saito, T.; et al. The Toll-like Receptor Family Protein RP105/MD1 Complex Is Involved in the Immunoregulatory Effect of Exopolysaccharides from Lactobacillus plantarum N14. Mol. Immunol. 2015, 64, 63–75. [Google Scholar] [CrossRef]
- Sun, M.; Liu, W.; Song, Y.; Tuo, Y.; Mu, G.; Ma, F. The Effects of Lactobacillus plantarum-12 Crude Exopolysaccharides on the Cell Proliferation and Apoptosis of Human Colon Cancer (HT-29) Cells. Probiotics Antimicrob. Prot. 2021, 13, 413–421. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. A Bioactive Exopolysaccharide from Marine Bacteria Alteromonas sp. PRIM-28 and Its Role in Cell Proliferation and Wound Healing In Vitro. Int. J. Biol. Macromol. 2019, 131, 10–18. [Google Scholar] [CrossRef]
- Alp, G.; Aslim, B. Relationship Between the Resistance to Bile Salts and Low pH with Exopolysaccharide (EPS) Production of Bifidobacterium spp. Isolated from Infants Feces and Breast Milk. Anaerobe 2010, 16, 101–105. [Google Scholar] [CrossRef]
- Imran, M.Y.M.; Reehana, N.; Jayaraj, K.A.; Ahamed, A.A.P.; Dhanasekaran, D.; Thajuddin, N.; Alharbi, N.S.; Muralitharan, G. Statistical Optimization of Exopolysaccharide Production by Lactobacillus plantarum NTMI05 and NTMI20. Int. J. Biol. Macromol. 2016, 93, 731–745. [Google Scholar] [CrossRef]
- Jia, K.; Tao, X.; Liu, Z.; Zhan, H.; He, W.; Zhang, Z.; Zeng, Z.; Wei, H. Characterization of Novel Exopolysaccharide of Enterococcus faecium WEFA23 from Infant and Demonstration of Its in Vitro Biological Properties. Int. J. Biol. Macromol. 2019, 128, 710–717. [Google Scholar] [CrossRef]
- Blache, P.; van de Wetering, M.; Duluc, I.; Domon, C.; Berta, P.; Freund, J.-N.; Clevers, H.; Jay, P. SOX9 Is an Intestine Crypt Transcription Factor, Is Regulated by the Wnt Pathway, and Represses the CDX2 and MUC2 Genes. J. Cell Biol. 2004, 166, 37–47. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, D.; Fang, L.; Liu, X.; Liu, C.; Zhao, F.; Wu, D.; Wang, X.; Wang, J.; Min, W. Hypoglycemic Effect of Exopolysaccharide from Lactiplantibacillus plantarum JLAU103 on Streptozotocin and High-Fat Diet-Induced Type 2 Diabetic Mice. Foods 2022, 11, 3571. [Google Scholar] [CrossRef]
- Li, J.; Qu, C.; Li, F.; Chen, Y.; Zheng, J.; Xiao, Y.; Jin, Q.; Jin, G.; Huang, X.; Jin, D. Inonotus Obliquus Polysaccharide Ameliorates Azoxymethane/Dextran Sulfate Sodium-Induced Colitis-Associated Cancer in Mice via Activation of the NLRP3 Inflammasome. Front. Pharmacol. 2020, 11, 621835. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, J.; Yan, Q.; Gu, Z.; August, A.; Huang, W.; Jiang, Z. Konjac Glucomannan Oligosaccharides Prevent Intestinal Inflammation Through SIGNR1-Mediated Regulation of Alternatively Activated Macrophages. Mol. Nutr. Food Res. 2021, 65, e2001010. [Google Scholar] [CrossRef]
- Hutsko, S.L.; Meizlisch, K.; Wick, M.; Lilburn, M.S. Early Intestinal Development and Mucin Transcription in the Young Poult with Probiotic and Mannan Oligosaccharide Prebiotic Supplementation. Poult. Sci. 2016, 95, 1173–1178. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Yao, D.; Zhao, J.; Wu, Y.; Lu, B.; Zheng, X. Physicochemical Characteristics and In Vitro and In Vivo Antioxidant Activity of a Cell-Bound Exopolysaccharide Produced by Lactobacillus fermentum S1. Int. J. Biol. Macromol. 2019, 139, 252–261. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Chen, W.; Lu, W. Lactic Acid Bacteria-Derived Exopolysaccharide: Formation, Immunomodulatory Ability, Health Effects, and Structure-Function Relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-H.; Song, A.-X.; Li, L.-Q.; Siu, K.-C.; Yao, Z.-P.; Wu, J.-Y. Effects of Exopolysaccharide Fractions with Different Molecular Weights and Compositions on Fecal Microflora During In Vitro Fermentation. Int. J. Biol. Macromol. 2020, 144, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Q.; Song, A.-X.; Wong, W.-T.; Wu, J.-Y. Isolation and Assessment of a Highly-Active Anti-Inflammatory Exopolysaccharide from Mycelial Fermentation of a Medicinal Fungus Cs-HK1. Int. J. Mol. Sci. 2021, 22, 2450. [Google Scholar] [CrossRef]
- Rubino, S.J.; Geddes, K.; Girardin, S.E. Innate IL-17 and IL-22 Responses to Enteric Bacterial Pathogens. Trends Immunol. 2012, 33, 112–118. [Google Scholar] [CrossRef]
- Kopan, R.; Ilagan, M.X.G. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K.; Ojediran, J.O.; Ogunsakin, A.O.; Banwo, K. Extracellular Polysaccharide from Weissella Confusa OF126: Production, Optimization, and Characterization. Int. J. Biol. Macromol. 2018, 111, 514–525. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | mRNA ID |
|---|---|---|---|
| β-actin | TGAGCTGCGTTTTACACCCT | GCCTTCACCGTTCCAGTTTT | NM_007393.5 |
| CXCL-1 | ACTGCACCCAAACCGAAGTC | TGGGGACACCTTTTAGCATCTT | NM_008176.3 |
| IL-6 | GTCGGAGGCTTAATTACACA | TTTTCTGCAAGTGCATCATC | NM_001314054.1 |
| PCNA | GCCGAGACCTTAGCCACATT | GTAGGAGACAGTGGAGTGGC | NM_011045.2 |
| Ki67 | ACCGTGGAGTAGTTTATCTGGG | TGTTTCCAGTCCGCTTACTTCT | NM_026472.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; You, Y.; Ren, L.; Fu, G.; Zhou, N.; Xiao, Y.; Shi, D. Exploration of a Postbiotic Derived from Enterococcus faecium HDRsEf1 and Its Probiotic Mechanisms. Microorganisms 2025, 13, 1518. https://doi.org/10.3390/microorganisms13071518
Chen Y, You Y, Ren L, Fu G, Zhou N, Xiao Y, Shi D. Exploration of a Postbiotic Derived from Enterococcus faecium HDRsEf1 and Its Probiotic Mechanisms. Microorganisms. 2025; 13(7):1518. https://doi.org/10.3390/microorganisms13071518
Chicago/Turabian StyleChen, Yingying, Yingting You, Lizhen Ren, Guilin Fu, Naiji Zhou, Yuncai Xiao, and Deshi Shi. 2025. "Exploration of a Postbiotic Derived from Enterococcus faecium HDRsEf1 and Its Probiotic Mechanisms" Microorganisms 13, no. 7: 1518. https://doi.org/10.3390/microorganisms13071518
APA StyleChen, Y., You, Y., Ren, L., Fu, G., Zhou, N., Xiao, Y., & Shi, D. (2025). Exploration of a Postbiotic Derived from Enterococcus faecium HDRsEf1 and Its Probiotic Mechanisms. Microorganisms, 13(7), 1518. https://doi.org/10.3390/microorganisms13071518







