EBV-Derived miR-BART20-3p Influences Proliferation and Migration in EBV-Positive Gastric Cancer Models by Suppressing PPARα
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Construction of AGS-EBV Cell Line
2.3. In Situ Hybridization for EBER
2.4. Immunohistochemistry (IHC)
2.5. Bioinformatics Analysis of DEGs Between EBVnGC and EBVaGC
2.6. Analysis of EBV-Encoded miRNAs Mediated Regulatory Network
2.7. Target Prediction
2.8. Luciferase Reporter Assay
2.9. Quantitative Reverse Transcription PCR (qRT-PCR)
2.10. ELISA
2.11. RNA Interference
2.12. CCK8 Assay
2.13. Wound Healing Assay
2.14. Transwell Assay
2.15. Statistical Analyses
3. Results
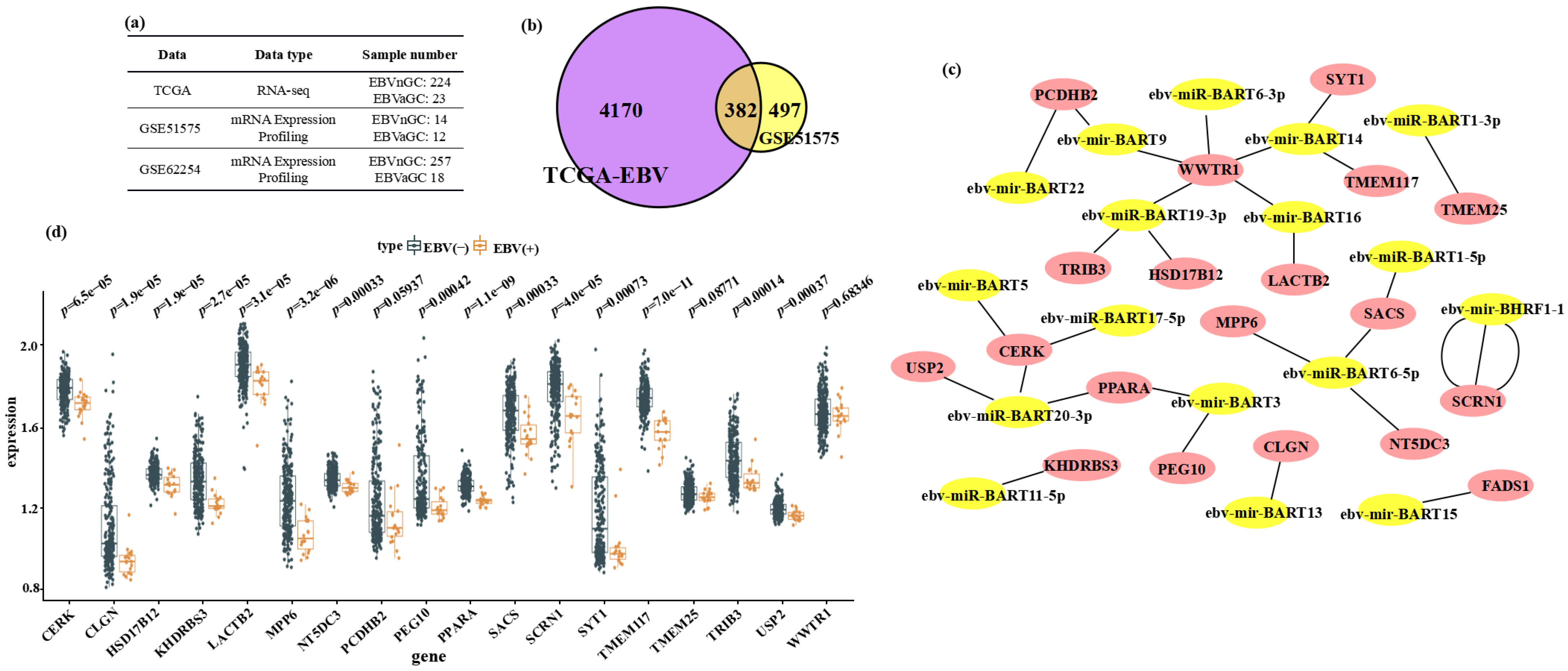
3.1. Bioinformatic Identification of Host Genes Downregulated in EBVaGC and Putatively Targeted by EBV miRNAs
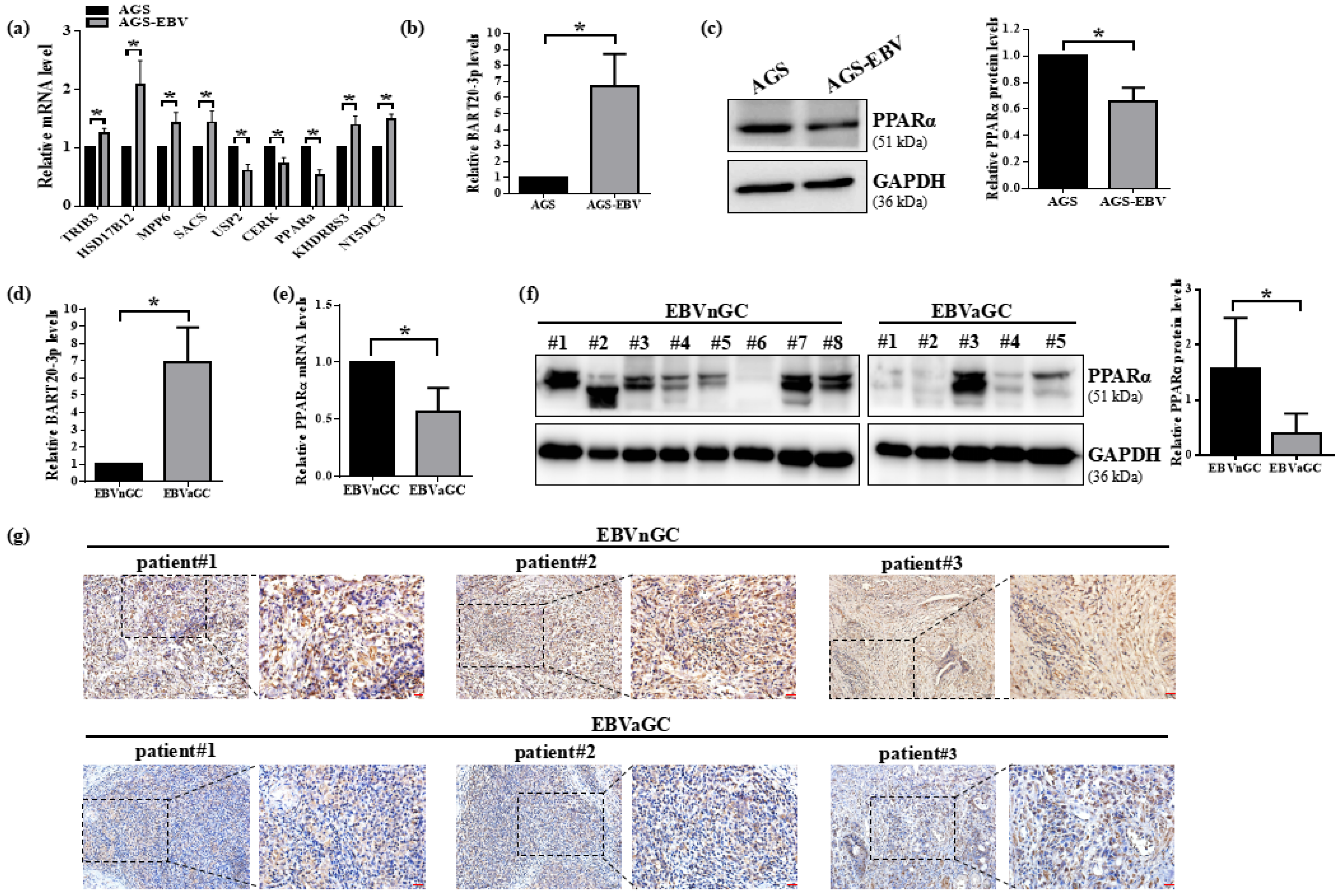
3.2. PPARα Expression Is Suppressed by EBV in Gastric Cancer Cells and Clinical Specimens
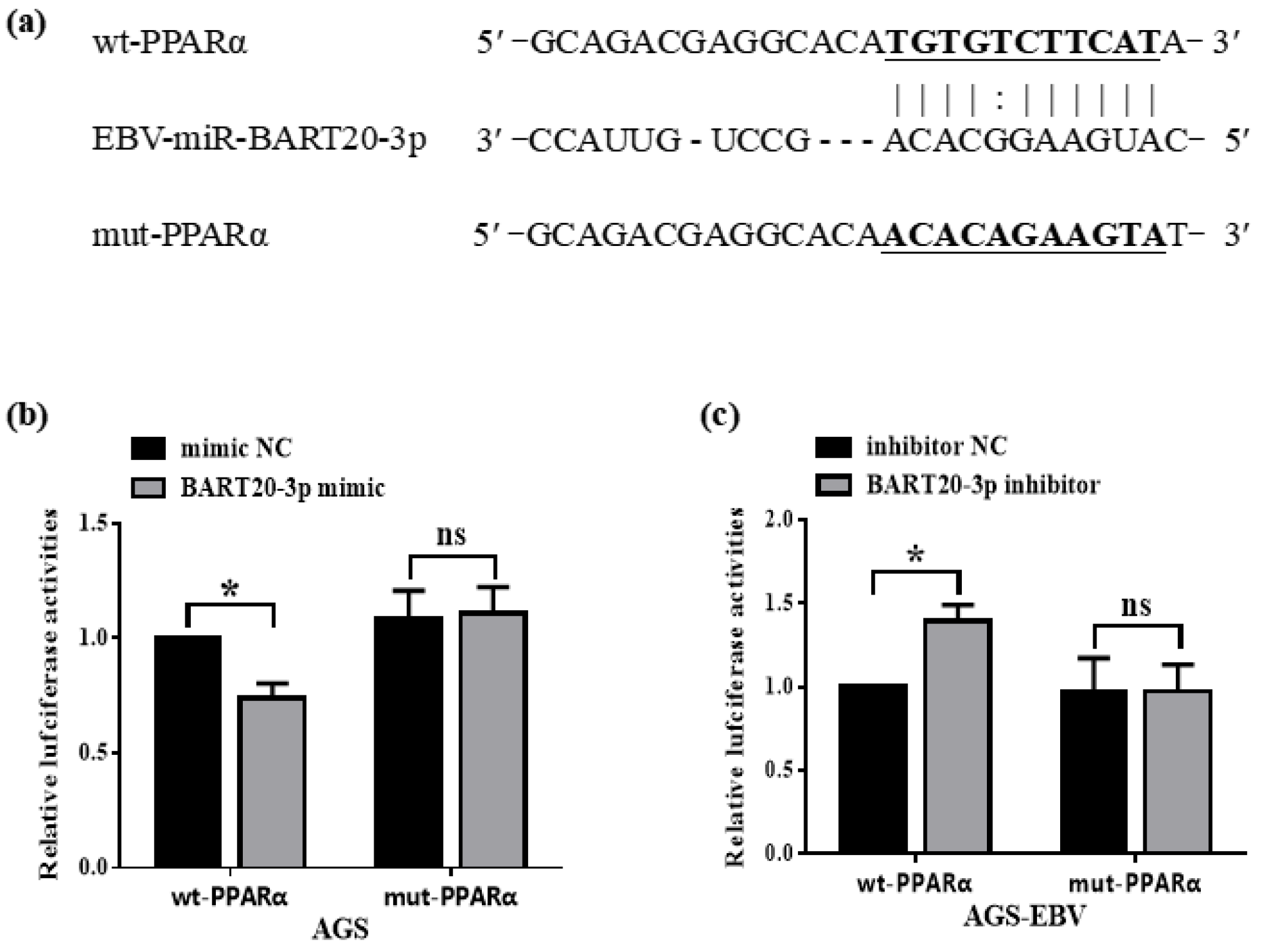
3.3. miR-BART20-3p Directly Binds the PPARα 3′-UTR to Suppress Its Expression
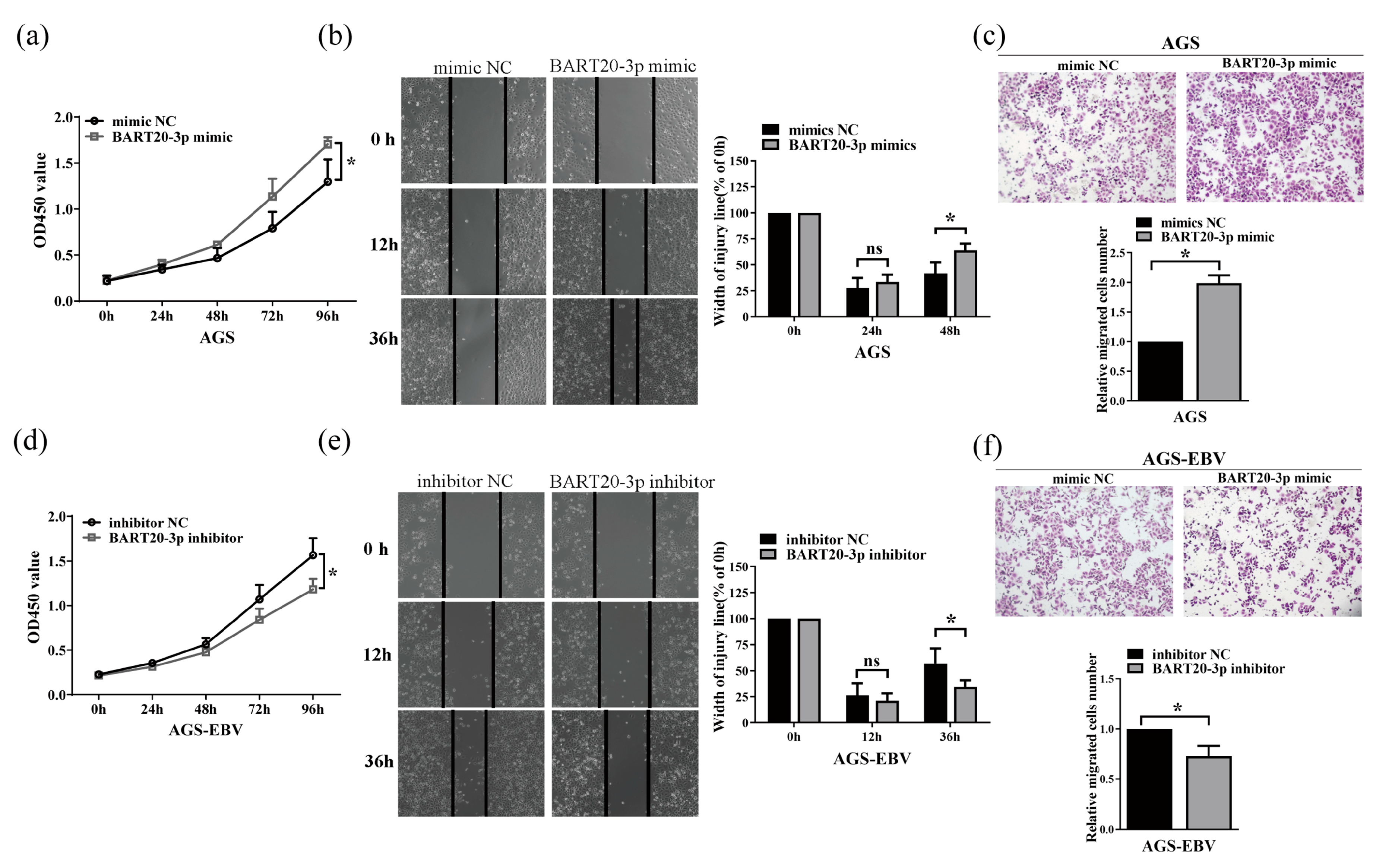
3.4. miR-BART20-3p Enhances Gastric Cancer Cell Proliferation and Migration
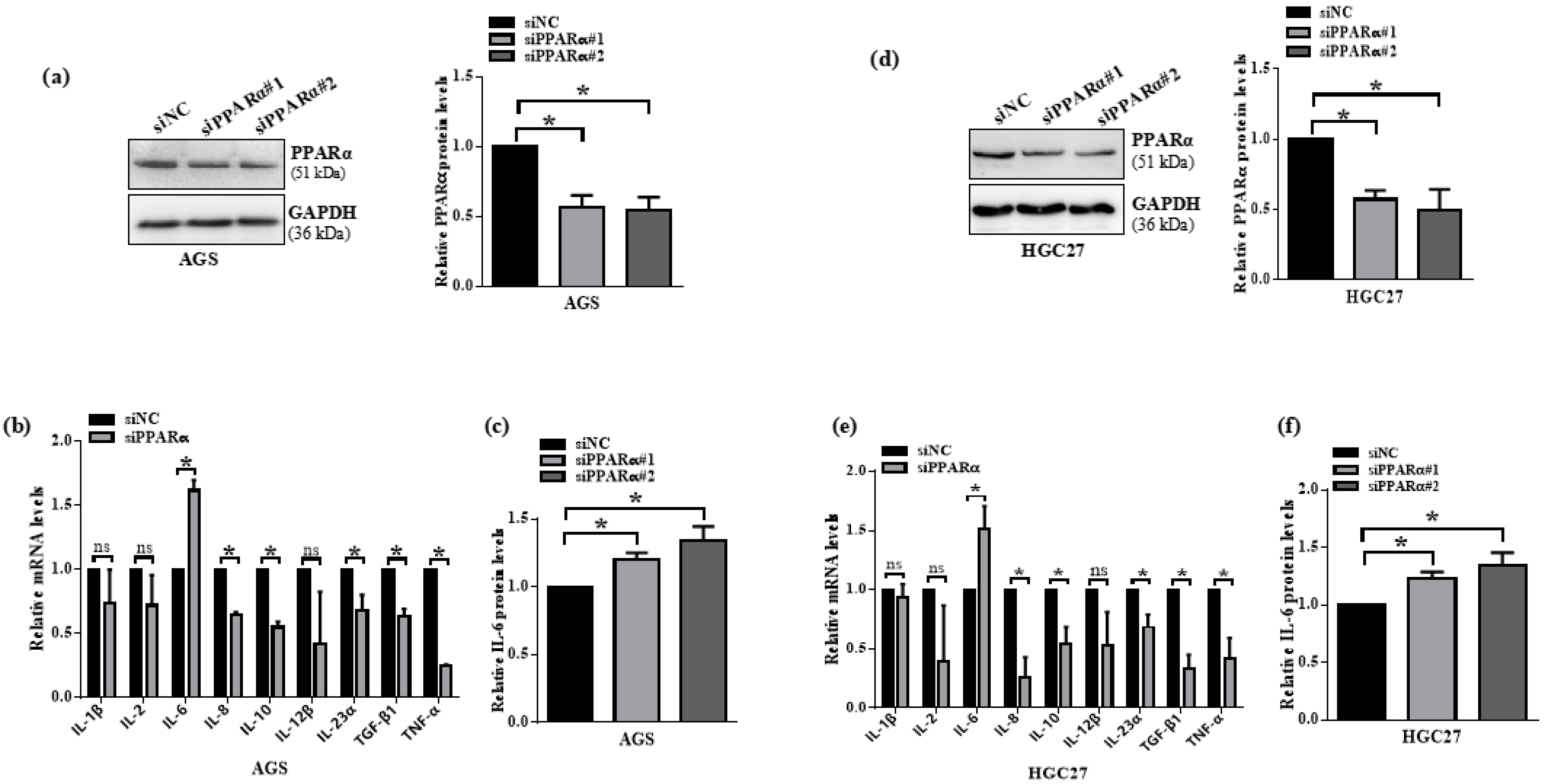
3.5. PPARα Knockdown Induces IL-6 Expression in Gastric Cancer Cells
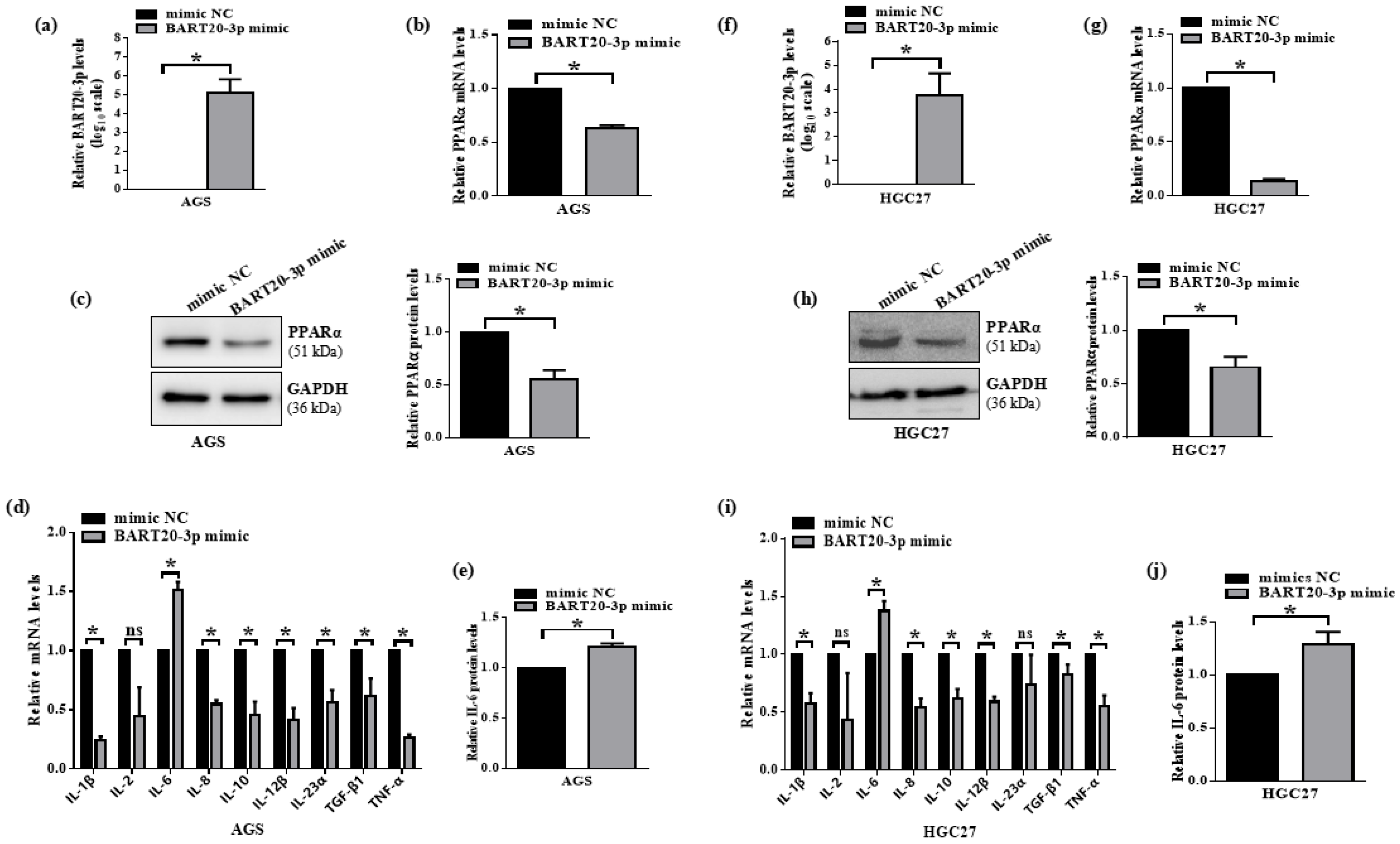
3.6. miR-BART20-3p Suppresses PPARα and Induces IL-6 in EBV-Negative Gastric Cancer Cells
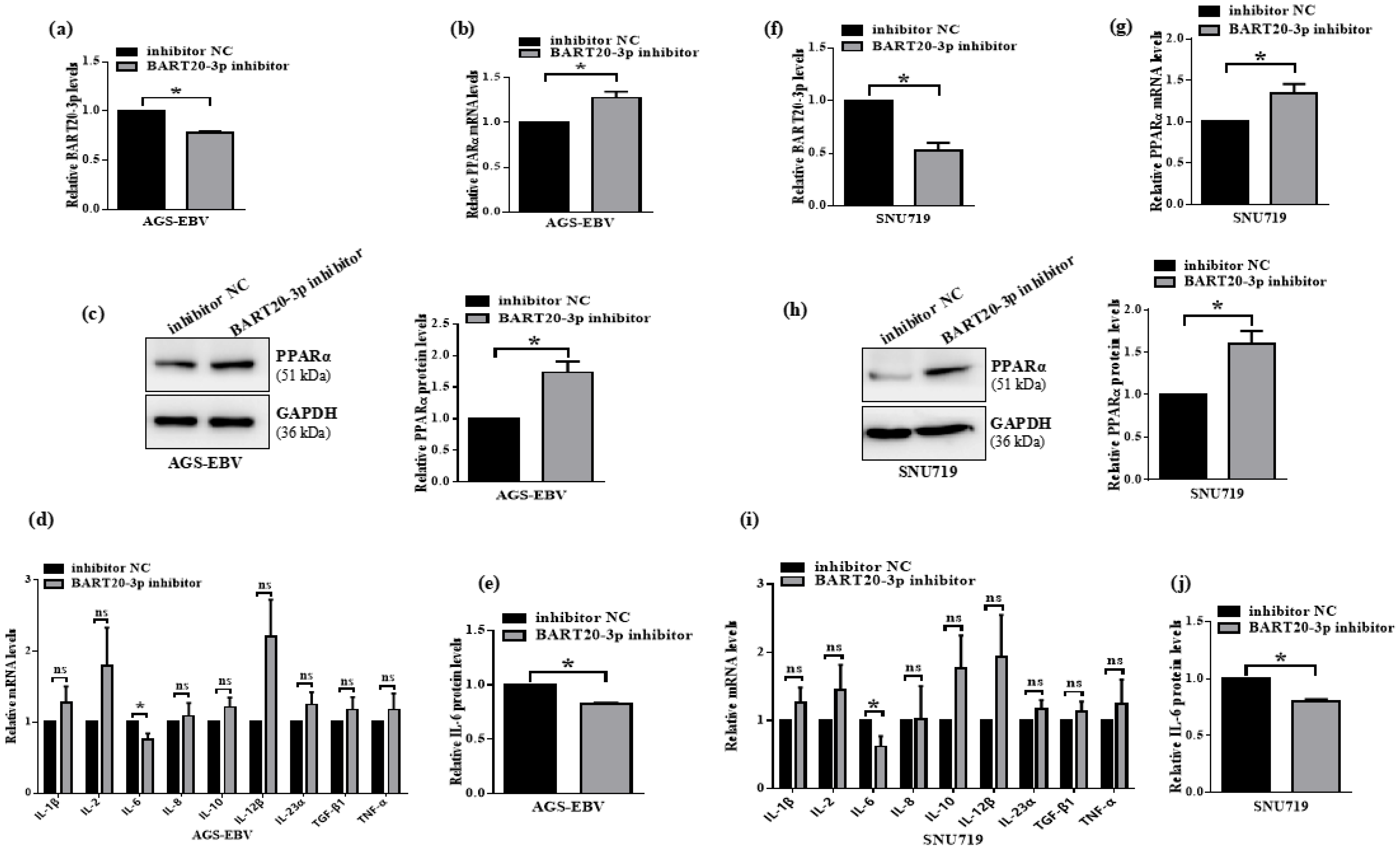
3.7. miR-BART20-3p Inhibition Restores PPARα and Suppresses IL-6 in EBV-Positive Gastric Cancer Cells
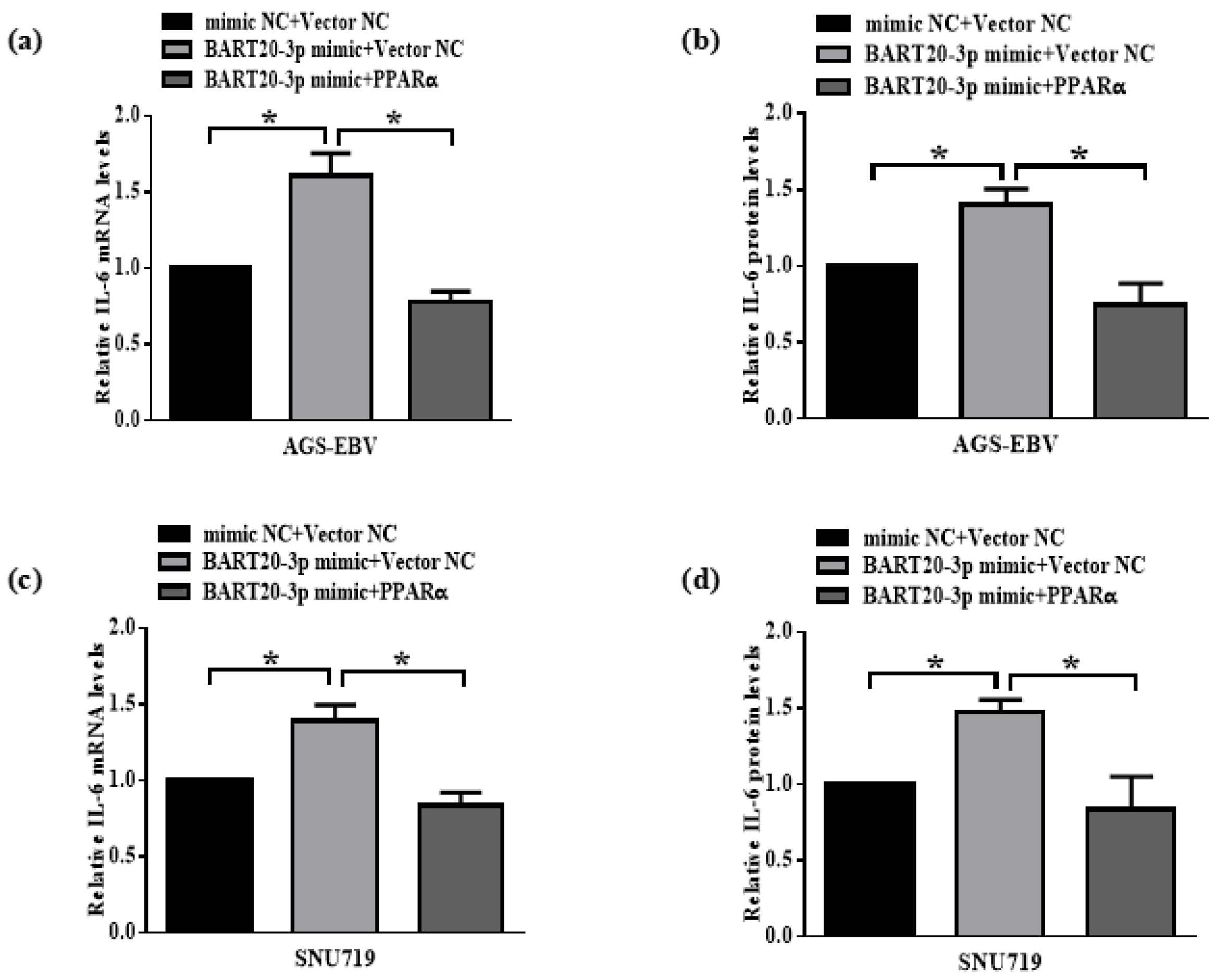
3.8. miR-BART20-3p Regulates IL-6 Expression Through PPARα Modulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Wenker, T.N.; El-Serag, H.B. Global burden of gastric cancer: Epidemiological trends, risk factors, screening and prevention. Nat. Rev. Clin. Oncol. 2023, 20, 338–349. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Tavakoli, A.; Monavari, S.H.; Solaymani Mohammadi, F.; Kiani, S.J.; Armat, S.; Farahmand, M. Association between Epstein-Barr virus infection and gastric cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 493. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- Cohen, J.I. Epstein-Barr virus infection. N. Engl. J. Med. 2000, 343, 481–492. [Google Scholar] [CrossRef]
- Lieberman, P.M. Virology. Epstein-Barr virus turns 50. Science 2014, 343, 1323–1325. [Google Scholar] [CrossRef]
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H., Jr. Primary Epstein-Barr virus infection. J. Clin. Virol. 2018, 102, 84–92. [Google Scholar] [CrossRef]
- Babcock, G.J.; Hochberg, D.; Thorley-Lawson, A.D. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 2000, 13, 497–506. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, T.; Zhou, Y.; Cheng, A.S.L.; Yu, J.; To, K.F.; Kang, W. The oncogenic role of Epstein-Barr virus-encoded microRNAs in Epstein-Barr virus-associated gastric carcinoma. J. Cell Mol. Med. 2018, 22, 38–45. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Zeng, B.; Hu, G.; Gan, R. Epstein-Barr virus-associated gastric cancer: A distinct subtype. Cancer Lett. 2020, 495, 191–199. [Google Scholar] [CrossRef]
- Shinozaki-Ushiku, A.; Kunita, A.; Isogai, M.; Hibiya, T.; Ushiku, T.; Takada, K.; Fukayama, M. Profiling of Virus-Encoded MicroRNAs in Epstein-Barr Virus-Associated Gastric Carcinoma and Their Roles in Gastric Carcinogenesis. J. Virol. 2015, 89, 5581–5591. [Google Scholar] [CrossRef]
- Auboeuf, D.; Rieusset, J.; Fajas, L.; Vallier, P.; Frering, V.; Riou, J.P.; Staels, B.; Auwerx, J.; Laville, M.; Vidal, H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: No alteration in adipose tissue of obese and NIDDM patients. Diabetes 1997, 46, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Pyper, S.R.; Viswakarma, N.; Yu, S.; Reddy, J.K. PPARalpha: Energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal 2010, 8, e002. [Google Scholar] [CrossRef]
- Drew, P.D.; Xu, J.; Storer, P.D.; Chavis, J.A.; Racke, M.K. Peroxisome proliferator-activated receptor agonist regulation of glial activation: Relevance to CNS inflammatory disorders. Neurochem. Int. 2006, 49, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Storer, P.D.; Chavis, J.A.; Racke, M.K.; Drew, P.D. Agonists for the peroxisome proliferator-activated receptor-alpha and the retinoid X receptor inhibit inflammatory responses of microglia. J. Neurosci. Res. 2005, 81, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, Y.; Ma, H. Combined aspirin and apatinib treatment suppresses gastric cancer cell proliferation. Oncol. Lett. 2017, 14, 5409–5417. [Google Scholar] [CrossRef]
- Planagumà, A.; Titos, E.; López-Parra, M.; Gaya, J.; Pueyo, G.; Arroyo, V.; Clària, J. Aspirin (ASA) regulates 5-lipoxygenase activity and peroxisome proliferator-activated receptor alpha-mediated CINC-1 release in rat liver cells: Novel actions of lipoxin A4 (LXA4) and ASA-triggered 15-epi-LXA4. Faseb J. 2002, 16, 1937–1939. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Senol, K.; Ozkan, M.B.; Vural, S.; Tez, M. The role of inflammation in gastric cancer. Adv. Exp. Med. Bio 2014, 816, 235–257. [Google Scholar]
- Kim, D.N.; Chae, H.S.; Oh, S.T.; Kang, J.H.; Park, C.H.; Park, W.S.; Takada, K.; Lee, J.M.; Lee, W.K.; Lee, S.K. Expression of viral microRNAs in Epstein-Barr virus-associated gastric carcinoma. J. Virol. 2007, 81, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, X.; Dang, C.; Wang, X.; Qi, Y.; Li, H. The Epstein-Barr virus-miRNA-BART6-5p regulates TGF-β/SMAD4 pathway to induce glycolysis and enhance proliferation and metastasis of gastric cancer cells. Oncol. Res. 2024, 32, 999–1009. [Google Scholar] [CrossRef]
- Yoon, J.H.; Min, K.; Lee, S.K. Epstein-Barr Virus miR-BART17-5p Promotes Migration and Anchorage-Independent Growth by Targeting Kruppel-Like Factor 2 in Gastric Cancer. Microorganisms 2020, 8, 258. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, K.C.; Wu, W.; Subramaniam, S.; Shyy, J.Y.; Chiu, J.J.; Li, J.Y.; Chien, S. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc. Natl. Acad. Sci. USA 2011, 108, 10355–10360. [Google Scholar] [CrossRef]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.C.; Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Won, T.W. Fenofibrate, a peroxisome proliferator-activated receptor α-agonist, blocks lipopolysaccharide-induced inflammatory pathways in mouse liver. Korean J. Hepatobiliary Pancreat. Surg. 2013, 17, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; De Bosscher, K.; Besnard, S.; Vanden Berghe, W.; Peters, J.M.; Gonzalez, F.J.; Fruchart, J.C.; Tedgui, A.; Haegeman, G.; Staels, B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J. B Chem. 1999, 274, 32048–32054. [Google Scholar] [CrossRef]
- Simondurairaj, C.; Krishnakumar, R.; Sundaram, S.; Venkatraman, G. Interleukin-6 Receptor (IL-6R) Expression in Human Gastric Carcinoma and its Clinical Significance. Cancer Investig. 2019, 37, 293–298. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, G.; Huang, Y.; Zheng, W.; Hua, J.; Yang, S.; Zhuang, J.; Ye, J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol. Rep. 2016, 35, 1787–1795. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Ye, G.; Xu, X.; Zeng, X.; Wu, B.; Xin, F.; Zhang, L.; Lin, X.; Lin, X.; Chen, W. EBV-Derived miR-BART20-3p Influences Proliferation and Migration in EBV-Positive Gastric Cancer Models by Suppressing PPARα. Microorganisms 2025, 13, 1514. https://doi.org/10.3390/microorganisms13071514
Wu Q, Ye G, Xu X, Zeng X, Wu B, Xin F, Zhang L, Lin X, Lin X, Chen W. EBV-Derived miR-BART20-3p Influences Proliferation and Migration in EBV-Positive Gastric Cancer Models by Suppressing PPARα. Microorganisms. 2025; 13(7):1514. https://doi.org/10.3390/microorganisms13071514
Chicago/Turabian StyleWu, Qiong, Guiying Ye, Xiazhen Xu, Xianchang Zeng, Biyun Wu, Fan Xin, Lu Zhang, Xu Lin, Xinjian Lin, and Wannan Chen. 2025. "EBV-Derived miR-BART20-3p Influences Proliferation and Migration in EBV-Positive Gastric Cancer Models by Suppressing PPARα" Microorganisms 13, no. 7: 1514. https://doi.org/10.3390/microorganisms13071514
APA StyleWu, Q., Ye, G., Xu, X., Zeng, X., Wu, B., Xin, F., Zhang, L., Lin, X., Lin, X., & Chen, W. (2025). EBV-Derived miR-BART20-3p Influences Proliferation and Migration in EBV-Positive Gastric Cancer Models by Suppressing PPARα. Microorganisms, 13(7), 1514. https://doi.org/10.3390/microorganisms13071514





