Cysteine-Mediated Root Growth Promotion in Strawberry (Fragaria × ananassa) Induced by TgSWO-Overexpressing Trichoderma
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Cultivation
2.2. Treatment of Strawberry with T. guizhouense NJAU4742
2.3. Root Colonization of NJAU4742 via Quantitative PCR
2.4. Observation of Root Structure via SEM
2.5. Transcriptome Analysis
2.6. Root Metabolite Composition Analysis
2.7. Statistical Analysis
3. Results
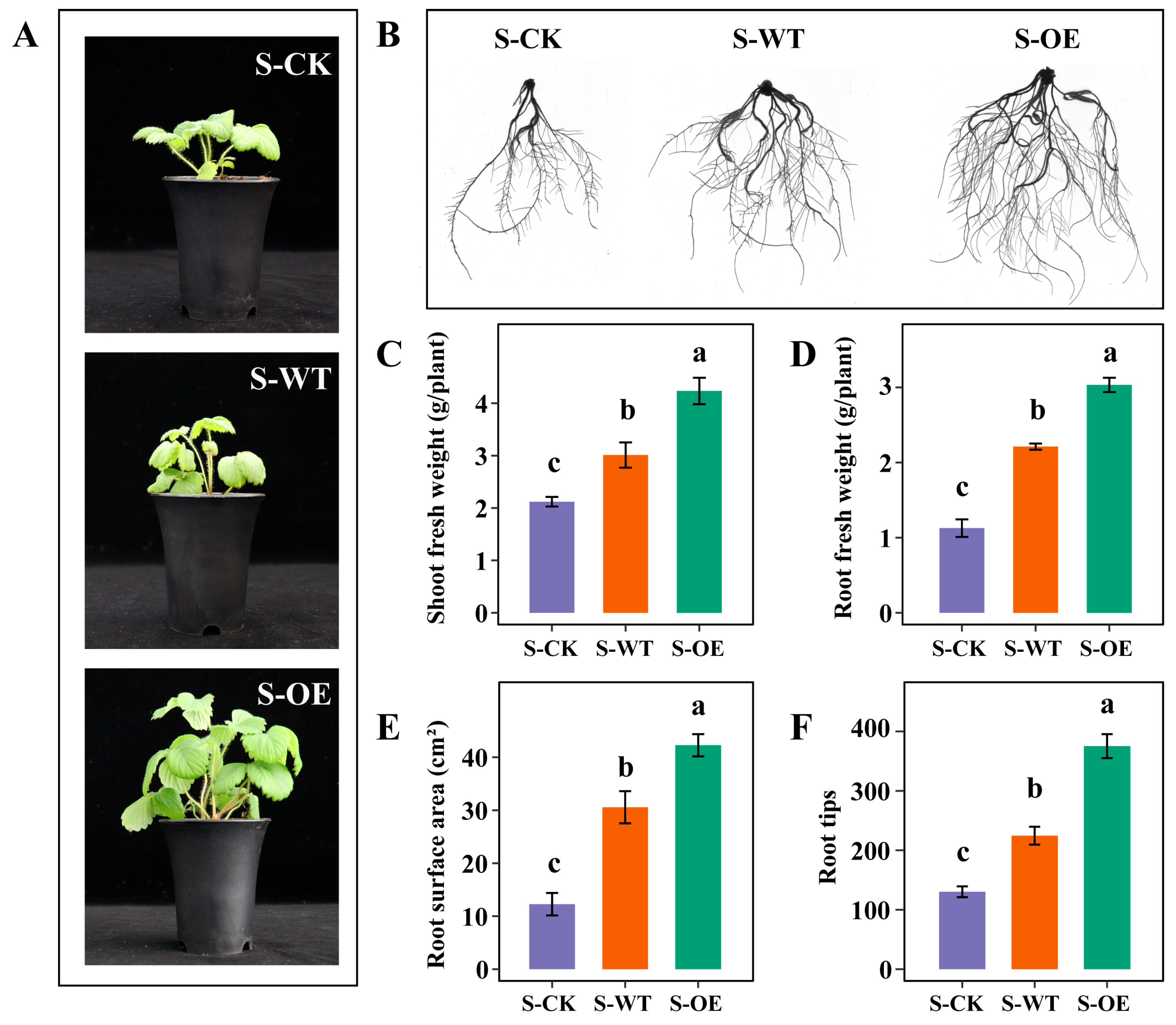
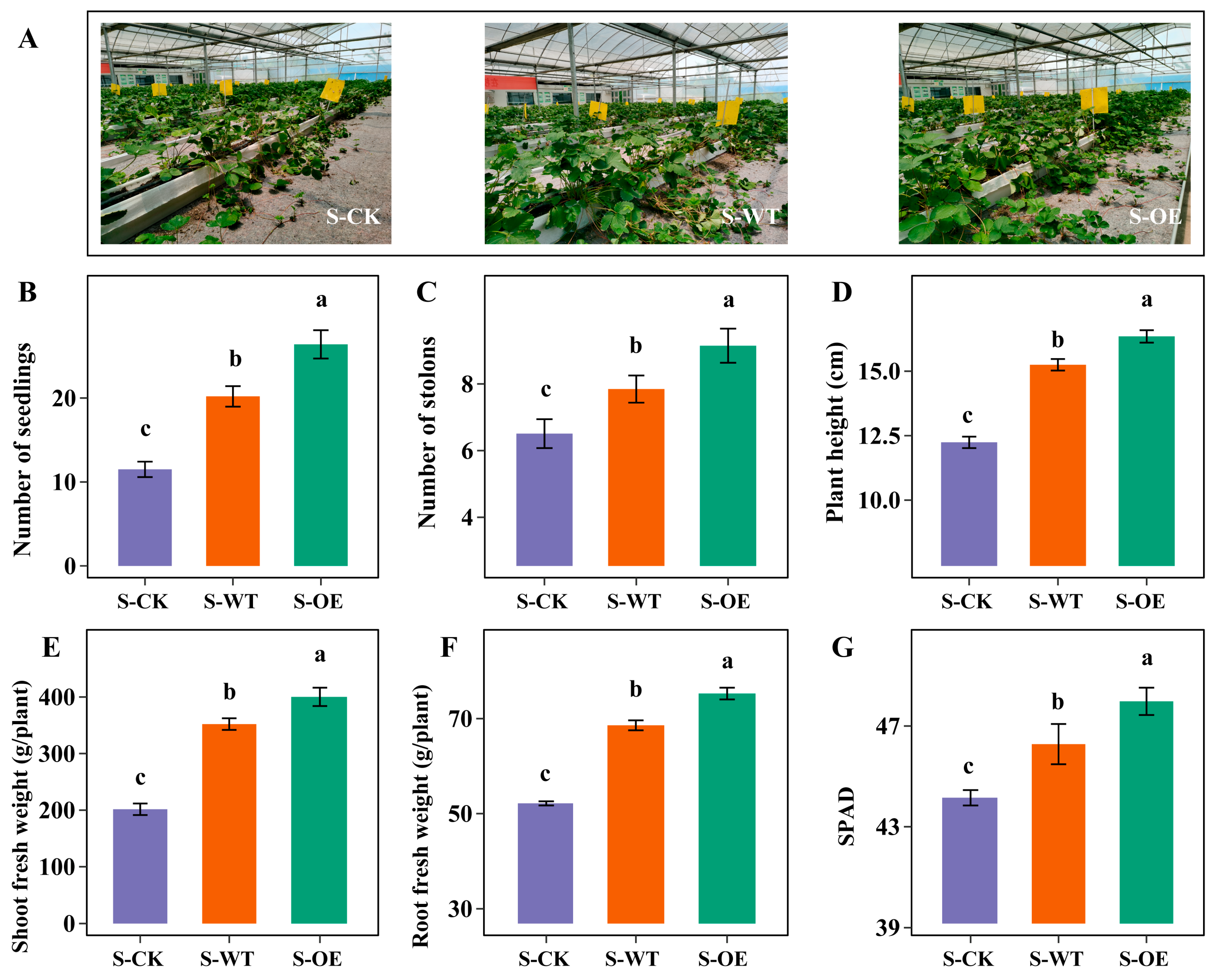
3.1. TgSWO-Overexpressing Trichoderma Improves the Growth of Strawberry Seedlings and Field Seedlings
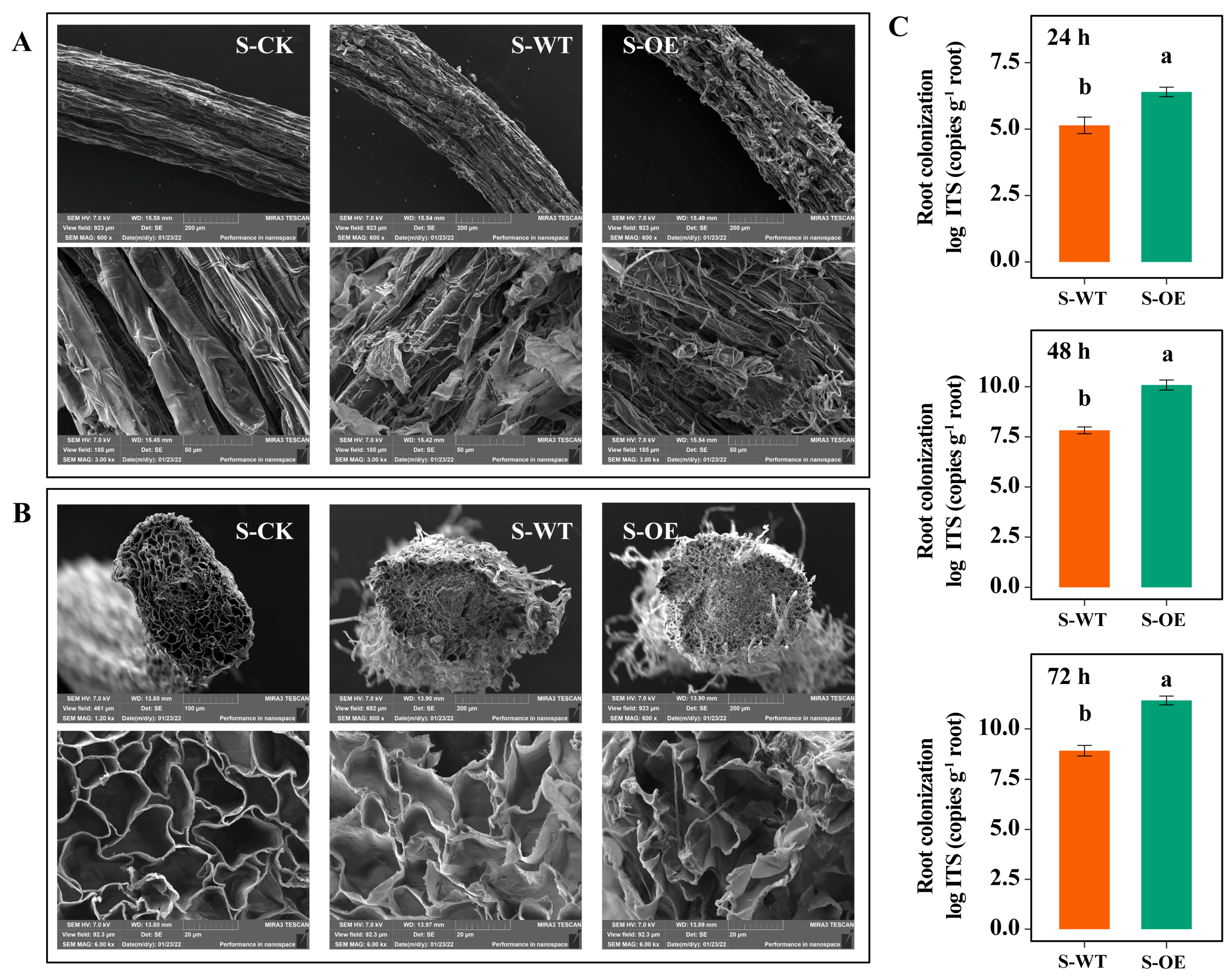
3.2. TgSWO-Overexpressing Trichoderma Significantly Affects the Root Colonization and Root Architecture
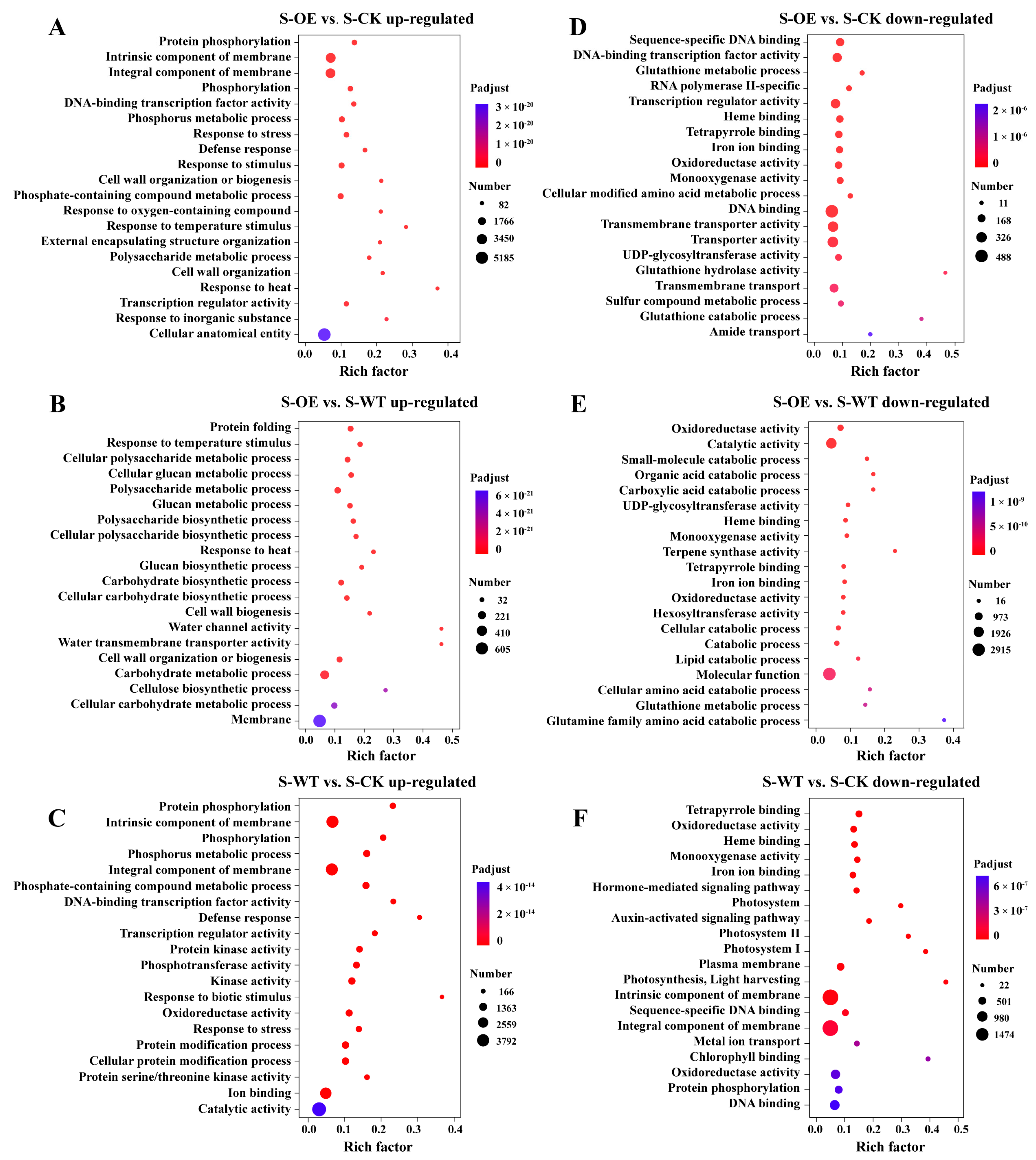
3.3. Transcriptomic Analysis Reveals TgSWO-Induced Molecular Mechanisms Underlying Trichoderma-Mediated Strawberry Growth Promotion
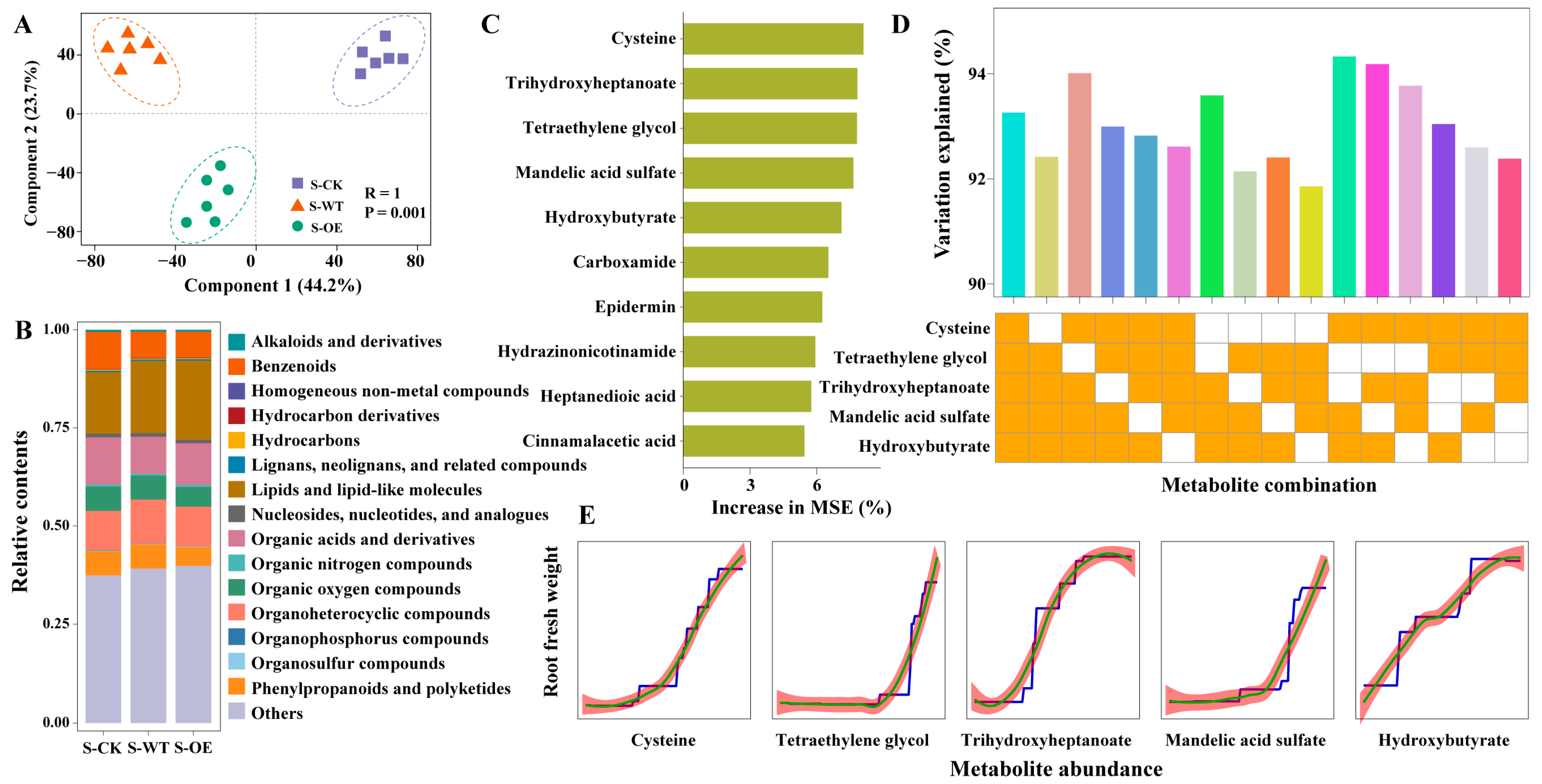
3.4. Root Metabolic Profiling Uncovers Cysteine Associated with TgSWO-Enhanced Strawberry Performance
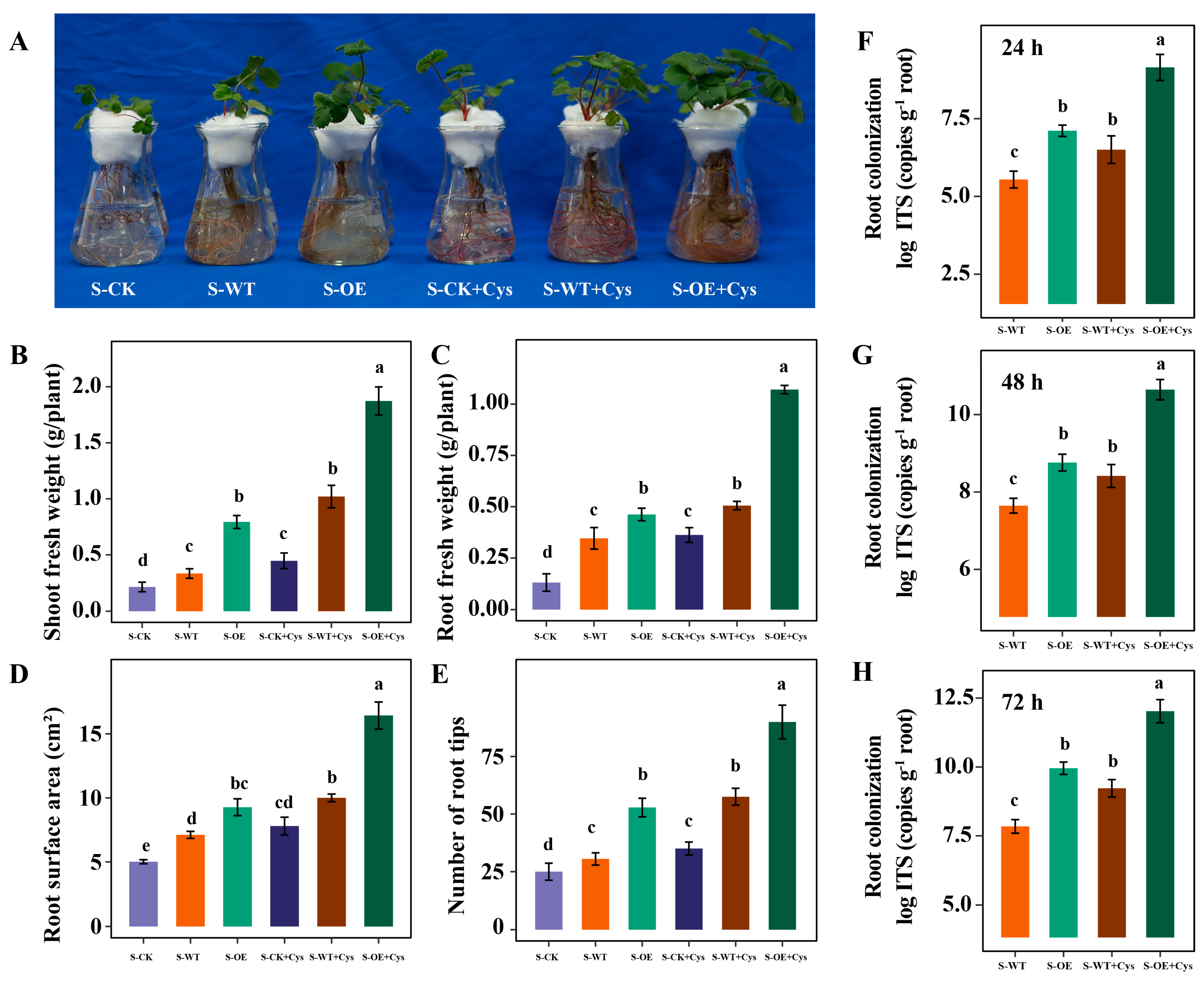
3.5. Cysteine Application Further Promotes the Trichoderma Colonization and Strawberry Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, M.; Oh, D.H.; Haas, J.S.; Hernandez, A.; Hong, H.; Ali, S.; Yun, D.J.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J.; et al. The genome of the extremophile crucifer Thellungiella parvula. Nat. Genet. 2011, 43, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, T.; Kang, C. Molecular bases of strawberry fruit quality traits: Advances, challenges, and opportunities. Plant Physiol. 2023, 193, 900–914. [Google Scholar] [CrossRef]
- de Souza Ribeiro, M.; Rodrigues, F.A.; De Araujo, R.C.; Nadal, M.C.; Andrade, G.V.S.; Pasqual, M.; Rodrigues, J.D. Growth-Promoting Bacteria Induce Salt Stress Tolerance in Strawberry Plants. J. Plant Growth Regul. 2023, 42, 7606–7613. [Google Scholar] [CrossRef]
- Kleifeld, O.; Chet, I. Trichoderma harzianum—Interaction with plants and effect on growth response. Plant Soil 1992, 144, 267–272. [Google Scholar] [CrossRef]
- Meng, X.; Miao, Y.; Liu, Q.; Ma, L.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. TgSWO from Trichoderma guizhouense NJAU4742 promotes growth in cucumber plants by modifying the root morphology and the cell wall architecture. Microb. Cell Factories 2019, 18, 148. [Google Scholar] [CrossRef]
- Stewart, A.; Hill, R. Applications of Trichoderma in plant growth promotion. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Bean, K.M.; Kisiala, A.B.; Morrison, E.N.; Emery, R.N. Trichoderma synthesizes cytokinins and alters cytokinin dynamics of inoculated Arabidopsis seedlings. J. Plant Growth Regul. 2022, 41, 2678–2694. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Chen, J.; Wang, R.; Zhang, J.; Hou, J.; Liu, T. Insights into the molecular mechanism of Trichoderma stimulating plant growth and immunity against phytopathogens. Physiol. Plant. 2023, 175, e14133. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Boamah, S.; Ojangba, T.; Zhang, S.; Zhu, N.; Osei, R.; John Tiika, R.; Boakye, T.A.; Khurshid, A.; Inayat, R.; Effah, Z. Evaluation of salicylic acid (SA) signaling pathways and molecular markers in Trichoderma-treated plants under salinity and Fusarium stresses. A Review. Eur. J. Plant Pathol. 2023, 166, 259–274. [Google Scholar] [CrossRef]
- Galeano, R.M.S.; de Oliveira Simas, A.L.; Ribeiro, J.V.S.; de Alencar Guimarães, N.C.; Viana, T.F.C.; Masui, D.C.; Corrêa, B.O.; Giannesi, G.C.; de Lima, S.F.; da Silva Brasil, M. Phosphorus-solubilizing Trichoderma strains: Mechanisms to promote soybean growth and support sustainable agroecosystems. Plant Soil. 2025, 1–21. [Google Scholar] [CrossRef]
- de Assis, M.A.; da Silva, J.J.; de Carvalho, L.M.; Parreiras, L.S.; Cairo, J.P.L.; Marone, M.P.; Gonçalves, T.A.; Silva, D.S.; Dantzger, M.; de Figueiredo, F.L. A Multiomics Perspective on Plant Cell Wall-Degrading Enzyme Production: Insights from the Unexploited Fungus Trichoderma erinaceum. J. Fungi 2024, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Turaeva, B.; Soliev, A.; Eshboev, F.; Kamolov, L.; Azimova, N.; Karimov, H.; Zukhritdinova, N.; Khamidova, K. The use of three fungal strains in producing of indole-3-acetic acid and gibberellic acid. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 32–43. [Google Scholar]
- Miao, J.; Wang, M.; Ma, L.; Li, T.; Huang, Q.; Liu, D.; Shen, Q. Effects of amino acids on the lignocellulose degradation by Aspergillus fumigatus Z5: Insights into performance, transcriptional, and proteomic profiles. Biotechnol. Biofuels 2019, 12, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Zhu, H.; Zhou, Y.; Shen, Q.; Liu, D. Cysteine facilitates the lignocellulolytic response of Trichoderma guizhouense NJAU4742 by indirectly up-regulating membrane sugar transporters. Biotechnol. Biofuels Bioprod. 2023, 16, 159. [Google Scholar] [CrossRef]
- Panicker, S.; Sayyed, R. Hydrolytic enzymes from PGPR against plant fungal pathogens. In Antifungal Metabolites of Rhizobacteria for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2022; pp. 211–238. [Google Scholar]
- Moreno-Sánchez, I.; Pejenaute-Ochoa, M.D.; Navarrete, B.; Barrales, R.R.; Ibeas, J.I. Ustilago maydis secreted endo-xylanases are involved in fungal filamentation and proliferation on and inside plants. J. Fungi 2021, 7, 1081. [Google Scholar] [CrossRef]
- Viterbo, A.; Chet, I. TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol. Plant Pathol. 2006, 7, 249–258. [Google Scholar] [CrossRef]
- Morán-Diez, E.; Hermosa, R.; Ambrosino, P.; Cardoza, R.E.; Gutiérrez, S.; Lorito, M.; Monte, E. The ThPG1 endopolygalacturonase is required for the Trichoderma harzianum–plant beneficial interaction. Mol. Plant-Microbe Interact. 2009, 22, 1021–1031. [Google Scholar] [CrossRef]
- Yan, J.; Wang, M.; Zeng, H.; Yang, H.; Lv, K.; Zhou, Z.; Hou, Y.; Zhang, J.; Kong, N.; Wu, J. Ti3C2Tx MXene nanosheets protect Torreya grandis against root rot disease. Chem. Eng. J. 2024, 481, 148687. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Liu, X.; Wang, R.; Chen, W.; Suo, J.; Yan, J.; Wu, J. Agrobacterium-mediated transient expression in Torreya grandis cones: A simple and rapid tool for gene expression and functional gene assay. Sci. Hortic. 2024, 338, 113664. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Zhao, J.; Liu, Y.; Chen, Z.; Tang, Z.; Tian, W.; Xi, Y.; Zhang, J. Long-term fertilization lowers the alkaline phosphatase activity by impacting the phoD-harboring bacterial community in rice-winter wheat rotation system. Sci. Total Environ. 2022, 821, 153406. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Haggblom, M.; Krumins, V.; Dong, Y.; Sun, X.; Li, F.; Wang, Q.; Li, B.; Yan, B. Bacterial Survival Strategies in an Alkaline Tailing Site and the Physiological Mechanisms of Dominant Phylotypes As Revealed by Metagenomic Analyses. Environ. Sci. Technol. 2018, 52, 13370–13380. [Google Scholar] [CrossRef]
- Wei, X.; Hu, Y.; Cai, G.; Yao, H.; Ye, J.; Sun, Q.; Veresoglou, S.D.; Li, Y.; Zhu, Z.; Guggenberger, G.; et al. Organic phosphorus availability shapes the diversity of phoD-harboring bacteria in agricultural soil. Soil. Biol. Biochem. 2021, 161, 108364. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root development and nutrient uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges and perspective. Plant J. 2022, 110, 23–42. [Google Scholar] [CrossRef]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- He, Z.; Webster, S.; He, S.Y. Growth–defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Bidirectional interaction between phyllospheric microbiotas and plant volatile emissions. Trends Plant Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dean, R.A. Movement of small RNAs in and between plants and fungi. Mol. Plant Pathol. 2020, 21, 589–601. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Q.; Zhang, S.; Noll, L.; Wanek, W. Significant release and microbial utilization of amino sugars and D-amino acid enantiomers from microbial cell wall decomposition in soils. Soil Biol. Biochem. 2018, 123, 115–125. [Google Scholar] [CrossRef]
- Sukumar, P.; Legue, V.; Vayssieres, A.; Martin, F.; Tuskan, G.A.; Kalluri, U.C. Involvement of auxin pathways in modulating root architecture during beneficial plant–microorganism interactions. Plant Cell Environ. 2013, 36, 909–919. [Google Scholar] [CrossRef]
- Raspanti, E.; Cacciola, S.O.; Gotor, C.; Romero, L.C.; García, I. Implications of cysteine metabolism in the heavy metal response in Trichoderma harzianum and in three Fusarium species. Chemosphere 2009, 76, 48–54. [Google Scholar] [CrossRef]
- Tikhomirova, A.; Rahman, M.M.; Kidd, S.P.; Ferrero, R.L.; Roujeinikova, A. Cysteine and resistance to oxidative stress: Implications for virulence and antibiotic resistance. Trends Microbiol. 2024, 32, 93–104. [Google Scholar] [CrossRef]
- Quarantin, A.; Castiglioni, C.; Schäfer, W.; Favaron, F.; Sella, L. The Fusarium graminearum cerato-platanins loosen cellulose substrates enhancing fungal cellulase activity as expansin-like proteins. Plant Physiol. Biochem. 2019, 139, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Perini, M.A.; Sin, I.N.; Villarreal, N.M.; Marina, M.; Powell, A.; Martinez, G.A.; Civello, P.M. Overexpression of the carbohydrate binding module from Solanum lycopersicum expansin 1 (Sl-EXP1) modifies tomato fruit firmness and Botrytis cinerea susceptibility. Plant Physiol. Biochem. 2017, 113, 122–132. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Wang, Y.; Zhang, X.; Yang, H.; Lu, Y.; Xu, Y.; Zhang, X.; Yan, Z. Cysteine-Mediated Root Growth Promotion in Strawberry (Fragaria × ananassa) Induced by TgSWO-Overexpressing Trichoderma. Microorganisms 2025, 13, 1480. https://doi.org/10.3390/microorganisms13071480
Meng X, Wang Y, Zhang X, Yang H, Lu Y, Xu Y, Zhang X, Yan Z. Cysteine-Mediated Root Growth Promotion in Strawberry (Fragaria × ananassa) Induced by TgSWO-Overexpressing Trichoderma. Microorganisms. 2025; 13(7):1480. https://doi.org/10.3390/microorganisms13071480
Chicago/Turabian StyleMeng, Xiaohui, Yuanhua Wang, Xu Zhang, Hongjun Yang, Yilei Lu, Ye Xu, Xiong Zhang, and Zhiming Yan. 2025. "Cysteine-Mediated Root Growth Promotion in Strawberry (Fragaria × ananassa) Induced by TgSWO-Overexpressing Trichoderma" Microorganisms 13, no. 7: 1480. https://doi.org/10.3390/microorganisms13071480
APA StyleMeng, X., Wang, Y., Zhang, X., Yang, H., Lu, Y., Xu, Y., Zhang, X., & Yan, Z. (2025). Cysteine-Mediated Root Growth Promotion in Strawberry (Fragaria × ananassa) Induced by TgSWO-Overexpressing Trichoderma. Microorganisms, 13(7), 1480. https://doi.org/10.3390/microorganisms13071480




