Germinated Spores of the Probiotic Bacterium Bacillus coagulans JBI-YZ6.3 Support Dynamic Changes in Intestinal Epithelial Communication and Resilience to Mechanical Wounding
Abstract
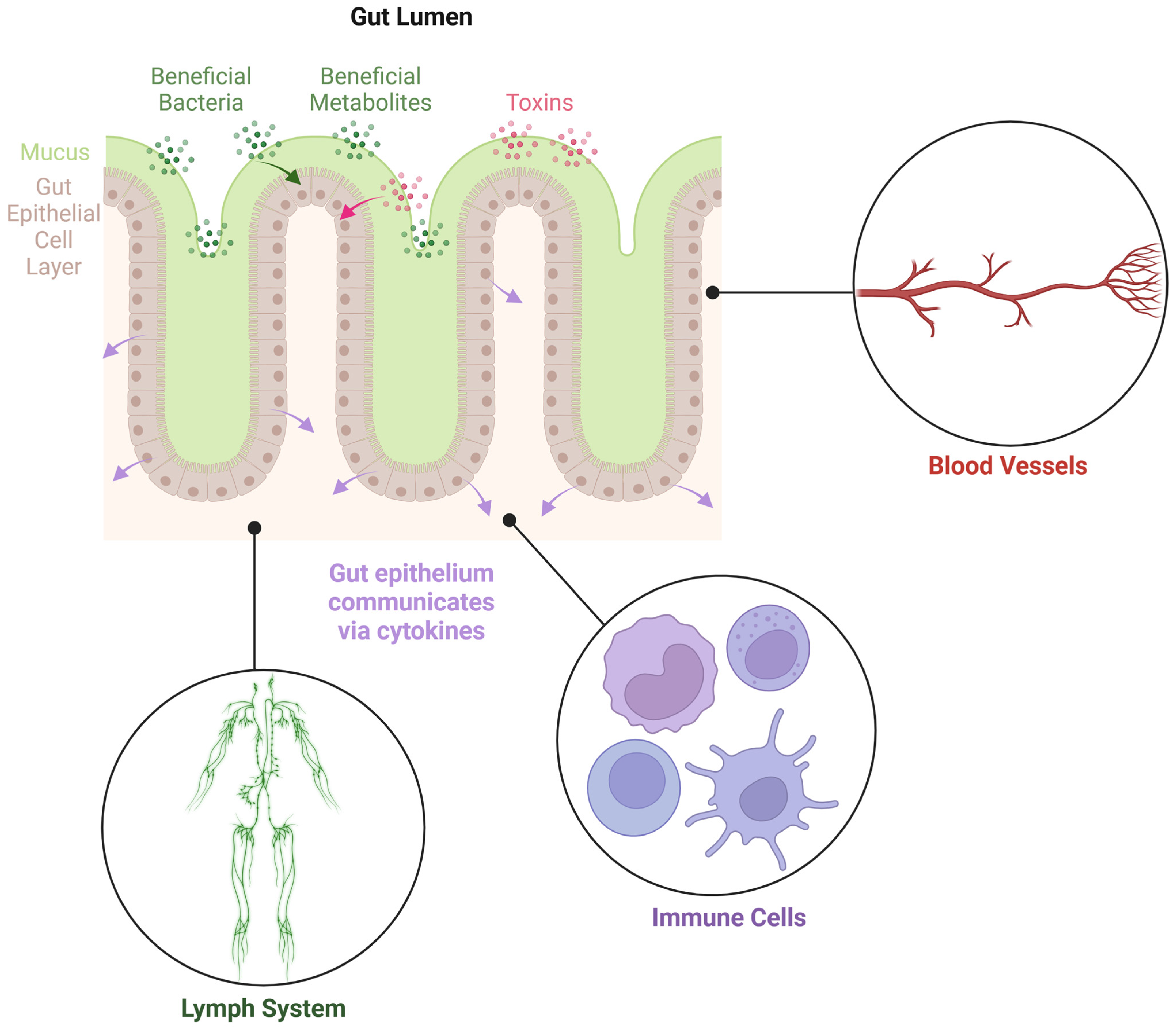
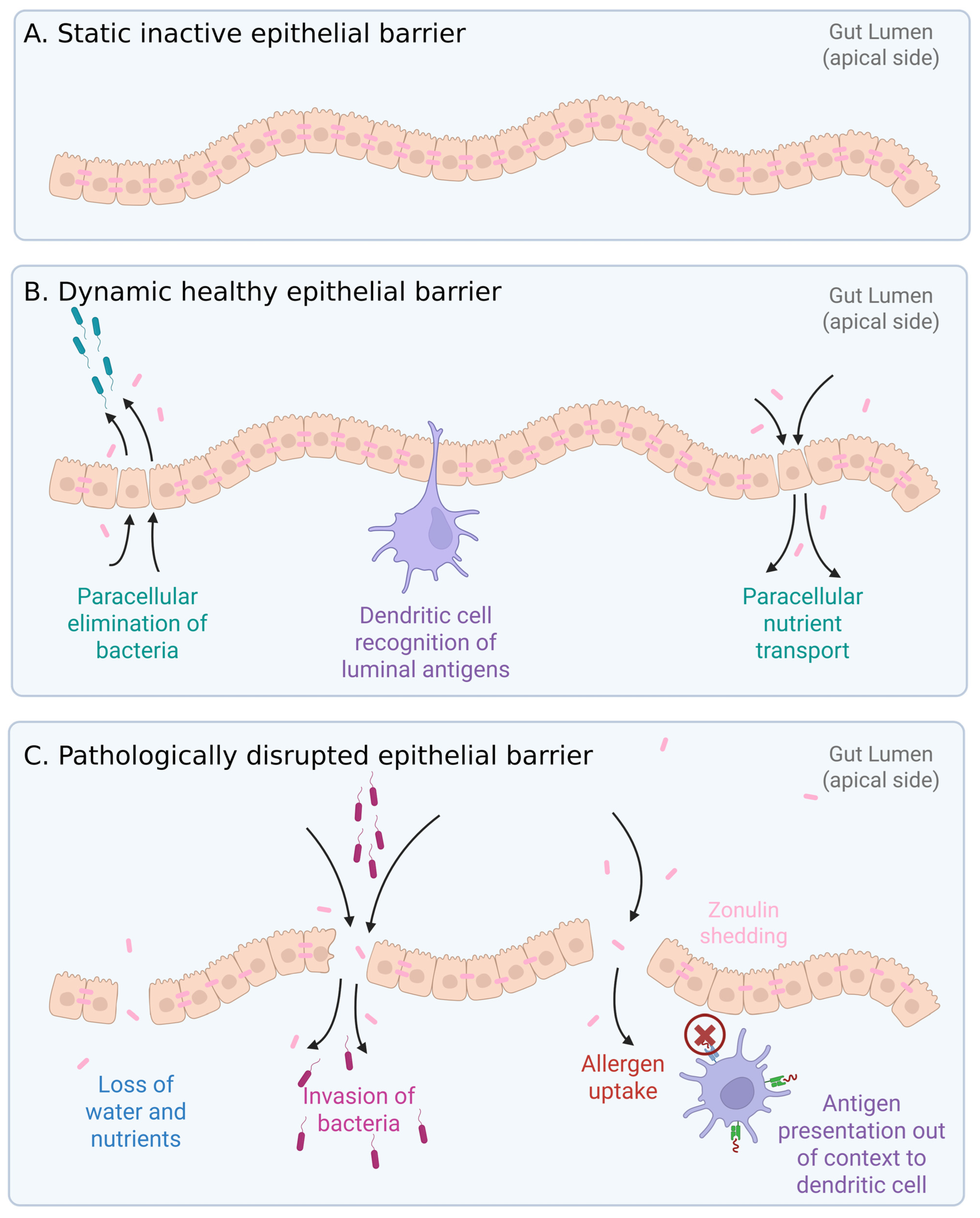
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. B. coagulans Germinated Spores
2.3. B. Coagulans Metabolites and Cell Wall Fractions
2.4. Gut Epithelial Cell Cultures
2.5. Metabolic Activity of Germinated Spores
2.6. Treatment of T84 Cells with Germinated Spores, Metabolite Fraction, and Cell Wall Fraction
2.7. Mechanical Wounding and Wound Recovery
2.8. Zonulin Release
2.9. Production of Cytokines and Chemokines
2.10. Statistical Analysis
3. Results
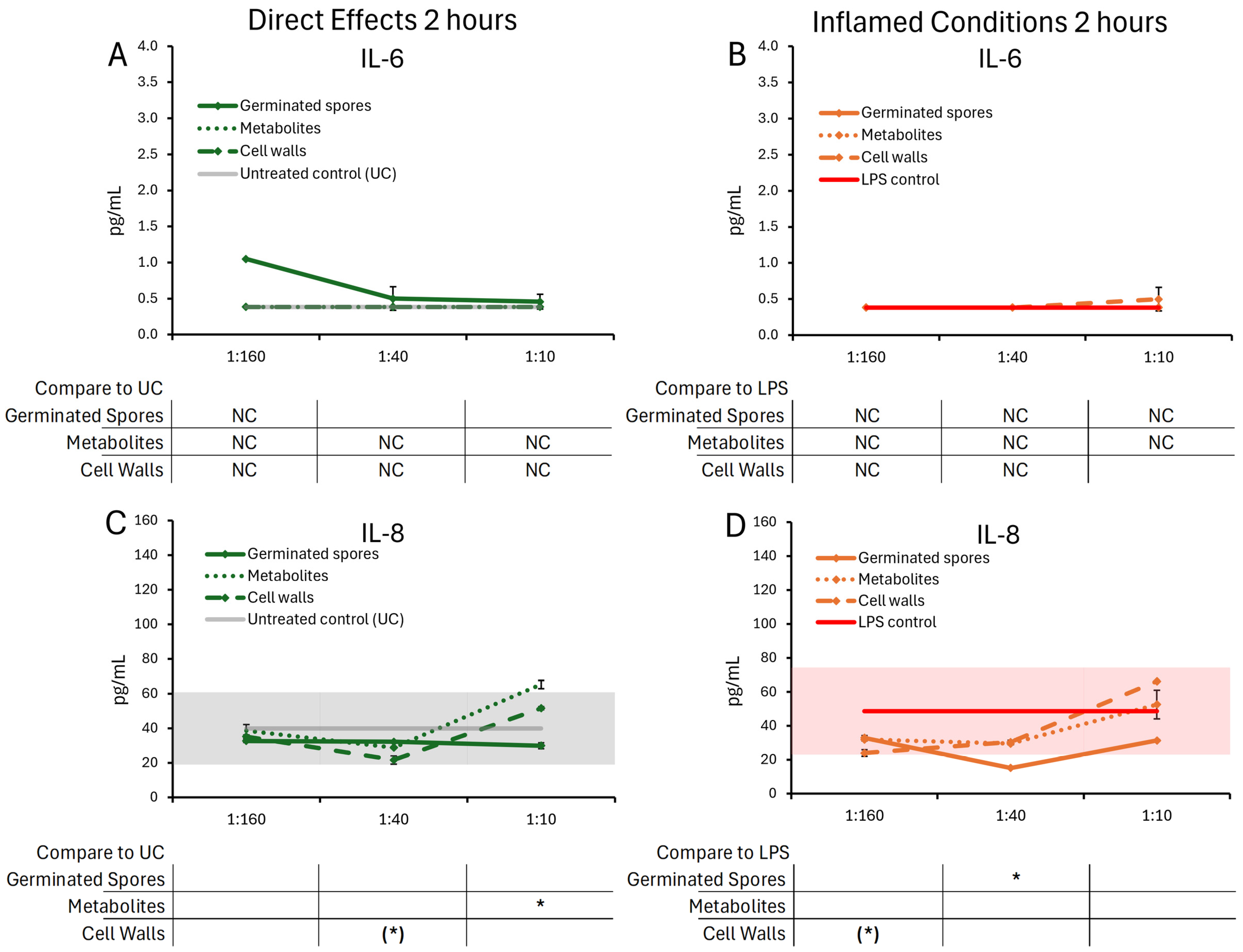
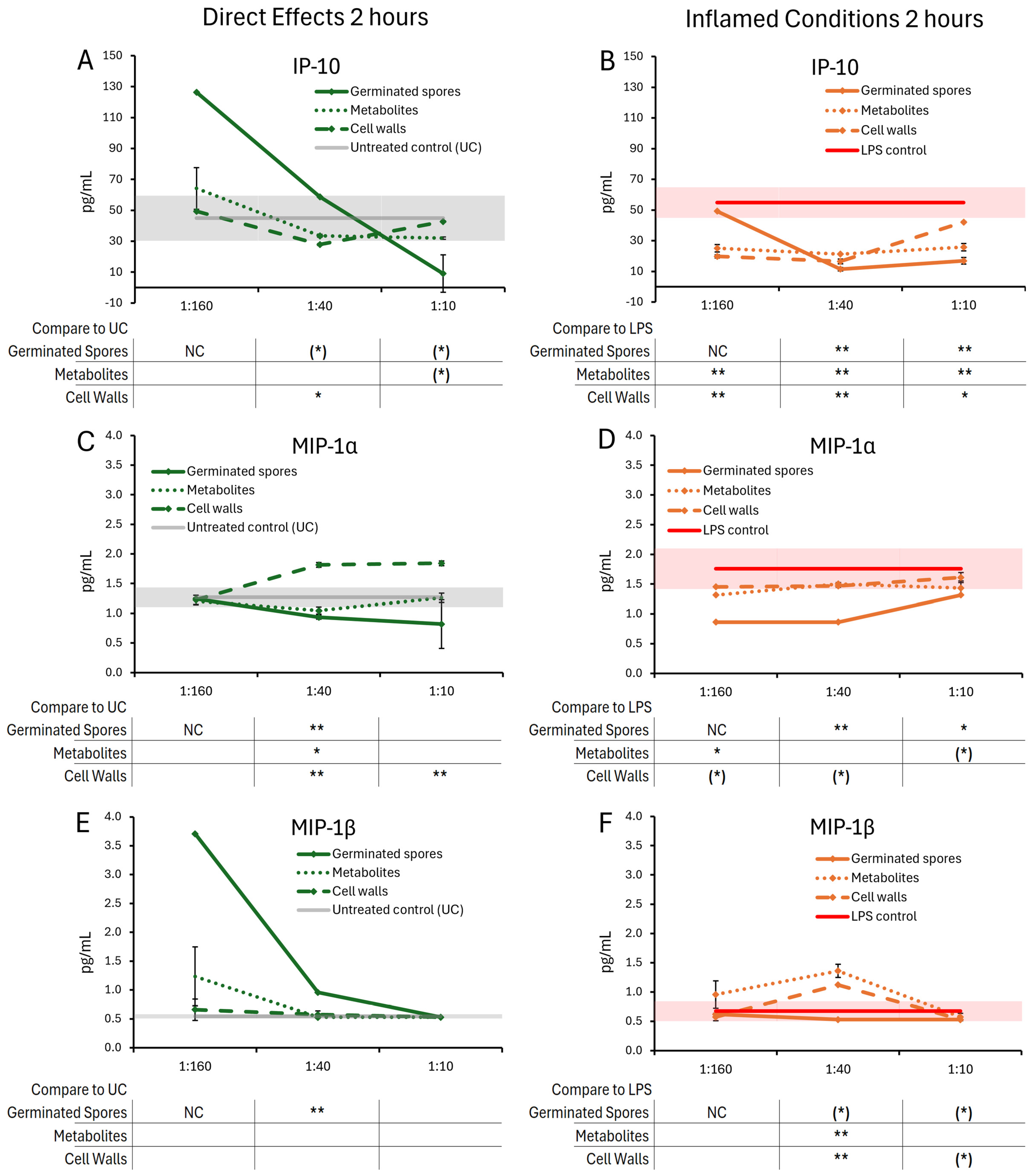
3.1. Cytokine and Chemokine Levels, 2 h Responses
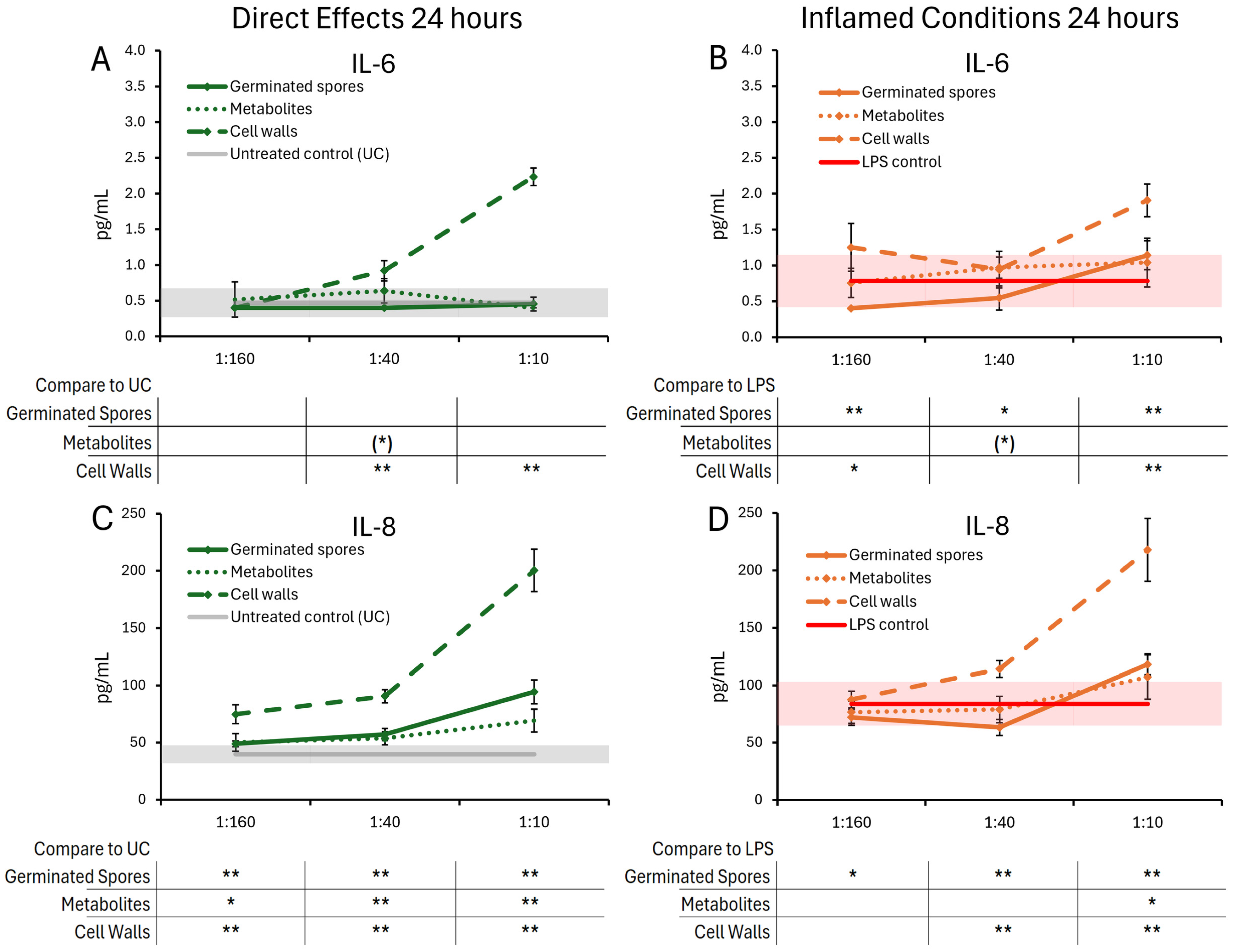
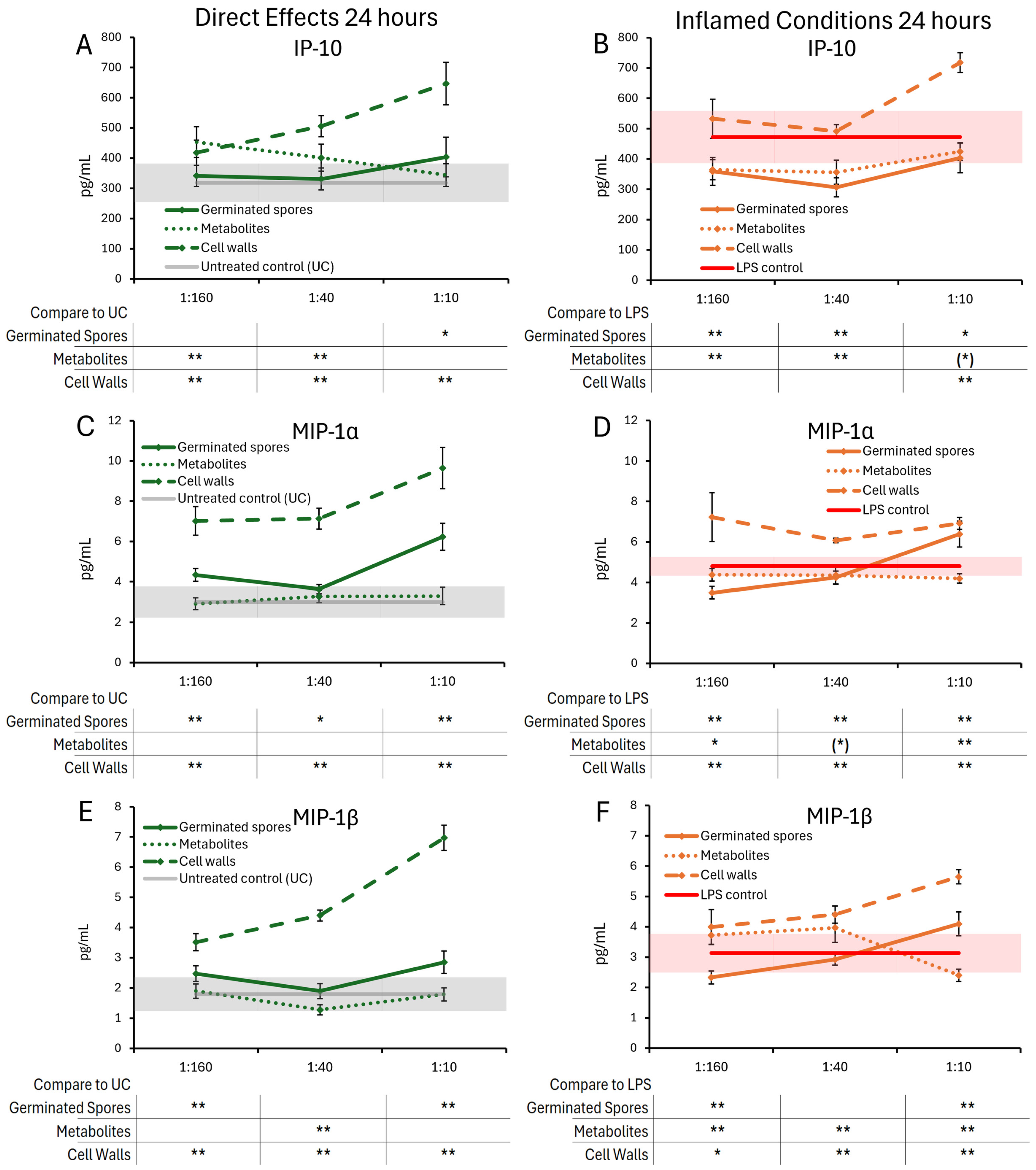
3.2. Cytokine and Chemokine Levels, 24 h Responses
3.3. Wound Healing
3.4. Zonulin Release
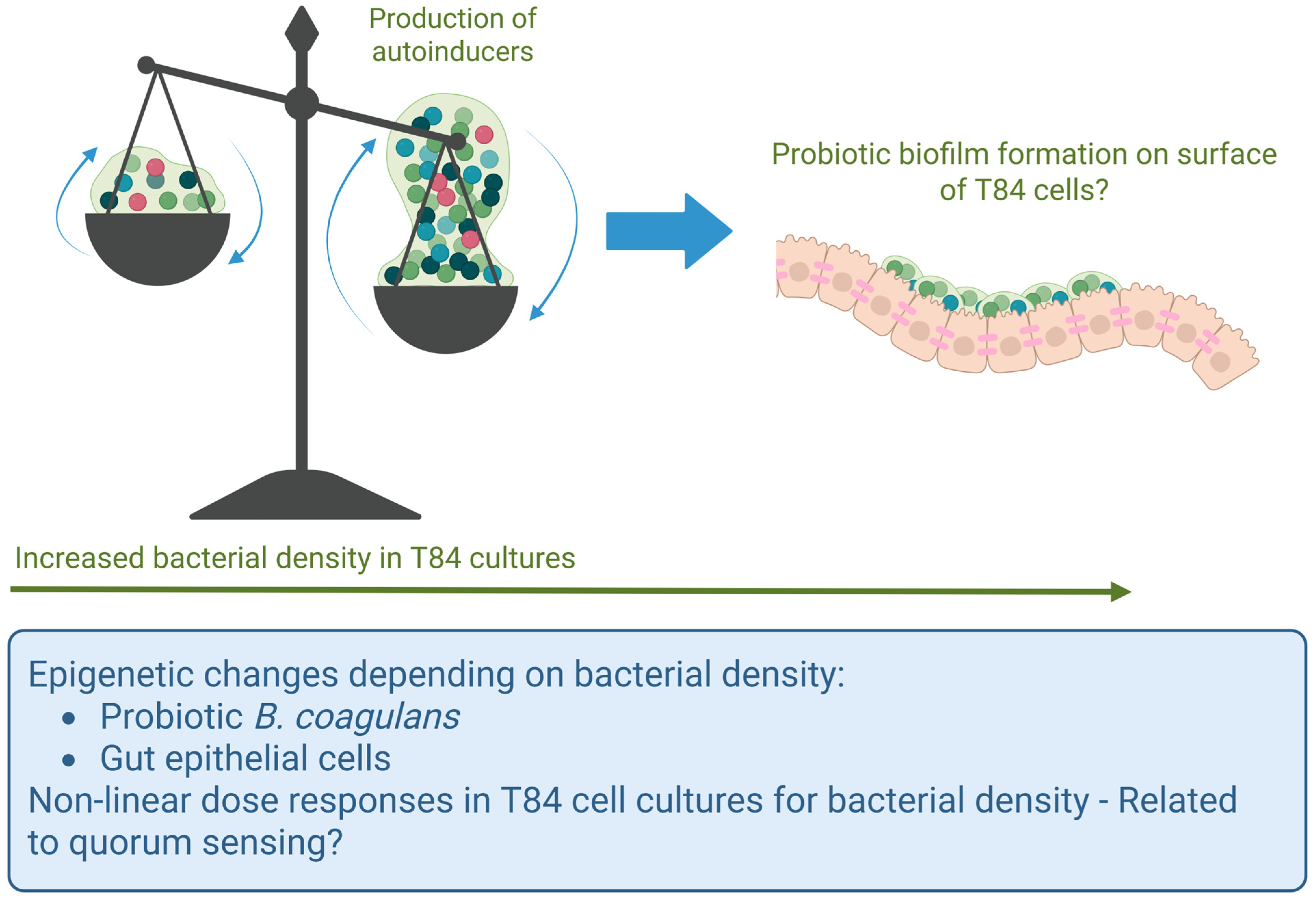
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMEM | Dulbecco’s modified Eagle’s medium |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IP-10 | Interferon gamma-induced protein 10 |
| MIP-1α | Macrophage inflammatory protein-1 alpha |
| MIP-1β | Macrophage inflammatory protein-1 beta |
References
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.J.; Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Ahn, J.; Hayes, R.B. Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu. Rev. Public Health 2021, 42, 277–292. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Q. Factors Affecting Gut Microbiome in Daily Diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, D.Q.; Chen, L.; Liu, J.R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Parsaei, M.; Sarafraz, N.; Moaddab, S.Y.; Ebrahimzadeh Leylabadlo, H. The importance of Faecalibacterium prausnitzii in human health and diseases. New Microbes New Infect. 2021, 43, 100928. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. The Gut Microbiota in Multiple Sclerosis: An Overview of Clinical Trials. Cell Transplant. 2019, 28, 1507–1527. [Google Scholar] [CrossRef]
- Feldman, G.J.; Mullin, J.M.; Ryan, M.P. Occludin: Structure, function and regulation. Adv. Drug Deliv. Rev. 2005, 57, 883–917. [Google Scholar] [CrossRef]
- Furuse, M.; Takai, Y. Recent advances in understanding tight junctions. Fac. Rev. 2021, 10, 18. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Veres-Székely, A.; Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Vannay, Á. Zonulin as a Potential Therapeutic Target in Microbiota-Gut-Brain Axis Disorders: Encouraging Results and Emerging Questions. Int. J. Mol. Sci. 2023, 24, 7548. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Chaudhari, A.; Dwivedi, M.K. Probiotics in the Prevention and Management of Human Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 1–11. [Google Scholar]
- Marteau, P.R. Probiotics in clinical conditions. Clin. Rev. Allergy Immunol. 2002, 22, 255–273. [Google Scholar] [CrossRef]
- Gionchetti, P.; Rizzello, F.; Venturi, A.; Brigidi, P.; Matteuzzi, D.; Bazzocchi, G.; Poggioli, G.; Miglioli, M.; Campieri, M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology 2000, 119, 305–309. [Google Scholar] [CrossRef]
- Weizman, Z.; Asli, G.; Alsheikh, A. Effect of a probiotic infant formula on infections in child care centers: Comparison of two probiotic agents. Pediatrics 2005, 115, 5–9. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ 2002, 324, 1361. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Stanton, C.; Desmond, C.; Coakley, M.; Collins, J.V.; Fitzgerald, G.F.; Ross, R.P. Challenges facing development of probiotic-containing functional foods. In Handbook of Fermented Functional Foods; CRC Press: Boca Raton, FL, USA, 2003; p. 27. [Google Scholar]
- Ayichew, T.; Belete, A.; Alebachew, T.; Tsehaye, H.; Berhanu, H.; Minwuyelet, A. Bacterial probiotics their importances and limitations: A review. J. Nutr. Health Sci. 2017, 4, 202. [Google Scholar]
- Keller, D.; Verbruggen, S.; Cash, H.; Farmer, S.; Venema, K. Spores of Bacillus coagulans GBI-30, 6086 show high germination, survival and enzyme activity in a dynamic, computer-controlled in vitro model of the gastrointestinal tract. Benef. Microbes 2019, 10, 77–87. [Google Scholar] [CrossRef]
- Bora, P.S.; Puri, V.; Bansal, A.K. Physicochemical Properties and Excipient Compatibility studies of Probiotic Bacillus coagulans Spores. Sci. Pharm. 2009, 77, 625–638. [Google Scholar] [CrossRef]
- Bomko, T.V.; Nosalskaya, T.N.; Kabluchko, T.V.; Lisnyak, Y.V.; Martynov, A.V. Immunotropic aspect of the Bacillus coagulans probiotic action. J. Pharm. Pharmacol. 2017, 69, 1033–1040. [Google Scholar] [CrossRef]
- Koh, Y.C.; Chang, Y.C.; Lin, W.S.; Leung, S.Y.; Chen, W.J.; Wu, S.H.; Wei, Y.S.; Gung, C.L.; Chou, Y.C.; Pan, M.H. Efficacy and Mechanism of the Action of Live and Heat-Killed Bacillus coagulans BC198 as Potential Probiotic in Ameliorating Dextran Sulfate Sodium-Induced Colitis in Mice. ACS Omega 2024, 9, 10253–10266. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, S.; Simon, A.; Khokhlova, E.; Colom, J.; Leeuwendaal, N.; Deaton, J.; Rea, K. In vitro safety and functional characterization of the novel Bacillus coagulans strain CGI314. Front. Microbiol. 2024, 14, 1302480. [Google Scholar] [CrossRef]
- Chang, X.; Kang, M.; Shen, Y.; Yun, L.; Yang, G.; Zhu, L.; Meng, X.; Zhang, J.; Su, X. Bacillus coagulans SCC-19 maintains intestinal health in cadmium-exposed common carp (Cyprinus carpio L.) by strengthening the gut barriers, relieving oxidative stress and modulating the intestinal microflora. Ecotoxicol. Environ. Saf. 2021, 228, 112977. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhiming, Y.; Wenyin, L.; Jianxin, Z.; Hao, Z.; Qixiao, Z.; Wei, C. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Mu, Y.; Cong, Y. Bacillus coagulans and its applications in medicine. Benef. Microbes 2019, 10, 679–688. [Google Scholar] [CrossRef]
- Iloba, I.; McGarry, S.V.; Yu, L.; Cruickshank, D.; Jensen, G.S. Differential Immune-Modulating Activities of Cell Walls and Secreted Metabolites from Probiotic Bacillus coagulans JBI-YZ6.3 under Normal versus Inflamed Culture Conditions. Microorganisms 2023, 11, 2564. [Google Scholar] [CrossRef]
- Jensen, G.S.; Benson, K.F.; Carter, S.G.; Endres, J.R. GanedenBC30 cell wall and metabolites: Anti-inflammatory and immune modulating effects in vitro. BMC Immunol. 2010, 11, 15. [Google Scholar] [CrossRef]
- Benson, K.F.; Redman, K.A.; Carter, S.G.; Keller, D.; Farmer, S.; Endres, J.R.; Jensen, G.S. Probiotic metabolites from Bacillus coagulans GanedenBC30™ support maturation of antigen-presenting cells in vitro. World J. Gastroenterol. 2012, 18, 1875–1883. [Google Scholar] [CrossRef]
- Devriese, S.; Van den Bossche, L.; Van Welden, S.; Holvoet, T.; Pinheiro, I.; Hindryckx, P.; De Vos, M.; Laukens, D. T84 monolayers are superior to Caco-2 as a model system of colonocytes. Histochem. Cell Biol. 2017, 148, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Mallegol, J.; Van Niel, G.; Lebreton, C.; Lepelletier, Y.; Candalh, C.; Dugave, C.; Heath, J.K.; Raposo, G.; Cerf-Bensussan, N.; Heyman, M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 2007, 132, 1866–1876. [Google Scholar] [CrossRef]
- Zhang, Y.; Gandhi, N.N. Complete Genomic Sequence of Bacillus coagulans Strain JBI-YZ6.3: A Natural Spore-Forming Isolate from Food-Grade Tapioca Starch. Microbiol. Resour. Announc. 2023, 12, e0100322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Overbeck, T.J.; Skebba, V.L.P.; Gandhi, N.N. Genomic and Phenotypic Safety Assessment of Probiotic Bacillus coagulans Strain JBI-YZ6.3. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef] [PubMed]
- McGarry, S.V.; Yu, L.; Cruickshank, D.; Iloba, I.; Jensen, G.S. Immune Activation by a Nutraceutical Blend: Rapid Increase in Immune-Modulating Cytokines, Followed by Induction of Anti-Inflammatory and Restorative Biomarkers. Nutraceuticals 2024, 4, 35–49. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Elemam, N.M.; Talaat, I.M.; Maghazachi, A.A. CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses 2022, 14, 2445. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor. Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Chen, J.W. Emerging role of chemokine CC motif ligand 4 related mechanisms in diabetes mellitus and cardiovascular disease: Friends or foes? Cardiovasc. Diabetol. 2016, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.N. The role of MIP-1 alpha in inflammation and hematopoiesis. J. Leukoc. Biol. 1996, 59, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bressuire-Isoard, C.; Broussolle, V.; Carlin, F. Sporulation environment influences spore properties in Bacillus: Evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol. Rev. 2018, 42, 614–626. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, X.M.; Liu, J.; Wang, H. Quorum Sensing, Biofilm, and Intestinal Mucosal Barrier: Involvement the Role of Probiotic. Front. Cell Infect. Microbiol. 2020, 10, 538077. [Google Scholar] [CrossRef]
- Wu, L.; Luo, Y. Bacterial Quorum-Sensing Systems and Their Role in Intestinal Bacteria-Host Crosstalk. Front. Microbiol. 2021, 12, 611413. [Google Scholar] [CrossRef]
- Bandyopadhaya, A.; Tsurumi, A.; Rahme, L.G. NF-κBp50 and HDAC1 Interaction Is Implicated in the Host Tolerance to Infection Mediated by the Bacterial Quorum Sensing Signal 2-Aminoacetophenone. Front. Microbiol. 2017, 8, 1211. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Ai, Q.; Tan, L. Autoinducer-2 promotes Pseudomonas aeruginosa PAO1 acute lung infection via the IL-17A pathway. Front. Microbiol. 2022, 13, 948646. [Google Scholar] [CrossRef]
- Motta, J.P.; Wallace, J.L.; Buret, A.G.; Deraison, C.; Vergnolle, N. Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 314–334. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, M.; Cui, X.; Chen, J.; Liu, C.; Zhang, X. Bacillus coagulans MZY531 alleviates intestinal mucosal injury in immunosuppressive mice via modulating intestinal barrier, inflammatory response, and gut microbiota. Sci. Rep. 2023, 13, 11181. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Salton, M.R.J.; Marshall, B. The Composition of the Spore Wall and the Wall of Vegetative Cells of Bacillus subtilis. Microbiology 1959, 21, 415–420. [Google Scholar] [CrossRef]
- Ghosh, S.; Setlow, P. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 2009, 191, 1787–1797. [Google Scholar] [CrossRef]
- Delbrück, A.I.; Zhang, Y.; Hug, V.; Trunet, C.; Mathys, A. Isolation, stability, and characteristics of high-pressure superdormant Bacillus subtilis spores. Int. J. Food Microbiol. 2021, 343, 109088. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGarry, S.V.; Grinage, E.A.F.; Sanchez, K.; Cruickshank, D.; Anderson, L.; Jensen, G.S. Germinated Spores of the Probiotic Bacterium Bacillus coagulans JBI-YZ6.3 Support Dynamic Changes in Intestinal Epithelial Communication and Resilience to Mechanical Wounding. Microorganisms 2025, 13, 1466. https://doi.org/10.3390/microorganisms13071466
McGarry SV, Grinage EAF, Sanchez K, Cruickshank D, Anderson L, Jensen GS. Germinated Spores of the Probiotic Bacterium Bacillus coagulans JBI-YZ6.3 Support Dynamic Changes in Intestinal Epithelial Communication and Resilience to Mechanical Wounding. Microorganisms. 2025; 13(7):1466. https://doi.org/10.3390/microorganisms13071466
Chicago/Turabian StyleMcGarry, Sage V., Earvin A. F. Grinage, Krista Sanchez, Dina Cruickshank, Liang Anderson, and Gitte S. Jensen. 2025. "Germinated Spores of the Probiotic Bacterium Bacillus coagulans JBI-YZ6.3 Support Dynamic Changes in Intestinal Epithelial Communication and Resilience to Mechanical Wounding" Microorganisms 13, no. 7: 1466. https://doi.org/10.3390/microorganisms13071466
APA StyleMcGarry, S. V., Grinage, E. A. F., Sanchez, K., Cruickshank, D., Anderson, L., & Jensen, G. S. (2025). Germinated Spores of the Probiotic Bacterium Bacillus coagulans JBI-YZ6.3 Support Dynamic Changes in Intestinal Epithelial Communication and Resilience to Mechanical Wounding. Microorganisms, 13(7), 1466. https://doi.org/10.3390/microorganisms13071466





