Minimum Inhibitory Concentration Increase in Clostridioides difficile Isolates from Patients with Recurrence: Results from a Retrospective Single-Centre Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. Statistics
3. Results
3.1. Patients
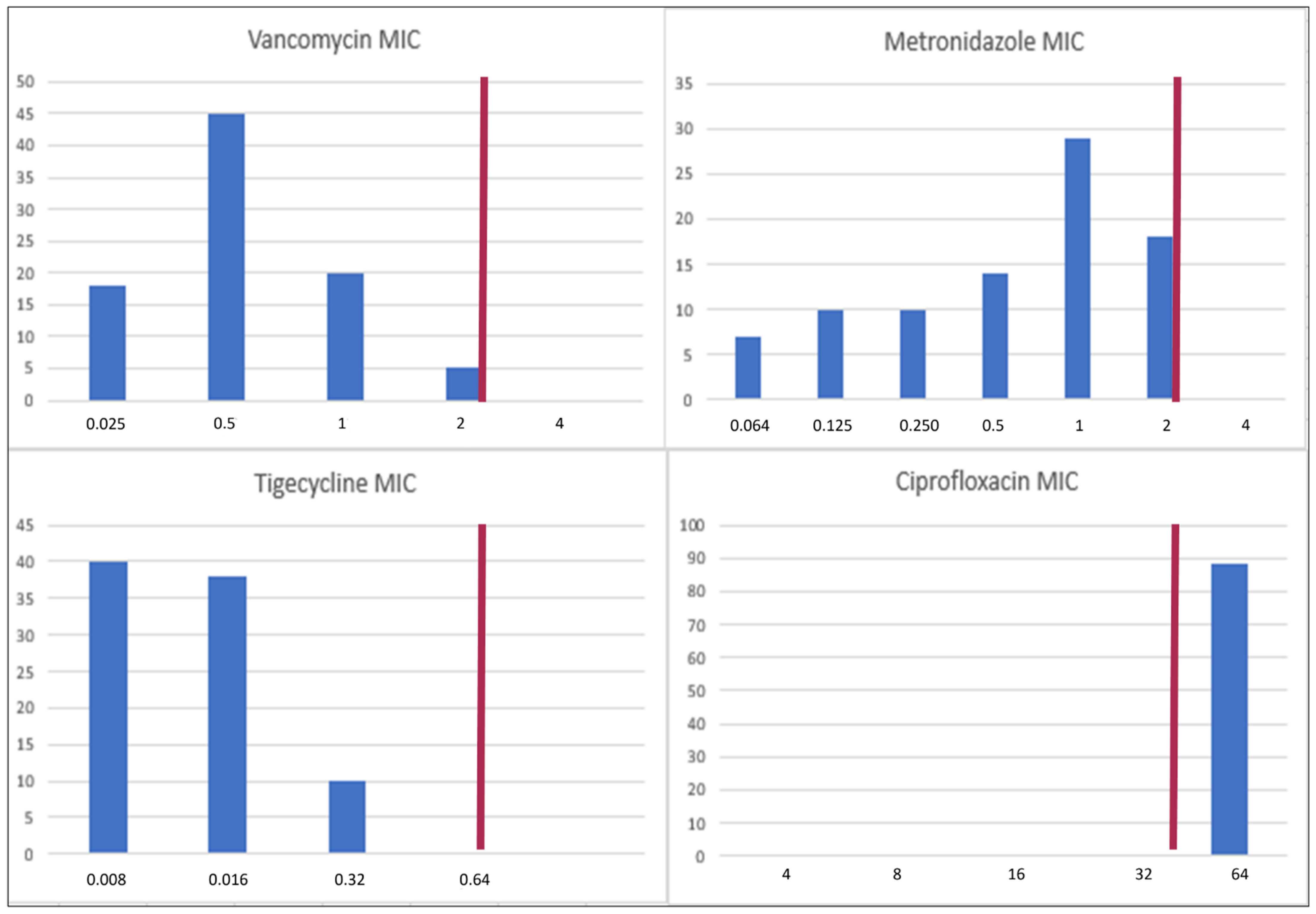
3.2. MICs of Vancomycin, Metronidazole, Tigecycline, and Ciprofloxacin
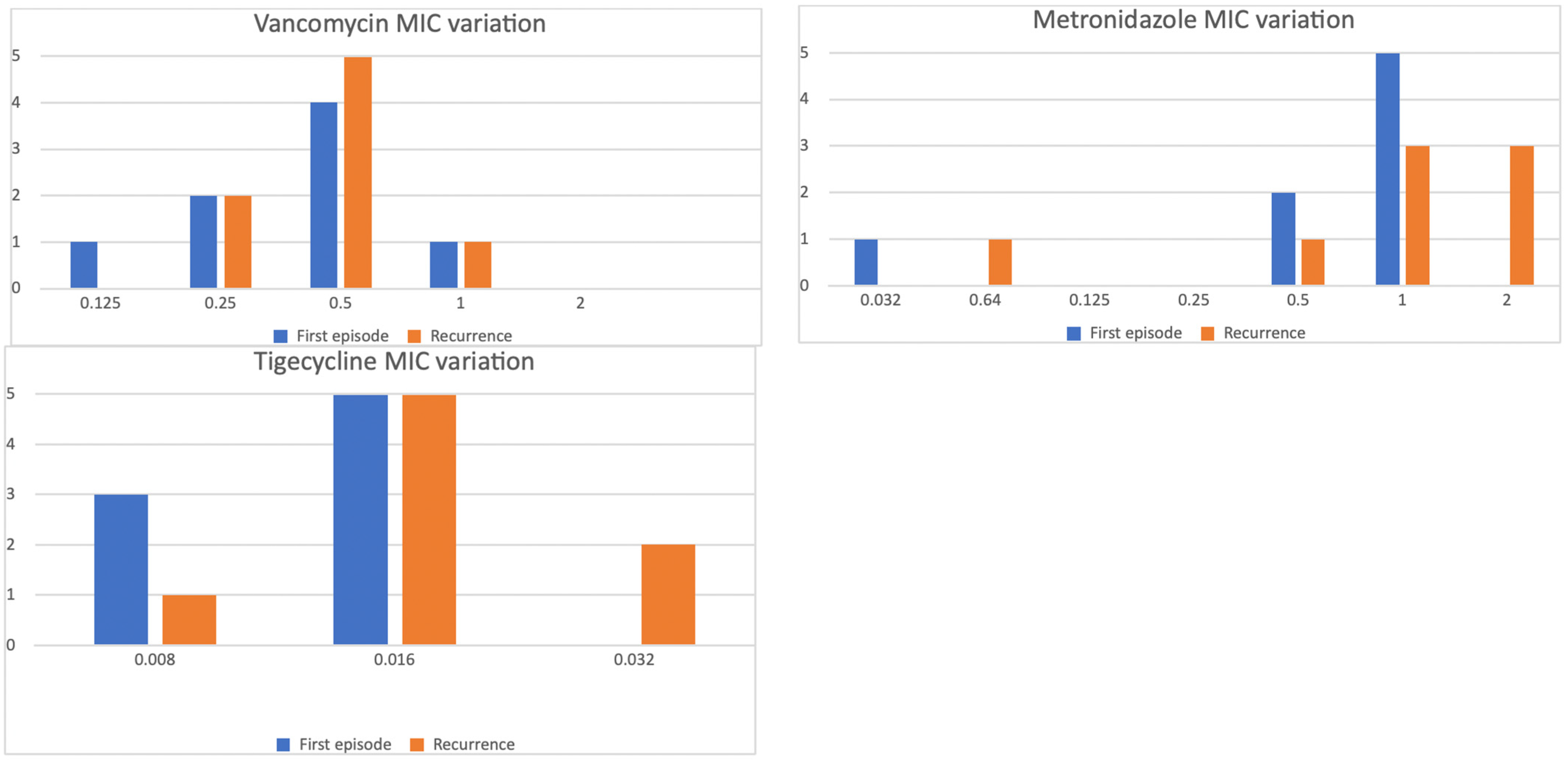
3.3. MIC Variation in Subsequent Episodes
3.4. Variables Associated with MIC Distributions
3.5. Relationship of Vancomycin, Metronidazole, and Tigecycline MICs with Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawson, P.A.; Citron, D.M.; Tyrrell, K.L.; Finegold, S.M. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 2016, 40, 95–99. [Google Scholar] [CrossRef]
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile Toxins: Mechanisms of Action and Antitoxin Therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef]
- Yu, H.; Alfred, T.; Nguyen, J.L.; Zhou, J.; Olsen, M.A. Incidence, Attributable Mortality, and Healthcare and Out-of-Pocket Costs of Clostridioides difficile Infection in US Medicare Advantage Enrollees. Clin. Infect. Dis. 2023, 76, E1476–E1483. [Google Scholar] [CrossRef]
- Tsigrelis, C. Recurrent Clostridioides difficile Infection: Recognition, Management, Prevention. Cleve. Clin. J. Med. 2020, 87, 347–359. [Google Scholar] [CrossRef]
- van Rossen, T.M.; Ooijevaar, R.E.; Vandenbroucke-Grauls, C.M.J.E.; Dekkers, O.M.; Kuijper, E.J.; Keller, J.J.; van Prehn, J. Prognostic Factors for Severe and Recurrent Clostridioides difficile Infection: A Systematic Review. Clin. Microbiol. Infect. 2022, 28, 321–331. [Google Scholar] [CrossRef]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef] [PubMed]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 Update on the Treatment Guidance Document for Clostridioides difficile Infection in Adults. Clin. Microbiol. Infect. 2021, 27, S1–S21. [Google Scholar] [CrossRef]
- Patel, D.; Senecal, J.; Spellberg, B.; Morris, A.M.; Saxinger, L.; Footer, B.W.; McDonald, E.G.; Lee, T.C. Fidaxomicin to Prevent Recurrent Clostridioides difficile: What Will It Cost in the USA and Canada? JAC Antimicrob. Resist. 2023, 5, dlac138. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Laboratory Procedures for Diagnosis and Typing of Human Clostridium difficile Infection; ECDC: Stockholm, Sweden, 2018.
- Dilnessa, T.; Getaneh, A.; Hailu, W.; Moges, F.; Gelaw, B. Prevalence and Antimicrobial Resistance Pattern of Clostridium difficile among Hospitalized Diarrheal Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0262597. [Google Scholar] [CrossRef] [PubMed]
- Sholeh, M.; Krutova, M.; Forouzesh, M.; Mironov, S.; Sadeghifard, N.; Molaeipour, L.; Maleki, A.; Kouhsari, E. Antimicrobial Resistance in Clostridioides (Clostridium) difficile Derived from Humans: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control 2020, 9, 158. [Google Scholar] [CrossRef]
- Eubank, T.A.; Gonzales-Luna, A.J.; Hurdle, J.G.; Garey, K.W. Genetic Mechanisms of Vancomycin Resistance in Clostridioides difficile: A Systematic Review. Antibiotics 2022, 11, 258. [Google Scholar] [CrossRef]
- Peng, Z.; Jin, D.; Kim, H.B.; Stratton, C.W.; Wu, B.; Tang, Y.-W.; Sun, X.; Kraft, C.S. Update on Antimicrobial Resistance in Clostridium difficile: Resistance Mechanisms and Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 2017, 55, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Cho, J.M.; Cunningham, S.A.; Behera, G.K.; Becker, S.; Amjad, T.; Greenwood-Quaintance, K.E.; Mendes-Soares, H.; Jones-Hall, Y.; Jeraldo, P.R.; et al. Plasmid Acquisition Alters Vancomycin Susceptibility in Clostridioides difficile. Gastroenterology 2021, 160, 941–945.e8. [Google Scholar] [CrossRef]
- Marchandin, H.; Anjou, C.; Poulen, G.; Freeman, J.; Wilcox, M.; Jean-Pierre, H.; Barbut, F. In Vivo Emergence of a Still Uncommon Resistance to Fidaxomicin in the Urgent Antimicrobial Resistance Threat Clostridioides difficile. J. Antimicrob. Chemother. 2023, 78, 1992–1999. [Google Scholar] [CrossRef]
- Eubank, T.A.; Dureja, C.; Gonzales-Luna, A.J.; Hurdle, J.G.; Garey, K.W. Reduced Vancomycin Susceptibility in Clostridioides difficile Is Associated with Specific Ribotypes. Open Forum Infect. Dis. 2024, 11, ofae588. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Pham, N.V.S.; Hays, R.A.; Bolick, D.T.; Goldbeck, S.M.; Poulter, M.D.; Hoang, S.C.; Shin, J.H.; Wu, M.; Warren, C.A. Influence of Binary Toxin Gene Detection and Decreased Susceptibility to Antibiotics Among Clostridioides difficile Strains on Disease Severity: A Single-Center Study. Antimicrob. Agents Chemother. 2022, 66, e00489-22. [Google Scholar] [CrossRef]
- Rahmoun, L.A.; Azrad, M.; Peretz, A. Antibiotic Resistance and Biofilm Production Capacity in Clostridioides difficile. Front. Cell. Infect. Microbiol. 2021, 11, 683464. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-J.; Deshpande, A.; E Hevener, K.; Endres, B.T.; Garey, K.W.; Palmer, K.L.; Hurdle, J.G. Constitutive Expression of the Cryptic vanGCd Operon Promotes Vancomycin Resistance in Clostridioides difficile Clinical Isolates. J. Antimicrob. Chemother. 2020, 75, 859–867. [Google Scholar] [CrossRef]
- Krutova, M.; Wilcox, M.; Kuijper, E. Clostridioides difficile Infection: Are the Three Currently Used Antibiotic Treatment Options Equal from Pharmacological and Microbiological Points of View? Int. J. Infect. Dis. 2022, 124, 118–123. [Google Scholar] [CrossRef]
- Barbanti, F.; Spigaglia, P. Microbiological Characteristics of Human and Animal Isolates of Clostridioides difficile in Italy: Results of the Istituto Superiore di Sanità in the Years 2006–2016. Anaerobe 2020, 61, 102136. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, Emerging Infections Program, Healthcare Associated Infections—Community Interface Surveillance Report, Clostridioides difficile Infection (CDI). 2019. Available online: https://www.cdc.gov/healthcare-associated-infections/?CDC_AAref_Val=https://www.cdc.gov/hai/eip/Annual-CDI-Report-2019.htm (accessed on 22 June 2023).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0; European Committee on Antimicrobial Susceptibility Testing (EUCAST): Växjö, Sweden, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 31st Informational Supplement; CLSI Document M100-S31; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Poilane, I.; Cruaud, P.; Torlotin, J.C.; Collignon, A. Comparison of the E Test to the Reference Agar Dilution Method for Antibiotic Susceptibility Testing of Clostridium difficile. Clin. Microbiol. Infect. 2000, 6, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Qin, X. Comparative Study of Clostridium difficile Clinical Detection Methods in Patients with Diarrhoea. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 8753284. [Google Scholar] [CrossRef]
- Dhand, N.K.; Khatkar, M.S. Statulator: An Online Statistical Calculator. Sample Size Calculator for Estimating a Single Proportion. Available online: http://statulator.com/SampleSize/ss1P.html (accessed on 22 June 2023).
- R Core Team. R 4.4.0: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 14 April 2024).
- Eubank, T.A.; Dureja, C.; Garey, K.W.; Hurdle, J.G.; Gonzales-Luna, A.J. Reduced Vancomycin Susceptibility in Clostridioides difficile Is Associated with Lower Rates of Initial Cure and Sustained Clinical Response. Clin. Infect. Dis. 2024, 79, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Vernon, J.; Pilling, S.; Morris, K.; Nicolson, S.; Shearman, S.; Clark, E.; Palacios-Fabrega, J.A.; Wilcox, M.; The Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium difficile Ribotypes’ Study Group. Five-Year Pan-European, Longitudinal Surveillance of Clostridium difficile Ribotype Prevalence and Antimicrobial Resistance: The Extended ClosER Study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 169–177. [Google Scholar]
- Freeman, J.; Viprey, V.; Ewin, D.; Spittal, W.; Clark, E.; Vernon, J.; Fawley, W.; Davis, G.; Tkalec, V.; Rupnik, M.; et al. Antimicrobial Susceptibility in Clostridioides difficile Varies According to European Region and Isolate Source. JAC Antimicrob. Resist. 2024, 6, dlae112. [Google Scholar] [CrossRef]
- Szabo, B.G.; Kadar, B.; Lenart, K.S.; Dezsenyi, B.; Kunovszki, P.; Fried, K.; Kamotsay, K.; Nikolova, R.; Prinz, G. Use of Intravenous Tigecycline in Patients with Severe Clostridium difficile Infection: A Retrospective Observational Cohort Study. Clin. Microbiol. Infect. 2016, 22, 990–995. [Google Scholar] [CrossRef]
- Spigaglia, P.; Barbanti, F.; Dionisi, A.M.; Mastrantonio, P. Clostridium difficile Isolates Resistant to Fluoroquinolones in Italy: Emergence of PCR Ribotype 018. J. Clin. Microbiol. 2010, 48, 2892–2896. [Google Scholar] [CrossRef]
- Spigaglia, P.; Barbanti, F.; Mastrantonio, P.; Brazier, J.S.; Barbut, F.; Delmée, M.; Kuijper, E.; Poxton, I.R.; on behalf of the European Study Group on (ESGCD). Fluoroquinolone Resistance in Clostridium difficile Isolates from a Prospective Study of C. difficile Infections in Europe. J. Med. Microbiol. 2008, 57, 784–789. [Google Scholar] [CrossRef]
- Aldape, M.J.; Packham, A.E.; Nute, D.W.; Bryant, A.E.; Stevens, D.L. Effects of Ciprofloxacin on the Expression and Production of Exotoxins by Clostridium difficile. J. Med. Microbiol. 2013, 62, 741–747. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Gomez-Simmonds, A.; Yin, M.T.; Freedberg, D.E. Antibiotic-Specific Risk for Community-Acquired Clostridioides difficile Infection in the United States from 2008 to 2020. Antimicrob. Agents Chemother. 2022, 66, e01129-22. [Google Scholar] [CrossRef]
- Richardson, C.; Kim, P.; Lee, C.; Bersenas, A.; Weese, J.S. Comparison of Clostridium difficile Isolates from Individuals with Recurrent and Single Episode of Infection. Anaerobe 2015, 33, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Snydman, D.R.; A McDermott, L.; Thorpe, C.M.; Chang, J.; Wick, J.; Walk, S.T.; Vickers, R.J. Antimicrobial Susceptibility and Ribotypes of Clostridium difficile Isolates from a Phase 2 Clinical Trial of Ridinilazole (SMT19969) and Vancomycin. J. Antimicrob. Chemother. 2018, 73, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Gargis, A.S.; Karlsson, M.; Paulick, A.L.; Anderson, K.F.; Adamczyk, M.; Vlachos, N.; Kent, A.G.; McAllister, G.; McKay, S.L.; Halpin, A.L.; et al. Reference Susceptibility Testing and Genomic Surveillance of Clostridioides difficile, United States, 2012–17. Clin. Infect. Dis. 2023, 76, 890–896. [Google Scholar] [CrossRef] [PubMed]
| Patients (Total) | 108 |
|---|---|
| Epidemiology | |
| Age [years] | 76 (62–84) |
| Males/females | 50/58 (46.0%/54.0%) |
| Charlson Comorbidity Index | 5 (4–7) |
| Cardiovascular disease | 32 (37.0%) |
| Peripheral venous disease | 12 (13.8%) |
| Stroke | 12 (13.8%) |
| Chronic Obstructive Pulmonary Disease | 21 (24.1%) |
| Liver disease | 13 (14.9%) |
| Solid malignancy | 16 (18.4%) |
| Haematological malignancy | 2 (2.4%) |
| Previous hospitalization | 38 (44.2%) |
| Current episode of CDI | |
| Hospital/community-acquired CDI | 74/12 (86.0%/14.0%) |
| Severe CDI | 39 (47.0%) |
| Episode: first/second/third or more | 67/14/5 (77.9%/16.3%/5.8%) |
| Duration of therapy [days] | 10 (10–13.5) |
| NAP1/R027 | 30 (30.0%) |
| Binary toxin | 42 (42.0%) |
| TcdB+ | 83 (83.0%) |
| ICU admission | 2 (2.4%) |
| Recurrence | 14 (16.9%) |
| Length of stay [days] | 26 (15–42%) |
| 28-day mortality | 6 (7.3%) |
| Therapies | |
| Use of proton pump inhibitors before CDI | 58 (69.1%) |
| Proton pump inhibitors started during CDI | 7 (8.4%) |
| Recent use of antibiotics | 76 (89.4%) |
| Previous use of vancomycin | 17 (19.8%) |
| Previous use of metronidazole | 7 (8.4%) |
| Previous use of fidaxomicin | 3 (3.5%) |
| Treatment with vancomycin | 59 (74.7%) |
| Treatment with fidaxomicin | 5 (6.3%) |
| Treatment with metronidazole | 1 (1.3%) |
| Treatment with vancomycin and metronidazole | 14 (17.7%) |
| Outcome | Vancomycin MIC (μg/mL) | |||||
|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | p | ||
| Recurrence | 3/14 (21.4%) | 6/35 (17.1%) | 4/17 (23.5%) | 1/5 (20%) | 0.9 | |
| 28-day mortality | 1/14 (7.1%) | 0/35 (0%) | 2/17 (11.8%) | 2/5 (40%) | 0.009 | |
| Metronidazole MIC (μg/mL) | ||||||
| 0.064 | 0.125 | 0.250 | 0.5 | 1 | ||
| Recurrence | 2/6 (33.3%) | 1/7 (14.3%) | 6/9 (11.1%) | 3/10 (30%) | 6/25 (24%) | 0.8 |
| 28-day mortality | 0/6 (0%) | 0/7 (0%) | 3/9 (33.3%) | 0/10 (0%) | 2/25 (8%) | 0.13 |
| Tigecycline MIC (μg/mL) | ||||||
| 0.008 | 0.016 | 0.032 | 0.064 | |||
| Recurrence | 6/35 (17.1%) | 8/29 (27.6%) | 0/7 (0%) | - | 0.3 | |
| 28-day mortality | 3/35 (8.6%) | 1/29 (3.6%) | 1/7 (14.3%) | - | 0.38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valsecchi, P.; Asperges, E.; Corbella, M.; Banfi, G.; Maffezzoni, M.; Amarasinghe, N.; Drago, R.; Virga, F.; Costanzo, F.; Calabretta, F.; et al. Minimum Inhibitory Concentration Increase in Clostridioides difficile Isolates from Patients with Recurrence: Results from a Retrospective Single-Centre Cohort Study. Microorganisms 2025, 13, 1515. https://doi.org/10.3390/microorganisms13071515
Valsecchi P, Asperges E, Corbella M, Banfi G, Maffezzoni M, Amarasinghe N, Drago R, Virga F, Costanzo F, Calabretta F, et al. Minimum Inhibitory Concentration Increase in Clostridioides difficile Isolates from Patients with Recurrence: Results from a Retrospective Single-Centre Cohort Study. Microorganisms. 2025; 13(7):1515. https://doi.org/10.3390/microorganisms13071515
Chicago/Turabian StyleValsecchi, Pietro, Erika Asperges, Marta Corbella, Greta Banfi, Marcello Maffezzoni, Nicolò Amarasinghe, Riccardo Drago, Flavia Virga, Filippo Costanzo, Francesca Calabretta, and et al. 2025. "Minimum Inhibitory Concentration Increase in Clostridioides difficile Isolates from Patients with Recurrence: Results from a Retrospective Single-Centre Cohort Study" Microorganisms 13, no. 7: 1515. https://doi.org/10.3390/microorganisms13071515
APA StyleValsecchi, P., Asperges, E., Corbella, M., Banfi, G., Maffezzoni, M., Amarasinghe, N., Drago, R., Virga, F., Costanzo, F., Calabretta, F., Sacchi, P., Cambieri, P., Di Sabatino, A., Baldanti, F., & Bruno, R. (2025). Minimum Inhibitory Concentration Increase in Clostridioides difficile Isolates from Patients with Recurrence: Results from a Retrospective Single-Centre Cohort Study. Microorganisms, 13(7), 1515. https://doi.org/10.3390/microorganisms13071515








