Postbiotics: An Alternative for Improving Health and Performance of Poultry Production
Abstract
1. Introduction
2. Postbiotics: Understanding the Concept and the Mechanisms of Action
- Immunomodulation: postbiotics promote selectivity, cytotoxicity against tumor cells, protection of the intestinal epithelium through apoptosis of normal epithelial cells, and improvement in IgA, IFN-γ, and IL-10 secretion [27]. In addition, they induce differentiation of T lymphocytes to establish the balance between Th1 and Th2 lymphocytes [8].
- Prevention of intestinal infection: postbiotics act as an intestinal barrier by altering the expression of host genes or modulating the local environment [28]. Also, they exercise the inactivation of pathogens through the production of short-chain organic acids and/or antimicrobial peptides, causing antagonistic intervention with pathogens by adhering to intestinal epithelial cells and promoting the production of IgA [29].
- Oxidative stress: postbiotics display antioxidant and anti-inflammatory actions to combat diseases that affect the gastrointestinal tract [12].
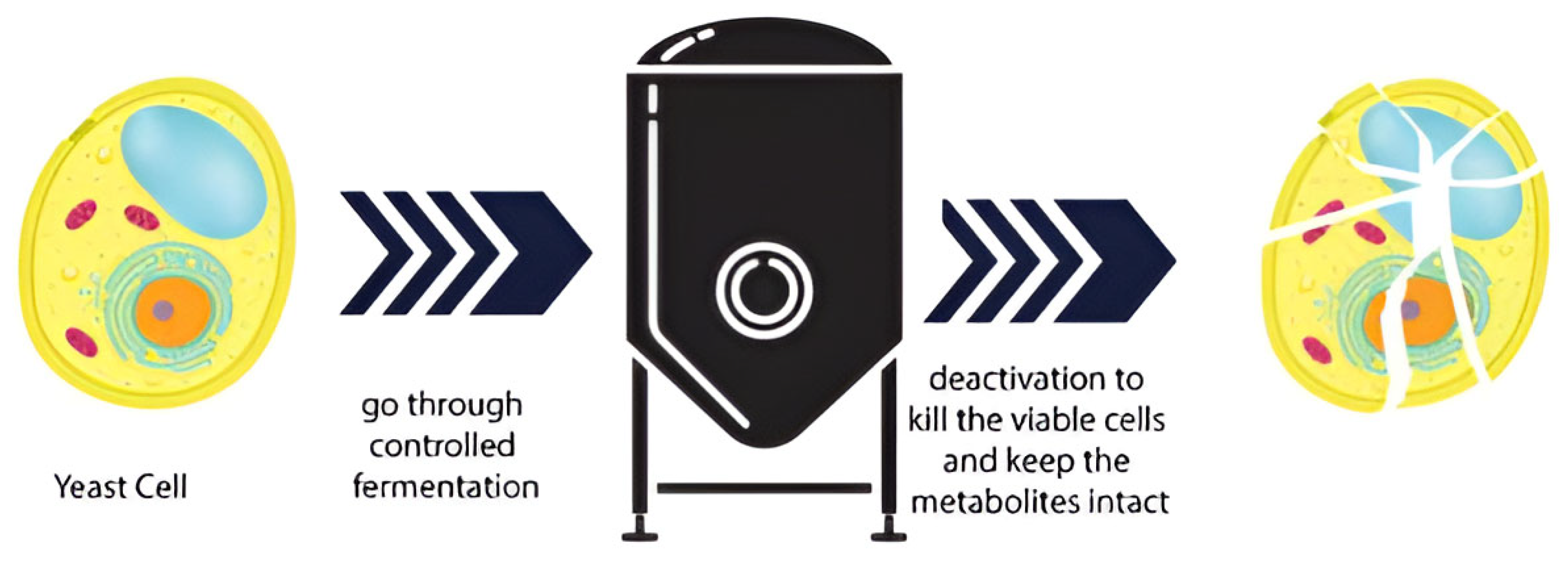
3. Postbiotics: Methods of Production and the Main Compounds
4. Eubiosis and Gut Health: Microbiota and Gut Barrier
5. Reported Impacts on Productivity and Poultry Health
5.1. Antioxidant Action and Meat Quality Interactions
5.2. Intestinal Morphology and Performance Enhance
5.3. Immune System Modulation and Pathogen Control
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the Microbiota for Microbial and Metabolite-Based Immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of Functional Food Components on Gut Health. Crit. Rev. Food Sci. Nutr. 2018, 59, 1927–1936. [Google Scholar] [CrossRef]
- Sun, X.; Zhai, J. Research Status and Trends of Gut Microbiota and Intestinal Diseases Based on Bibliometrics. Microorganisms 2025, 13, 673. [Google Scholar] [CrossRef]
- Mosca, F.; Giannì, M.L.; Rescigno, M. Can Postbiotics Represent a New Strategy for NEC? Probiotics Child Gastrointest. Health: Adv. Microbiol. Infect. Dis. Public Health 2019, 10, 37–45. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Chambers, L.; Avery, A.; Dalrymple, J.; Farrell, L.; Gibson, G.; Harrington, J.; Rijkers, G.; Rowland, I.; Spiro, A.; Varela-Moreiras, G.; et al. Translating Probiotic Science into Practice. Nutr. Bull. 2019, 44, 165–173. [Google Scholar] [CrossRef]
- Rad, A.H.; Abbasi, A.; Kafil, H.S.; Ganbarov, K. Potential Pharmaceutical and Food Applications of Postbiotics: A Review. Curr. Pharm. Biotechnol. 2020, 21, 1576–1587. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary Supplementation of Postbiotics Mitigates Adverse Impacts of Heat Stress on Antioxidant Enzyme Activity, Total Antioxidant, Lipid Peroxidation, Physiological Stress Indicators, Lipid Profile and Meat Quality in Broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.-Y.; Lin, L.-J.; Hsieh, Y.-C.; Chang, S.-C.; Lee, T.-T. Effects of Saccharomyces Cerevisiae and Phytase Co-Fermentation of Wheat Bran on Growth, Antioxidation, Immunity and Intestinal Morphology in Broilers. Anim. Biosci. 2021, 34, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, E.; Frana, T.; Logue, C.M.; Smith, D.P.; Pavlidis, H.O.; Chaney, W.E. Effect of Feeding a Postbiotic Derived from Saccharomyces Cerevisiae Fermentation as a Preharvest Food Safety Hurdle for Reducing Salmonella Enteritidis in the Ceca of Layer Pullets. J. Food Prot. 2021, 84, 275–280. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Salminen, S.J. The Health Effects of Cultured Milk Products with Viable and Non-Viable Bacteria. Int. Dairy J. 1998, 8, 749–758. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Akit, H.; Samsudin, A.A. Effects of Dietary Postbiotic and Inulin on Growth Performance, IGF1 and GHR MRNA Expression, Faecal Microbiota and Volatile Fatty Acids in Broilers. BMC Vet. Res. 2016, 12, 163. [Google Scholar] [CrossRef]
- Zhang, M.; Li, D.; Yang, X.; Wei, F.; Wen, Q.; Feng, Y.; Jin, X.; Liu, D.; Guo, Y.; Hu, Y. Integrated Multi-Omics Reveals the Roles of Cecal Microbiota and Its Derived Bacterial Consortium in Promoting Chicken Growth. mSystems 2023, 8, e0084423. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Jastrząb, R.; Graczyk, D.; Siedlecki, P. Molecular and Cellular Mechanisms Influenced by Postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A Review on Preparation and Chemical Analysis of Postbiotics from Lactic Acid Bacteria. Enzym. Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- María José, H.-G.; Elena, F.-R. Postbiotics in Human Health: Possible New Functional Ingredients? Food Res. Int. 2020, 137, 109660. [Google Scholar] [CrossRef]
- Zawistowska-Rojek, A.; Tyski, S. How to Improve Health with Biological Agents—Narrative Review. Nutrients 2022, 14, 1700. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Cell-Free Supernatants from Cultures of Lactic Acid Bacteria Isolated from Fermented Grape as Biocontrol against Salmonella Typhi and Salmonella Typhimurium Virulence via Autoinducer-2 and Biofilm Interference. PeerJ 2019, 7, e7555. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut Microbiota Modulation: A Novel Strategy for Prevention and Treatment of Colorectal Cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Khodaii, Z.; Ghaderian, S.M.H.; Natanzi, M.M. Probiotic Bacteria and Their Supernatants Protect Enterocyte Cell Lines from Enteroinvasive Escherichia Coli (EIEC) Invasion. Int. J. Mol. Cell. Med. 2017, 6, 183–189. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Aghebati Maleki, L.; Samadi Kafil, H.; Abbasi, A. Postbiotics: A Novel Strategy in Food Allergy Treatment. Crit. Rev. Food Sci. Nutr. 2020, 61, 492–499. [Google Scholar] [CrossRef]
- Mantziari, A.; Salminen, S.; Szajewska, H.; Malagón-Rojas, J.N. Postbiotics against Pathogens Commonly Involved in Pediatric Infectious Diseases. Microorganisms 2020, 8, 1510. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P. Detection, Isolation, and Purification of Bifidobacterial Exopolysaccharides. Bifidobact. Methods Protoc. 2021, 2278, 101–115. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Di, H.; Deng, Z.; Liu, J.; Wang, H. Gut Health Benefit and Application of Postbiotics in Animal Production. J. Anim. Sci. Biotechnol. 2022, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef]
- León, E.D.; Francino, M.P. Roles of Secretory Immunoglobulin A in Host-Microbiota Interactions in the Gut Ecosystem. Front. Microbiol. 2022, 13, 880484. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
- Pfefferle, P.I.; Prescott, S.L.; Kopp, M. Microbial Influence on Tolerance and Opportunities for Intervention with Prebiotics/Probiotics and Bacterial Lysates. J. Allergy Clin. Immunol. 2013, 131, 1453–1463. [Google Scholar] [CrossRef]
- Fisher, J.F.; Mobashery, S. Constructing and Deconstructing the Bacterial Cell Wall. Protein Sci. 2020, 29, 629–646. [Google Scholar] [CrossRef]
- Hernández, S.B.; Dörr, T.; Waldor, M.K.; Cava, F. Modulation of Peptidoglycan Synthesis by Recycled Cell Wall Tetrapeptides. Cell Rep. 2020, 31, 107578. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Pedroso, A.A.; Mallo, J.J.; Puyalto, M.; Kim, W.K.; Applegate, T.J. Sodium Butyrate Improved Performance While Modulating the Cecal Microbiota and Regulating the Expression of Intestinal Immune-Related Genes of Broiler Chickens. Poult. Sci. 2017, 96, 3981–3993. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kano, H.; Sashihara, T.; Sugano, H.; Horiuchi, H.; Saito, T.; Oda, M. Immunomodulatory Effects of Polysaccharides Produced by Lactobacillus Delbrueckii Ssp. Bulgaricus OLL1073R-1. J. Dairy Sci. 2006, 89, 2873–2881. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, X.; Min, W.; Yang, Z. Characterization and Immunomodulatory Activity of an Exopolysaccharide Produced by Lactobacillus Plantarum JLK0142 Isolated from Fermented Dairy Tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, High Production and Antimicrobial Activity of Exopolysaccharides from Lactococcus Lactis F-Mou. Microb. Pathog. 2019, 132, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Pillay, C.; Nyaga, M.M.; Sabiu, S. Poultry gut health—Microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. J. Anim. Sci. Biotechnol. 2021, 12, 119. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10 (Suppl. 1), S17–S30. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Mardani, K.; Ezati, P. Efficacy of lyophilized cell-free supernatant of Lactobacillus salivarius (Ls-BU2) on Escherichia coli and shelf life of ground beef. Vet. Res. Forum 2019, 10, 193–198. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Alam, A.; Neish, A. Role of Gut Microbiota in Intestinal Wound Healing and Barrier Function. Tissue Barriers 2018, 6, 1539595. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Fischer, J.; Ganesan, R.; Hos, N.J.; Cildir, G.; Wolke, M.; Pessia, A.; Frommolt, P.; Desiderio, V.; Velagapudi, V.; et al. Salmonella Typhimurium Impairs Glycolysis-Mediated Acidification of Phagosomes to Evade Macrophage Defense. PLoS Pathog. 2021, 17, e1009943. [Google Scholar] [CrossRef] [PubMed]
- Hortová-Kohoutková, M.; Lázničková, P.; Frič, J. How Immune-Cell Fate and Function Are Determined by Metabolic Pathway Choice. BioEssays 2021, 43, 2000067. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Slack, E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr. Opin. Gas-troenterol. 2009, 23, 673–678. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- Karimi, S.; Rashidian, E.; Birjandi, M.; Mahmoodnia, L. Antagonistic Effect of Isolated Probiotic Bacteria from Natural Sources against Intestinal Escherichia Coli Pathotypes. Electron. Physician 2018, 10, 6534–6539. [Google Scholar] [CrossRef]
- Pascual, A.; Pauletto, M.; Giantin, M.; Radaelli, G.; Ballarin, C.; Birolo, M.; Zomeño, C.; Dacasto, M.; MBortoletti; Vascellari, M.; et al. Effect of Dietary Supplementation with Yeast Cell Wall Extracts on Performance and Gut Response in Broiler Chickens. J. Anim. Sci. Biotechnol. /J. Anim. Sci. Biotechnol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, T.T. The Laetiporus Sulphureus Fermented Product Enhances the Antioxidant Status, Intestinal Tight Junction, and Morphology of Broiler Chickens. Animals 2021, 11, 149. [Google Scholar] [CrossRef]
- Thanh, N.T.; Loh, T.C.; Foo, H.L.; Hair-bejo, M.; Azhar, B.K. Effects of Feeding Metabolite Combinations Produced by Lactobacillus Plantarum on Growth Performance, Faecal Microbial Population, Small Intestine Villus Height and Faecal Volatile Fatty Acids in Broilers. Br. Poult. Sci. 2009, 50, 298–306. [Google Scholar] [CrossRef]
- Soumeh, E.A.; Mohebodini, H.; Toghyani, M.; Shabani, A.; Ashayerizadeh, A.; Jazi, V. Synergistic Effects of Fermented Soybean Meal and Mannan-Oligosaccharide on Growth Performance, Digestive Functions, and Hepatic Gene Expression in Broiler Chickens. Poult. Sci. 2019, 98, 6797–6807. [Google Scholar] [CrossRef]
- Lai, L.P.; Lee, M.T.; Chen, C.S.; Yu, B.; Lee, T.T. Effects of Co-Fermented Pleurotus Eryngii Stalk Residues and Soybean Hulls by Aureobasidium Pullulans on Performance and Intestinal Morphology in Broiler Chickens. Poult. Sci. 2015, 94, 2959–2969. [Google Scholar] [CrossRef]
- Nelson, J.R.; Sobotik, E.B.; Athrey, G.; Archer, G.S. Effects of Supplementing Yeast Fermentate in the Feed or Drinking Water on Stress Susceptibility, Plasma Chemistry, Cytokine Levels, Antioxidant Status, and Stress- and Immune-Related Gene Expression of Broiler Chickens. Poult. Sci. 2020, 99, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.E.C.; Branton, S.L.; Evans, J.D.; Leigh, S.A.; Kim, E.J.; Olanrewaju, H.A.; Pharr, G.T.; Pavlidis, H.O.; Gerard, P.D.; Peebles, E.D. Growth and Humoral Immune Effects of Dietary Original XPC in Layer Pullets Challenged with Mycoplasma Gallisepticumabc. Poult. Sci. 2020, 99, 3030–3037. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.C.; Thanh, N.T.; Foo, H.L.; Hair-Bejo, M.; Azhar, B.K. Feeding of Different Levels of Metabolite Combinations Produced by Lactobacillus Plantarum on Growth Performance, Fecal Microflora, Volatile Fatty Acids and Villi Height in Broilers. Anim. Sci. J. 2010, 81, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Price, P.T.; Byrd, J.A.; Alvarado, C.Z.; Pavlidis, H.O.; McIntyre, D.R.; Archer, G.S. Utilizing Original XPCTM in Feed to Reduce Stress Susceptibility of Broilers. Poult. Sci. 2018, 97, 855–859. [Google Scholar] [CrossRef]
- Aristides, L.G.A.; Venancio, E.J.; Alfieri, A.A.; Otonel, R.A.A.; Frank, W.J.; Oba, A. Carcass Characteristics and Meat Quality of Broilers Fed with Different Levels of Saccharomyces Cerevisiae Fermentation Product. Poult. Sci. 2018, 97, 3337–3342. [Google Scholar] [CrossRef]
- Choe, D.W.; Loh, T.C.; Foo, H.L.; Hair-Bejo, M.; Awis, Q.S. Egg Production, Faecal PH and Microbial Population, Small Intestine Morphology, and Plasma and Yolk Cholesterol in Laying Hens given Liquid Metabolites Produced by Lactobacillus Plantarum Strains. Br. Poult. Sci. 2012, 53, 106–115. [Google Scholar] [CrossRef]
- West, C.; Stanisz, A.M.; Wong, A.; Kunze, W.A. Effects of Saccharomyces Cerevisiae or Boulardii Yeasts on Acute Stress Induced Intestinal Dysmotility. World J. Gastroenterol. 2016, 22, 10532. [Google Scholar] [CrossRef]
- Takadanohara, H.; Catanzaro, R.; Chui, D.H.; He, F.; Yadav, H.; Ganguli, A.; Sakata, Y.; Solimene, U.; Minelli, E.; Kobayashi, R.; et al. Beneficial Effect of a Symbiotic Preparation with S. Boulardii Lysate in Mild Stress-Induced Gut Hyper-Permeability. Acta Biomed 2012, 83, 208–216. [Google Scholar]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How Do Intestinal Probiotics Restore the Intestinal Barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Van Coillie, E.; Goris, J.; Cleenwerck, I.; Grijspeerdt, K.; Botteldoorn, N.; Van Immerseel, F.; De Buck, J.; Vancanneyt, M.; Swings, J.; Herman, L.; et al. Identification of Lactobacilli Isolated from the Cloaca and Vagina of Laying Hens and Characterization for Potential Use as Probiotics to Control Salmonella Enteritidis. J. Appl. Microbiol. 2006, 102, 1095–1106. [Google Scholar] [CrossRef]
- Canonici, A.; Siret, C.; Pellegrino, E.; Pontier-Bres, R.; Pouyet, L.; Montero, M.P.; Colin, C.; Czerucka, D.; Rigot, V.; André, F. Saccharomyces Boulardii Improves Intestinal Cell Restitution through Activation of the α2β1 Integrin Collagen Receptor. PLoS ONE 2011, 6, e18427. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarimi, R.; Daneshyar, M.; Aghazadeh, A. Thyme (Thymus Vulgaris) Extract Consumption Darkens Liver, Lowers Blood Cholesterol, Proportional Liver and Abdominal Fat Weights in Broiler Chickens. Ital. J. Anim. Sci. 2011, 10, e20. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of Feeding Different Postbiotics Produced by Lactobacillus Plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Seidler, Y.; Bailey, H.R.; Whitacre, L.K.; Bargo, F.; Lüersen, K.; Rimbach, G.; Pighetti, G.M.; Ipharraguerre, I.R.; Ríus, A.G. A Postbiotic from Aspergillus Oryzae Attenuates the Impact of Heat Stress in Ectothermic and Endothermic Organisms. Sci. Rep. 2021, 11, 6407. [Google Scholar] [CrossRef]
- Najafi, P.; Zulkifli, I.; Soleimani, A.F.; Goh, Y.M. Acute Phase Proteins Response to Feed Deprivation in Broiler Chickens. Poult. Sci. 2016, 95, 760–763. [Google Scholar] [CrossRef]
- Jansseune, S.C.; Lammers, A.; van Baal, J.; Blanc, F.; van der Laan, M.H.P.; Calenge, F.; Hendriks, W.H. Diet composition influences probiotic and postbiotic effects on broiler growth and physiology. Poult. Sci. 2024, 103, 103650. [Google Scholar] [CrossRef]
- Jansseune, S.C.; Blanc, F.; Lammers, A.; van Baal, J.; Bruneau, N.; Pinard-van der Laan, M.H.; Hendriks, W.H.; Calenge, F. Microbiota but not immune modulation by a pro- and postbiotic was associated with the diet-additive interaction in broilers. Poult. Sci. 2024, 103, 104184. [Google Scholar] [CrossRef]
- Forder, R.E.; Willson, N.-L.; Angove, J.A.; McWhorter, T.J.; McQueen, M.A.; Cadogan, D.J. Dietary inclusion of a saccharomyces cerevisiae metabolite improved reproductive performance but did not affect intestinal permeability in two chicken meat breeder lines. Poult. Sci. 2024, 103, 103595. [Google Scholar] [CrossRef]
- Ramlucken, U.; Lalloo, R.; Roets, Y.; Moonsamy, G.; van Rensburg, C.J.; Thantsha, M.S. Advantages of bacillus-based probiotics in poultry production. Livest. Sci. 2020, 241, 104215. [Google Scholar] [CrossRef]
- Ramlucken, U.; Ramchuran, S.O.; Moonsamy, G.; Lalloo, R.; Thantsha, M.S.; Jansen van Rensburg, C. A novel bacillus based multi-strain probiotic improves growth performance and intestinal properties of clostridium perfringens challenged broilers. Poult. Sci. 2020, 99, 331–341. [Google Scholar] [CrossRef]
- Ghareeb, K.; Awad, W.A.; Mohnl, M.; Porta, R.; Biarnés, M.; Böhm, J.; Schatzmayr, G. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in Broiler Chickens. Poult. Sci. 2012, 91, 1825–1832. [Google Scholar] [CrossRef] [PubMed]


| Terms 1 | Definition |
|---|---|
| Probiotic | Live microorganisms, administered in adequate amounts: confer a health benefit on the host |
| Prebiotic | Substrate selectively utilized by host microorganisms conferring a health benefit |
| Symbiotic | A mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit to the host |
| Postbiotic | Preparation of inanimate microorganisms and/or their components that confer a health benefit to the host |
| Parabiotic | Non-viable microbial cells, administered in adequate amounts: confer a benefit to the host |
| Exopolysaccharide (EP) | Original Bacteria | Potential Fields of Application | Reference |
|---|---|---|---|
| D-Glucose, D-Galactose, Mannose, Xylose | Lactobacillus delbrueckii ssp. bulgaricus (OLL1073R-1) | (Non-poultry) Fermented milk products, such as yogurt for human consumption | [40] |
| Glucose, Galactose | Lactobacillus plantarum JLK0142 | Potential use as functional food or feed additive for macrophage immunomodulation against pathogens | [41] |
| D-Glucopyranose, D-Galactopyranose | Lactococcus lactis ssp. F-mou (LT898177.1) | Feed additive against pathogens, such as E. coli and Lysteria monocytogenes | [42] |
| Postbiotics | Results | References |
|---|---|---|
| Saccharomyces cerevisiae and phytase co-fermented wheat bran | Birds fed with 10% wheat bran fermented alongside postbiotics showed higher BW, BWG, and FC, improved villus height and number of Lactobacillus spp. in the cecum, and lower expression of pro-inflammatory cytokines. | [13] |
| Yeast cell wall extracts | Postbiotic supplementation decreased feed intake and improved feed conversion, increased villus height and the number of goblet cells, and reduced the density of intestinal inflammatory cells. | [57] |
| Laetiporus sulphureus fermented product | The postbiotic supplementation increased the activity of superoxide dismutase (SOD) and at the same time decreased the concentration of malondialdehyde. | [58] |
| Lactobacillus plantarum RI11 | The postbiotic supplementation increased plasma activities of total antioxidant capacity, catalase, and glutathione and reduced the decrease in α-1-acid-glycoprotein and ceruloplasmin in heat-stressed broilers. | [12] |
| Blend of Lactobacillus plantarum (RS5, RI11, RG14 e RG11) | Results indicate that the blend of probiotics increased final BW, BWG, average daily weight gain and lower FC, increased lactic acid bacteria population, small intestine villus height, and fecal volatile fatty acid population. | [59] |
| Metabolic products synthesized by lactic acid bacteria + Inulin | Results indicate the synergistic effect under probiotics + inulin increased the final BW, and reduced FC, increased villus height in duodenum and ileum and fecal lactic acid bacteria, and decreased fecal pH Enterobacteriaceae. | [17] |
| Fermented soybean meal with or without mannan-oligosaccharides | The use of a postbiotic with a prebiotic increased BWG, reduced FC and reduced plasma 3-methylhistidine concentration, increased villus height and the villus height to crypt depth ratio in the duodenum, increased lactic acid bacteria (fecal, ileal, and cecal), reduced pH (fecal, ileal and cecal) and reduced Clostridium perfringens (ileal and cecal). | [60] |
| Pleurotus eryngii stalk residues | Supplementation of 0.5% postbiotic increased body weight gain, the rate of lactic acid bacteria in relation to pathogens in the cecum, villus height in the ileum, and jejunum and the villus height/crypt depth ratio in birds at 35 days of age. | [61] |
| Fermented Yeast | Yeast fermented supplementation resulted in lower cortisol concentrations in response to acute stress and lower heterophil/lymphocyte ratios in response to chronic stress in broilers when compared to controls. | [62] |
| Saccharomyces cerevisiae fermentation-based metabolites | Reduced the amount of Salmonella Enteritidis colonization compared to the positive control | [14] |
| Lactobacillus plantarum LTC-113 | Chicks challenged with Salmonella Typhimurium and fed Lactobacillus plantarum LTC-113 had less S. Typhimurium colonization in the liver, spleen, and caeca, and intestinal impermeability compared with the animals that did not receive the postbiotic | [41] |
| Saccharomyces cerevisiae fermentation-based metabolites | Dietary treatment of the birds did not significantly affect the weights of the ovaries, bursa, or cecum, but the challenge with Mycoplasma gallisepticum caused a significant impact on the weights at 12 weeks of age. | [63] |
| L. plantarum RS5, RI11, RG14 e RG11 | Broilers fed a combination of postbiotics reduced the number of Enterobacteriaceae and increased the number of lactic acid bacteria, probably due to the lower fecal pH. | [64] |
| Saccharomyces cerevisiae fermentation-based metabolites | Blood corticosterone concentration increased significantly in the absence of heat stress and decreased in the presence of heat stress and improved heterophil/lymphocyte ratio and composite asymmetry score (0.54–1.50; p < 0.0001) compared to control and at 42 days of age under heat stress or during normal rearing conditions | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roque, F.; Lopes, M.H.S.; Raffi, P.; Oliveira, R.; Caparroz, M.; Longhini, G.; Granghelli, C.; Faria, D.; Araujo, L.; Araujo, C. Postbiotics: An Alternative for Improving Health and Performance of Poultry Production. Microorganisms 2025, 13, 1472. https://doi.org/10.3390/microorganisms13071472
Roque F, Lopes MHS, Raffi P, Oliveira R, Caparroz M, Longhini G, Granghelli C, Faria D, Araujo L, Araujo C. Postbiotics: An Alternative for Improving Health and Performance of Poultry Production. Microorganisms. 2025; 13(7):1472. https://doi.org/10.3390/microorganisms13071472
Chicago/Turabian StyleRoque, Fabricia, Mário Henrique Scapin Lopes, Paulo Raffi, Ricardo Oliveira, Márcio Caparroz, Giovana Longhini, Carlos Granghelli, Douglas Faria, Lúcio Araujo, and Cristiane Araujo. 2025. "Postbiotics: An Alternative for Improving Health and Performance of Poultry Production" Microorganisms 13, no. 7: 1472. https://doi.org/10.3390/microorganisms13071472
APA StyleRoque, F., Lopes, M. H. S., Raffi, P., Oliveira, R., Caparroz, M., Longhini, G., Granghelli, C., Faria, D., Araujo, L., & Araujo, C. (2025). Postbiotics: An Alternative for Improving Health and Performance of Poultry Production. Microorganisms, 13(7), 1472. https://doi.org/10.3390/microorganisms13071472






