Abstract
Colletotrichum fructicola is a member of the gloeosporioides complex and can act as a pathogen, causing anthracnose in various plants and as an endophyte residing in healthy plants. As a plant pathogen, C. fructicola has been frequently reported to cause anthracnose in chili fruit and tea plants, bitter rot in apples and pears, crown rot in strawberries, and Glomerella leaf spot in apples, which are the most common diseases associated with this pathogen. Over the years, C. fructicola has been reported to infect a wide range of plants in tropical, subtropical, and temperate regions, including various types of fruit crops, ornamental and medicinal plants, tree nuts, peanuts, and weeds. Several reports have also been made regarding endophytic C. fructicola recovered from different plant parts. Endophytic C. fructicola has the ability to switch to a pathogenic state, which may contribute to the infection of host and other susceptible plants. Due to the economic importance of C. fructicola infections, the present review highlighted C. fructicola as a plant pathogen and endophyte, providing a summary of its infections in various plants and endophytic ability to inhabit plant tissues. Several control measures for managing C. fructicola infections have also been provided.
Keywords:
Colletotrichum fructicola; endophyte; pathogen; fruit crops; ornamental; medicinal plants; tree nuts; peanuts; weeds 1. Introduction
Colletotrichum species are mainly regarded as pathogens but have also been identified as endophytes in various plants and as saprophytes [1]. Plant pathogenic Colletotrichum have a wide host range, and almost all crops are susceptible to one or more species. The disease caused by Colletotrichum spp. is known as anthracnose, and its symptoms include leaf spot, stem rot, postharvest rot, and cankers [2,3]. Owing to its economic importance, the genus Colletotrichum is listed among the top 10 plant pathogenic fungi [2].
Endophytic Colletotrichum resides in healthy tissues of a wide range of host plants. Endophytes have been reported to provide advantages to host plants, such as promoting growth, resistance against diseases, and drought tolerance [4,5]. In certain conditions, such as during stress and senescence, endophytes can transform into pathogens [6].
Colletotrichum species are grouped into 15 species complexes or phylogenetic clades, namely, acutatum, agaves, boninense, caudatum, dematium, destructivum, dracaenophilum, gigasporum, gloeosporioides, graminicola, magnum, orbiculare, orchidearum, spaethianum, and truncatum [7,8,9]. A species complex is described as a group of species that form a monophyletic clade and display similar morphological characteristics [1]. Thus, to resolve the different Colletotrichum species complexes, as well as species within a complex, multiple loci are used as markers. Common markers used in the phylogenetic analysis of Colletotrichum species are actin (ACT), calmodulin (CAL), chitin synthase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), internal transcribed spacer (ITS), glutamine synthetase (GS), manganese superoxide dismutase, β-tubulin, and the DNA lyase and the mating-type locus MAT1-2-1 (APN2/MAT-IGS) [6,7,10,11,12,13].
Colletotrichum fructicola is member of the gloeosporioides complex that comprises 22 species and one subspecies [7]. Species in the gloeosporioides complex include many pathogens that cause anthracnose in various plants. Some species, including C. fructicola, can also act as endophytes that reside in many healthy plants. As the morphological and physiological characteristics of C. fructicola and other species of the gloeosporioides complex are similar, multiple markers have been used to reliably identify and distinguish them [7].
The following markers are suggested for successful species identification and phylogenetic analysis of species within the gloeosporioides complex: TUB2, GS, GAPDH, APN2, and ApMat [7,14,15] According to Vieira et al. [16] and dos Santos Vieira [17], ACT, CHS-1, ITS, and GAPDH are markers that are not suitable for species delimitation. The most suitable markers to utilize for species identification, however, are up for debate. To accurately identify species within the gloeosporioides complex, multiple markers are still needed.
In Northern Thailand, C. fructicola was first isolated from diseased coffee berries (Coffea arabica) and described by Pihastuti et al. [18]. Later, the species was isolated as a leaf endophyte of cacao (Theobroma cacao) in Central America [19]; however, the species was referred to as C. ignotum. During a reassessment of C. gloeosporioides taxonomy from various hosts worldwide, C. ignotum was synonymized with C. fructicola [7]. In the reassessment, both the morphological and cultural characteristics of C. ignotum and C. fructicola were similar. Based on the phylogenetic analysis of multiple loci, both species were monophyletic within the Musae clade. The type specimens of both species were also grouped into the same haplotype subgroup [7]. In addition, Glomerella cingulata var. minor, which was described from Ficus, was also synonymized with C. fructicola [7]. The ex-holotype culture of G. cingulata var. minor was found to phylogenetically match the type specimen of C. fructicola [7].
As a plant pathogen, C. fructicola has been frequently reported to cause anthracnose in chili fruit and tea plants, bitter rot in apples and pears, crown rot in strawberries, and Glomerella leaf spot in apples, which are the most common diseases associated with this pathogen. Over the years, C. fructicola has been reported to infect a wide range of plants in tropical, subtropical, and temperate regions, including various types of fruit crops, ornamental and medicinal plants, tree nuts, peanuts, and weeds. Several reports have also been made regarding endophytic C. fructicola recovered from different plant parts, which demonstrated its wide host range and worldwide distribution. The present review discusses C. fructicola as both a plant pathogen and endophyte, providing a comprehensive summary of the infections it causes in various plants, as well its endophytic host plants.
2. Plant Pathogenic Colletotrichum fructicola
Infections by C. fructicola primarily cause anthracnose in various plants. Symptoms of anthracnose commonly appear as dark lesions on infected plant parts. In severe infections, sunken lesions become visible, and during moist or wet conditions, conidial masses form in lesions. Depending on the plant part affected, anthracnose is also referred to as fruit rot, leaf blight, stem rot, crown rot, seedling blight, and twig blight [20,21]. Typically, C. fructicola grows as a saprophyte in soil and plant debris and infects plants through the water-splashing of conidia and air dissemination of ascospores [22].
Since the first description was made for C. fructicola isolated from coffee berries [18], this species has been identified as a pathogen in various other plants worldwide. Plant pathogenic C. fructicola not only causes anthracnose but also other diseases, such as bitter rot, crown rot, leaf spot, and leaf blotch. After the taxonomy of species within the gloeosporioides complex was revised, the C. fructicola reported as a pathogen was identified using multiple markers and morphological characteristics [7,9,18,21]. The pathogenicity of C. fructicola isolates was also documented, indicating the impact they have on production (Tables 1–15).
The species-specific detection of plant pathogenic C. fructicola using several PCR-based methods has been developed to facilitate detection and differentiation from other Colletotrichum species. A single marker derived from comparative genomics analysis was developed to differentiate C. fructicola among prevalent pathogens of strawberry [23]. A quantitative PCR assay employing ITS primers was utilized to detect C. fructicola in the leaves of tea oil trees [24]. Specific primers and hydrolysis probes were developed as components of a real-time PCR assay aimed at detecting C. fructicola associated with bitter rot in apples [25]. These PCR-based methods offer considerable benefits for the swift detection of C. fructicola, contributing to improved disease management.
Many types of plants are infected by C. fructicola, including agricultural crops, ornamental and medicinal plants, cacti, and weeds. The host range has broadened over time. This was likely due to species identification using molecular markers, of which several species of C. gloeosporioides sensu lato were phylogenetically described since the taxonomic revision of the gloeosporioides complex. Other possibilities may be related to its pathogenic and endophytic lifestyles. Moreover, the availability of host plants and suitable climatic conditions are favorable for C. fructicola growth and development, particularly in tropical and subtropical regions.
Several reasons may be attributed to the introduction and dispersal of C. fructicola into new areas, such as via the agricultural and plant trade of latent pathogens or endophytes, transportation, and through human-aided means [26,27]. These methods of the introduction and dispersal of fungal pathogens are also known as hitchhiking, in which hitchhikers are commonly hidden in a plant in the form of small fungal propagules [28,29]. Hitchhiking of fungal pathogens was reported to explain the introduction of Diaporthe spp. into new areas through the international trade of plant materials [30]. Fungal propagules can infect and establish host plants in the new environment due to host availability and climate suitability. The establishment of C. fructicola in some parts of European Union countries is associated with these two factors [31].
The ability of latent or endophytic C. fructicola to hitchhike poses epidemiological concern due to their hidden nature, which facilitate their undetected dissemination and subsequent emergence, potentially resulting in disease outbreak in a new environment. Once introduced, latent or endophytic C. fructicola can occasionally switch hosts, transitioning from their original host plants to either closely related or even more distantly related plants [32]. Furthermore, lack of host specificity of C. fructicola allows them to infect a variety of crops.
3. Common Fruit Crops Infected by Colletotrichum fructicola
Fruit crops are susceptible to C. fructicola infections, which result in anthracnose, fruit rot, crown rot, and leaf spot. Some of these fruit crops, such as apples, pears, citrus, strawberries, avocados, mangoes, papaya, dragon fruits, and pineapples, are economically important in several crop-producing countries. The pathogen not only infects fruits but also other plant parts, including the leaves, petals, petioles, and stems. The most noticeable anthracnose symptoms appear on the surface of fruits, manifesting as brown-to-black sunken lesions containing masses of conidia [33]. Dark, sunken lesions may also appear on the leaves and stems. Table 1 summarizes common symptoms caused by Colletotrichum on fruits, leaves, and stems.

Table 1.
Common symptoms of anthracnose on fruits, leaves, and stems.
Glomerella leaf spot on apples (Malus domestica) is a serious disease caused by C. fructicola, which often occurs in apple-producing countries (Table 2), in subtropical and humid environments, but is confined to a number of countries in Asia and America [34,37,38,39]. Bitter rot in apples is often associated with C. fructicola, and the disease occurs worldwide (Table 2). Apple bitter rot affects all cultivars, although they have different susceptibilities [40]. The pathogen infects fruits in orchards and after harvest during storage [41]. In addition to Glomerella leaf spot and bitter rot, C. fructicola causes apple anthracnose [42,43,44,45] and fruit rot [46].

Table 2.
Diseases in apples caused by plant pathogenic Colletotrichum fructicola.
Anthracnose and ripe rot can occur in C. fructicola-infected grapes (Vitis vinifera and other Vitis spp.) in several grape-producing countries, including China, Brazil, Korea, and Japan (Table 3). Initially, anthracnose in grapes was caused by Elsinoe ampelina, but later Colletotrichum spp., including C. fructicola, were found to be associated with the disease [54,55]. The anthracnose symptoms exhibited by E. ampelina and Colletotrichum spp. are similar, resulting in sunken, necrotic lesions on the berries, leaves, shoots, and stems [56]. The most prevalent species recovered from grape anthracnose in Zhejiang Province, China, was C. fructicola [57]. In addition to anthracnose, C. fructicola also causes ripe rot in grape berries [58]. In Southern Brazil, C. fructicola was the second-most dominant species causing ripe rot in grapes [59].

Table 3.
Ripe rot and anthracnose in grapes caused by plant pathogenic Colletotrichum fructicola.
After apples and grapes, pears (Pyrus spp.) are important temperate fruit crops broadly cultivated in China, Argentina, USA, Spain, and Italy. In a study by Fu et al. [64], C. fructicola was the most common species associated with infections of the fruits and leaves of P. pyrifolia and P. bretschneideri in six main pear cultivation provinces in China. Other reports of C. fructicola as a pathogen of pears are presented in Table 4. In pear fruits, C. fructicola can cause bitter rot and anthracnose. Anthracnose caused by C. fructicola in hybrid pear fruits (P. pyrifolia × P. communis) was reported in Korea. Black specks lesion on the fruits that become larger lead to fruit drop. On pear leaves, three types of symptoms appear to be associated with C. fructicola infections, namely large, necrotic lesions, small round spots, and tiny black spots [64,65,66].

Table 4.
Diseases in pears caused by plant pathogenic Colletotrichum fructicola.
In Citrus spp., C. fructicola can cause anthracnose in the fruits, leaves, stems, and petals (Table 5). Various Citrus spp. have been previously reported to be infected by C. fructicola, including Ci. bergamia (bergamot orange), Ci. grandis (pomelo), Ci. reticulata (mandarin orange), and Ci. sinensis (oranges), as well as kumquat (Fortunella margarita), during the preharvest and postharvest periods [69,70,71,72].

Table 5.
Anthracnose in Citrus spp. caused by plant pathogenic Colletotrichum fructicola.
Strawberries (Fragaria × ananassa) are susceptible to anthracnose infection, which commonly occurs soon after planting and at the seedling stage [76]. The most prevalent species isolated from the infected plant parts of strawberries [77,78] including the fruits, leaves, petioles, and stolons, was reported to be C. fructicola (Table 6). Based on a study by Gan et al. [79], C. fructicola was the main virulent species found in the field, although less-virulent isolates were also detected. Strawberry crown rot is also caused by C. fructicola (Table 6), resulting in the wilting and stunting of aboveground plant parts, which can cause the plant to collapse. Cutting into the crown tissues revealed reddish-brown lesions [80,81,82].

Table 6.
Anthracnose and crown rot of strawberry caused by plant pathogenic Colletotrichum fructicola.
Notable anthracnose infections of fruit crops caused by C. fructicola include a number of tropical and other fruit crops, such as kiwis, cherries, litchi, carambola, and pomegranates (Table 7). Shot hole leaf caused by C. fructicola was reported in Prunus sibirica [89].

Table 7.
Anthracnose and other diseases of fruit crops caused by plant pathogenic Colletotrichum fructicola.
Other Host Plants Infected by Colletotrichum fructicola
Various other plants, including coffee berries, Camellia spp., vegetable crops, ornamentals, peanuts, tree nuts, medicinal plants, and weeds, can also be infected by C. fructicola.
Anthracnose in coffee berries is caused by a complex of Colletotrichum spp., including C. fructicola (Table 8). Pathogenic C. fructicola associated with diseased coffee berries was first reported in Northern Thailand [18]. Another report of C. fructicola infection associated with coffee berries was made in Puerto Rico, and the resulting disease was referred to as coffee fruit rot [120]. The disease affected the maturity stages of coffee berries and caused internal and external rotting of green berries. Severe internal rot was incited by C. fructicola and the berries later become mummified [120,121].

Table 8.
Anthracnose and fruit rot of coffee berries caused by plant pathogenic Colletotrichum fructicola.
Leaf anthracnose is a serious disease that affects Camellia spp. [122,123,124]. Pathogenic C. fructicola has been found to cause leaf anthracnose in Ca. sinensis, a beverage crop, as well in the tea oil trees, Ca. oleifera and Ca. yuhsienensis, which would lead to economic losses (Table 9).

Table 9.
Anthracnose of Camellia spp. caused by plant pathogenic Colletotrichum fructicola.
Anthracnose in several vegetable crops is often caused by C. fructicola. In chili, anthracnose mainly affects the fruits but can also infect leaves (Table 10). Anthracnose caused by C. fructicola has been detected in Japanese pickling melons, culinary melons, luffa sponge gourds, and Chinese flowering cabbages (Table 10).

Table 10.
Anthracnose and fruit rot of vegetable crops caused by plant pathogenic Colletotrichum fructicola.
The leaves of various ornamental plants can be infected by C. fructicola, causing leaf spot or anthracnose (Table 11). However, in Phalaenopsis, C. fructicola can infect the petals, causing a ring of green tissue that borders necrotic areas [145]. Infected leaves eventually wither and can lead to defoliation, as observed during infections of Bletilla striata [146] and Salix babylonica [147].

Table 11.
Ornamental plants infected by plant pathogenic Colletotrichum fructicola.
Peanuts and tree nuts are also susceptible to C. fructicola infection (Table 12). In peanuts, C. fructicola can cause leaf spot similar to that observed during the infection of walnuts, pecan, macadamia, and Chinese hickory. Fruit anthracnose has been reported in walnuts and pecan [160,161]. Infection on the fruits can lead to mummified fruits and fruit drops.

Table 12.
Peanuts and tree nuts infected by plant pathogenic Colletotrichum fructicola.
Various types of medicinal plants can be infected by C. fructicola, causing leaf spot and stem rot (Table 13). Infection causes wilting and defoliation of the leaves [165,166,167,168,169,170,171].

Table 13.
Medicinal plants infected by plant pathogenic Colletotrichum fructicola.
Weeds can also be infected by C. fructicola, mainly causing leaf spot (Table 14). Besides leaf spot, the pathogen can also cause stem rot, crown rot, and petiole rot in the aquatic weed, water hyacinth (Eichhornia crassipes) [172]. Pigweed and Japanese brome can also be infected by C. fructicola [173,174]. The infected parts turn yellow, thinner, and eventually wither [172,174].

Table 14.
Weeds infected by plant pathogenic Colletotrichum fructicola.
Other plants and crops that can be infected by C. fructicola are presented in Table 15. Leaf spot or anthracnose is the most commonly reported disease for these plants/crops.

Table 15.
Other plants or crops infected by Colletotrichum fructicola.
4. Infection and Colonization by Colletotrichum fructicola
A similar infection and colonization behavior is shared between C. fructicola and other species of the gloeosporioides complex. The penetration and colonization of C. fructicola have been characterized in apples (M. domestica Gala) and Ca. oleifera leaves [194,195]. Penetration of the host begins with the germination of conidia and the formation of appressoria, whereafter the penetration peg expedites the entry of the pathogen through the epidermis and cuticle of the host [196,197]. In C. oleifera, the pathogen can also infect or invade through the stomata via germ tubes or hyphae [195]. After penetration, infection vesicles develop within the epidermal cells, followed by the formation of primary hyphae that spread into adjacent cells.
The pathogen remains in living plant tissues and is established therein by absorbing nutrients without killing the plant cells, which demonstrates the biotrophic stage. Subsequently, secondary hyphae form, initiating the necrotrophic stage. At this stage, the host tissues are destroyed, and symptoms of disease emerge [198,199]. Figure 1 shows the infection of C. fructicola on susceptible host plants with biotropic and nectrotropic stages.
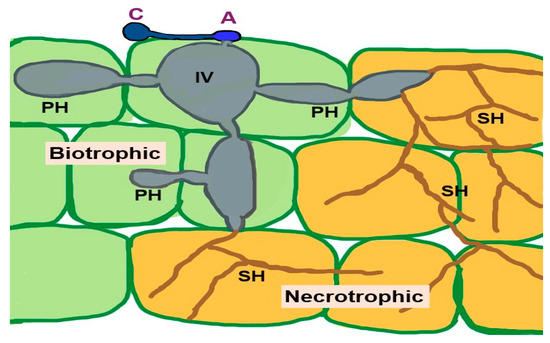
Figure 1.
Colletotrichum fructicola infection on susceptible host plant. Biotrophic stage: Conidium (C) on the host plant germinate and produce an appressorium (A) that assists in the penetration of the host tissues and forms an infection vesicle (IV) and primary hypha (PH) in the living tissues. During this stage, the host cell maintains its structural integrity. The fungus then transitions to the necrotrophic stage by developing thin secondary hyphae (SH), colonizing and killing the surrounding tissues. The fungus produces cell wall degrading enzymes, proteases, and nutrient transporters to aid in tissue degradation and nutrient acquisition from the dead host cells [198].
The latent or quiescent period is a transitional stage that occurs towards the biotrophic and necrotrophic stages. The latent period is important for species associated with postharvest diseases, particularly fruit rot or anthracnose. Pathogenic Colletotrichum remains dormant within the plant host before shifting to the active stages. The transition from dormant to biotrophic and necrotrophic stages occurs during fruit ripening in response to biochemical and physiological changes in the fruits [200].
Colletotrichum employs a latent period as a means of survival, waiting for the host to become susceptible following harvest. Since symptoms are not immediately visible, infected crops pass through sorting and inspection stages, entering the supply chain without detection [201]. The pathogen’s ability to remain latent allows it to be transported over long distances in crops that appear healthy, facilitating its dissemination to new areas. When the crops encounter stress factors, like mechanical damage, temperature and humidity changes, the pathogen switches from a latent state to active growth stages, rapidly multiplying and spreading during storage or transport, particularly in packed conditions with high moisture and limited airflow [202]. The appearance of symptoms during postharvest indicate the beginning of a postharvest disease outbreak.
The Colletotrichum life cycle includes asexual and sexual stages. During pathogen growth, acervuli develop in plant tissues and produce masses of conidia. These conidia serve as the primary inoculum during the new infection cycle and are dispersed to new hosts or the same host plant in a new location via rain splashes, irrigation water, wind-driven rain, and cultivation tools [203]. The perithecia and sexual fruiting structures are formed under favorable conditions, which release and disperse ascospores. In the absence of a susceptible host, the perithecia act as survival structures [18,19]. Figure 2 shows the life cycle and disease development of Colletotrichum spp., which is also applicable to C. fructicola. A detailed review of the Colletotrichum spp. life cycle was previously described by Latunde-Dada [204] and de Silva et al. [203].
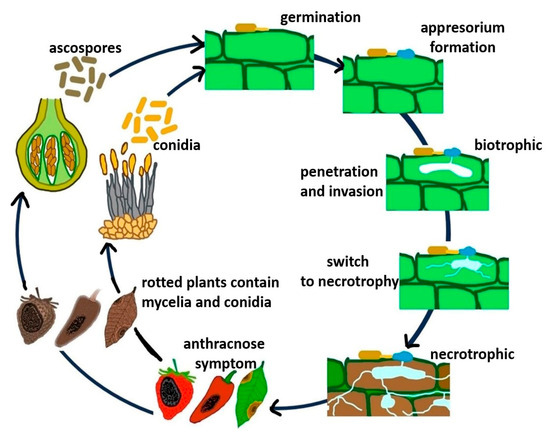
Figure 2.
Life cycle of C. fructicola and disease development on infected plants.
5. Molecular Pathogenesis of Colletotrichum fructicola
The molecular pathogenesis of C. fructicola in infected host plants has been studied in several plants, including tea oil trees (Ca. oleifera), strawberries, apples, and pears, at different stages of infection. In these studies, structure-specific genes, virulence factors, effector proteins, secreted proteases, transcription factors, and secondary metabolite enzymes involved in host–pathogen interactions were identified, and in some cases, the genes or proteins characterized. Data on the molecular mechanisms underlying C. fructicola infection provide insights into disease pathogenesis, the colonization of the pathogen, and the defense mechanisms of the plant. Some studies on the molecular mechanisms of C. fructicola infection have been performed on Ca. oleifera anthracnose, Glomerella leaf spot in apples, strawberry anthracnose, and pear anthracnose.
5.1. Pathogenesis of Colletotrichum fructicola
Based on comparative genome analysis, the C. fructicola genome contains the largest number of virulence factors, including plant cell wall-degrading enzymes, secondary metabolite biosynthetic enzymes, effectors, secreted proteinases, and small secreted proteins [205]. These virulence factors are expressed at different stages of the infection process to ensure successful colonization, starting before penetration, after appressoria penetration, during colonization by intracellular hyphae, and when switching from biotrophy to necrotrophy [206]. At different stages of infection, a range of genes and proteins are involved in plant cell wall degradation and secondary metabolism, during which effector expression is highly upregulated [207]. Several genes and proteins involved in the pathogenicity of C. fructicola have been identified and characterized, as presented in Table 16.

Table 16.
Genes/proteins identified and characterized during Colletotrichum fructicola pathogenesis and their functions.
The transcriptomic profiles of differentially expressed genes in the conidia, appressoria, and infectious hyphae of C. fructicola during infections in apple leaves were previously obtained. In C. fructicola-strawberry interactions, during plant cell wall degradation and penetration into plant tissues, genes encoding putative effectors, including the chitin-binding protein, were upregulated. The detection of chitin-binding protein at this stage might indicate that the pathogen attaches to the host by expressing chitinases or the chitin-binding protein [207,216]. During the early stage of strawberry infection, several pectin-degrading enzyme families were detected, including the carbohydrate esterase family 1, glycoside hydrolase family, and several pectate lyases. Cutinase-encoding genes were also upregulated [207].
For successful infection, pathogens produce numerous virulence effectors that facilitate infection and exploit plant physiology and immune responses [217,218]. Comparative genome studies have shown that Colletotrichum species contain hundreds of putative effectors that are unique, conserved, and vital for pathogen adaptation in the host plant [206,219,220].
Effectors are highly expressed during C. fructicola infection and host plant colonization. In hemibiotrophic infections caused by Colletotrichum, effectors are induced by the host plant and successively expressed. Most of these effectors are expressed during the early stage of host penetration by the appressorium and at the beginning of the necrotrophic stage, where cell death is induced, hastening a switch between the stages of infection [221]. During C. fructicola infection in apple leaves, many candidate effector proteins were detected, and their expression strongly induced. Several of these effector proteins were also upregulated during C. fructicola infection in strawberries [195]. A novel effector, CfEC92, was identified to be involved in C. fructicola infections causing leaf spot in apples and is thus considered important for pathogen virulence. CfEC92 expression was induced during the early stages of infection and the formation of appressoria to assist the pathogen in penetrating the host plant and inducing vesicle formation at the biotrophic stage [194].
The significant role of effectors in the pathogenicity of Colletotrichum positions them as potential targets for developing disease management strategies, such as plant breeding and biocontrol [222]. Certain effectors are conserved among various Colletotrichum species and play a crucial role in their overall virulence. These effectors are potential targets for resistance that might provide protection against multiple Colletotrichum species. For example, the conserved effector NIS1 has demonstrated its importance for virulence in multiple Colletotrichum species [223]. Several Colletotrichum effectors that exhibit antimicrobial properties against competing microbes have been discovered. Although these facilitate the colonization of the fungi, they also offer a promising source of new antimicrobial substances for biocontrol agents. For instance, the ribonucleases CfRibo1 and CfRibo2 from C. fructicola function as antimicrobial effectors, suggesting their potential application as antimicrobial effectors [224].
Transcription factors have been reported to play a role in the pathogenesis of C. fructicola causing Glomerella leaf spot in apples. During the pathogenesis of C. fructicola in apple leaves, the expression of the transcription factor CfSte12 was found to be upregulated throughout the germination of conidia, formation of appressoria, penetration, and development of structures required for sexual reproduction [209]. Another transcription factor, CfMcm1, was expressed during early infection and in conidia. The transcription factor, CfMcm1, is the main regulator that plays a role in pathogenicity, sexual and asexual reproduction, and melanin synthesis [213]. Moreover, the transcription factor, CfCpmd1, is important for strain compatibility in sexual reproduction, as it affects the growth of hyphae, sporulation, and the formation of appressoria, which play a notable role in the pathogenicity of C. fructicola causing leaf spot in apples [215].
Secondary metabolites also contribute to C. fructicola pathogenicity. During the pathogenesis of C. fructicola in strawberries, the expression of secondary metabolite backbone genes was upregulated, of which polyketide synthase-encoding genes involved in melanin biosynthesis were detected. Melanin biosynthesis plays an essential role in the development of appressoria during pathogenesis [207]. Other secondary metabolite backbone genes were also found to be highly regulated 96 h after inoculation, which corresponds with the necrotrophic stage of the pathogen [207]. Understanding the pathogenesis and interaction between C. fructicola and the host plant is pivotal for strategizing suitable disease management and crop protection practices.
5.2. Host Defense
During infection and colonization, C. fructicola encounters several defense responses by the host plant. Studies on the mechanisms of defense in response to C. fructicola pathogenesis have been conducted for strawberries, tea oil trees, and pears, providing insight into the defense strategies employed by host plants.
The pathogenesis of C. fructicola in strawberry and pear leaves is generally similar, with salicylic acid, jasmonic acid, reactive oxygen species, and peroxidases playing roles in pathogen resistance. Salicylic acid is the main plant defense hormone used against biotrophic pathogens, whereas jasmonic acid and ethylene are essential for resistance against necrotrophic pathogens [225]. The genes associated with salicylic and jasmonic acid biosynthesis and signaling pathways were upregulated during infection in strawberry and pear leaves, demonstrating that both plant hormones may constitute the core defense mechanism used by host plants [207,226,227].
Peroxidases are a notable class of pathogenesis-related proteins that are activated in host plants upon pathogen infection. Seven peroxidase-encoding genes were upregulated at 96 h postinfection by C. fructicola in strawberries, which may indicate the activation of plant defense mechanisms [207]. Peroxidases are expressed to restrict pathogen infection by forming structural barriers or producing a toxic environment through the activation of reactive oxygen species [228].
Infection by pathogens can cause oxidative stress due to the accumulation of reactive oxygen species in plants after pathogen recognition, which represents one of the earliest activated defense responses against pathogens [229]. Reactive oxygen species were found to be regulated, and genes involved in the necrotrophic stage of strawberry infection were identified [207]. The generation of reactive oxygen species often leads to symptoms that induce cell death [207,230]. In pear leaves infected with C. fructicola, genes related to lignin and flavonoid biosynthesis were also found to be upregulated, demonstrating that lignin and phytoalexin are involved in the defense pathway of the host plant [227].
Flavonoids and lignin are among the products of phenylpropanoid metabolism and are involved in plant defense against pathogens [231]. Genes related to flavonoid and lignin biosynthesis were upregulated at the necrotrophic stage of C. fructicola infection in pear leaves. These findings indicate that lignin and phytoalexin are involved in the defense pathway utilized by pear leaves in response to pathogen infection [227].
The brassinosteroid, 24-epibrassinolide, which is a steroid-type phytohormone, may activate lignin biosynthesis in tea plants (Ca. sinensis) during infection by C. fructicola causing anthracnose. Gene analysis has shown that 24-epibrassinolide activates genes related to lignin biosynthesis in response to C. fructicola infection [232]. Lignin accumulation in leaves potentially strengthens the cell wall structure and reduces C. fructicola infections, thereby enhancing tea plant resistance [232].
Transcription factors are also involved in host plant defense against fungal pathogens. WRKY transcription factors are among the largest families of transcriptional regulators in plants that trigger plant immune responses by inactivating defense-suppressing WRKY proteins [233]. Genes encoding WRKY factors have been identified during C. fructicola infection in strawberries, and several genes were found to be upregulated during the mid-to-late stages of infection [207]. In a study by Li et al. [195], multiple candidate WRKYs were found to be induced by C. fructicola in infected tea oil trees.
The infection response showed that plants employ various mechanisms of defense against C. fructicola. However, the pathogen utilizes a series of antagonistic mechanisms to successfully infect and colonize host plants.
6. Cross-Pathogenicity
Many Colletotrichum species, including C. fructicola, can infect various plants because they are not host-specific. Studies on C. fructicola infecting various hosts, often as first reports, have increased over the years, showing that many host plants are susceptible to C. fructicola infections and may also indicate cross-infection [234,235,236,237]. Cross-infection among species within the same species complex can lead to serious infections in the plant host. Therefore, the assessment of cross-infection in various host plants could provide insights into the host range and pathogenic potential of C. fructicola. Table 17 summarizes reported cross-infection of Colletotrichum spp. from primary host to other plants.

Table 17.
Cross-infection of Colletotrichum spp. from primary host to other plants.
Cross-infection of species belonging to the gloeosporioides complex has been reported in several studies [135,234,236,238]. In a study by Lakshmi et al. [238], isolates of C. gloeosporioides recovered from acid lime, cashew, custard apple, guava, and pomegranate were able to infect mango fruits and leaves, demonstrating cross-infection among the fruit crops. The C. gloeosporioides isolated from avocado fruits was pathogenic to mango fruits but showed a lower aggressiveness. Similarly, C. gloeosporioides isolated from mango fruits were pathogenic to avocado fruits but with a lower aggressiveness [220].
A cross-infection study on five Colletotrichum species, including C. fructicola, of several fruit crops and chili fruits, such as papaya (Carica papaya), orange (Ci. reticulata), rose apple (Eugenia javanica), mango (Mangifera indica), and guava (Psidium guajava), indicated that C. fructicola and other species of the gloeosporioides complex could infect all the fruit crops and chili fruits tested. The cross-infection study also showed that the tested species had a wide host range [236]. In a study by Freeman et al. [239], cross-infection by C. gloeosporioides isolated from Limonium occurred when tested on mangoes, strawberries, peaches, pears, and nectarines.
According to a study by Eaton et al. [240] on Colletotrichum spp. isolated from the anthracnose of apples, strawberries, and blueberries, mixed-fruit orchards may expedite the cross-infection of Colletotrichum spp. The study findings were in accordance with those of a cross-infection study by Liu et al. [135], which involved Colletotrichum spp., including C. fructicola, from chili fruits and showed that they were also able to infect pear fruits. In Sichuan Province, China, chili plants are often planted in pear orchards in which the Colletotrichum spp. associated with chili plants have the potential to cross-infect pear fruits [135].
The implications of cross-pathogenicity and the latent infection of Colletotrichum are particularly important in mixed-cropping systems. In mixed-cropping systems, certain crops may serve as asymptomatic hosts for Colletotrichum, harboring latent infections and elevating disease risk. These crops can serve as pathogen reservoirs, maintaining the pathogen populations and enabling carryover between seasons or plantings. On the other hand, if a crop is resistant or less susceptible, it may function as a barrier to the spread of disease [243].
Many species of the gloeosporioides complex, including C. fructicola, can cross-infect various hosts, as these pathogens generally have the potential to adapt to new hosts and cause infections [241,242,244,245]. However, virulence and aggressiveness may differ due to the adaptation of the pathogen in the new host and their capability to overcome host defense mechanisms [245]. Host range information from the occurrence of cross-infection provides useful data for suitable disease management methods in the field, as well as after harvest. This information will be useful for the development of resistant cultivars.
7. Endophytic Colletotrichum fructicola
The genus Colletotrichum comprises the most common endophytic fungal species found within plant tissues [19,246,247,248]. For instance, endophytic Colletotrichum spp. that are members of the gloeosporioides complex were the most common endophytes isolated from forest leaves [199,249]. Endophytic C. fructicola has been recovered from several plants, including citrus, grasses, mangoes, coffee, tea plants, cocoa, and aquatic plants (Table 18).
Endophytic Colletotrichum that inhabits host tissues may benefit the plants because endophytes can enhance plant growth, confer heat and drought tolerance, and provide disease resistance [249,250,251,252]. Based on several factors, including wounding, changes in plant physiology, host senescence, and changes in environmental factors, endophytic Colletotrichum may transform into a saprophyte or pathogen that induces disease symptoms [253,254,255,256,257]. According to Crouch et al. [257], endophytism of Colletotrichum spp. may serve as a vital part of their lifecycle; however, this is not fully understood.

Table 18.
Endophytic Colletotrichum fructicola isolated from various plants.
Table 18.
Endophytic Colletotrichum fructicola isolated from various plants.
| Host Plants | Plant Parts | Countries | References |
|---|---|---|---|
| Citrus | |||
| Citrus reticulata ‘Nanfengmiju’ | Leaves | Jiangxi Province, China | [70] |
| Fortunella margarita | Branches | Guangxi Province, China | [70] |
| Grasses | |||
| Dwarf napier (Pennisetum purpureum) and lemon grass (Cymbopogon citratus) | Leaves and sheaths | Muang District, Chiang Rai, Thailand | [258] |
| Lawn grass (Axonopus compressus) | Leaves | Penang, Malaysia | [259] |
| Coffee (Coffea arabica) | Berries | Chiang Mai, Thailand | [18] |
| Cacao (Theobroma cacao) | Leaves | Panama | [19] |
| Mango (Mangifera indica) | Young and mature leaves, stem fragments (with cork layer), and mature inflorescences | Pernambuco State, Brazil | [260] |
| Tea (Camellia sinensis) | Leaves | Fujian, Guizhou, Henan, Jiangxi, Sichuan, Yunnan, and Zhejiang, China | [6] |
| Dendrobium spp. | Leaves and roots | Mae Fah Luang District, Chiang Rai, Thailand | [261] |
| Licania tomentosa | Leaves | Brazil | [262] |
| Java plum (Syzygium cumini) | Fruits and seeds | Malang, East Java, Indonesia | [263] |
| Tabernaemontana heyneana (medicinal plant) | Leaves | Southern Western Ghats, India | [264] |
| Magnolia candolli and M. garrettii | Leaves | M. candolli: Yunnan Province, China M. garrettii: Chiang Mai Province, Thailand | [265] |
| Aquatic plants | Leaves | Yunnan, Guizhou, and Sichuan Provinces, China | [266] |
| Chinese boxthorn (Lycium chinense) | Leaves and fruits | South Chungcheong Province, Republic of Korea | [267] |
Endophytic Colletotrichum that colonize roots, stems, and leaves, and extensively occupy plant tissues, can be categorized as Class 2 endophytes based on the description by Rodriguez et al. [256]. Class 2 endophytes are described as endophytic fungi that colonize plants through direct penetration or via infectious structures and colonize plant tissues intercellularly, with little to no effect on the host plant [256,268,269]. In healthy plants, fungal sporulation is low; however, during senescence, fungi rapidly sporulate and emerge [270].
Several studies have demonstrated that species isolated as endophytes, including C. fructicola, can switch to a pathogenic state [99,259,260,265,271]. These findings highlight the lifestyle-switching from endophyte to pathogen, which may contribute to the infection of various hosts by C. fructicola. The switching of fungal lifestyles is described as a symbiotic continuum, in which the interaction involves a balance of antagonism [272,273,274]. When endophytism appears to be in a labile state, frequent switching from endophytism to pathogenicity, or from mutualism to parasitism, occurs [275].
Pathogenic species of the gloeosporioides complex may likely switch to an endophytic state, especially the species with a wide host range. This observation was based on studies by Redman et al. [276] and Redman et al. [251]. Pathogenic C. magna can infect cucurbits, causing anthracnose, but can also act as an endophyte when colonizing various noncucurbits. In contrast, mutant C. magna were found capable of infecting tomatoes, a nonhost, and colonizing cucurbit cultivars without inducing disease symptoms, which indicated its endophytic lifestyle or mutualistic effects [260,276]. The mutation of a single locus in pathogenic C. magna allowed the plant pathogen to switch to an endophytic state [277]. According to Redman et al. [251], environmental factors or host plant genetic variations may play a role in causing individual isolates of pathogenic Colletotrichum spp. to adopt an endophytic lifestyle in host plants and provide disease resistance, drought tolerance, and growth enhancement.
Although the major factors that cause lifestyle switching are difficult to determine, several have been predicted thus far, such as environmental factors and host plant genetic variations (Figure 3). Hazardous environmental conditions affect plant health, resulting in a weakening of plant defense responses. In turn, endophytes can grow abruptly and switch to a pathogenic form [278,279]. The host plant and microbial genotypes that are involved in fungal gene expression and plant-host recognition are regarded as important factors that influence whether fungi adopt a particular lifestyle [280]. In addition, higher nutrient conditions have been recognized to stimulate lifestyle switching [281]. Other factors, such as plant age and abiotic environments, have also been proposed to influence whether fungi act as pathogens or endophytes [273,274]. Under these conditions, the balance of antagonism between the endophyte and host plant is affected, resulting in disease occurrence and visible symptoms [274].
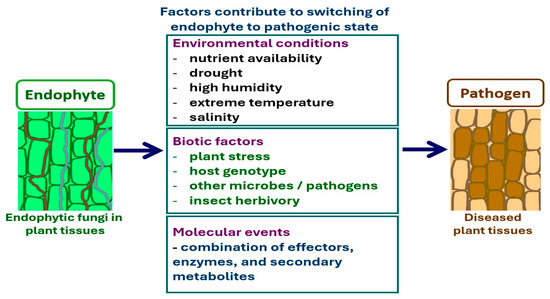
Figure 3.
Factors contribute to lifestyle switching from endophyte to pathogen.
The ecological role of endophytic C. fructicola can be understood through a comparative study with known beneficial endophytes, such as C. tofieldiae [282] and Epichloë sp. [283]. This comparative study would include genomic and transcriptomic analyses to examine, among other factors, the presence or absence of virulence factors, genes related to stress tolerance, profiling of secondary metabolites, and pathways for phytohormone synthesis. Such analysis could uncover regulatory mechanisms that dictate whether the fungus acts as a pathogen or remains endophytic; it would also identify the genes linked to pathogenicity and those associated with beneficial traits for host plants [284].
8. Fine Line Between Pathogenesis and Endophytism
Colletotrichum spp. can exhibit both pathogenic and nonpathogenic lifestyles, demonstrating a fine line between pathogenicity and endophytism, as demonstrated in studies by Redman et al. [276] and Redman et al. [251]. The wildtype C. magna isolate was pathogenic to cucurbits; however, disruption of the pathogenicity gene resulted in a nonpathogenic, symbiotic C. magna mutant that expressed either commensal, mutualistic, or intermediate-mutualistic lifestyles [276]. Previously identified as a commensal on peppers and tomatoes in disease-resistance experiments, C. gloeosporioides was found to switch to mutualism in both host plants during drought and growth studies. Furthermore, C. musae was designated as an intermediate mutualist in disease studies on the tomato varieties, Big Beef and California Wonder, but found to be a commensal on both tomato varieties in growth enhancement experiments, as well as a mutualist on peppers in a drought-tolerance study [251].
Redman et al. [251] concluded that the host plant determines the outcome of fungal-plant symbiosis, as well as the fungal lifestyles expressed. This may be due to differences in fungal gene expression in response to the host plant or differences in the ability of a plant to respond to fungal infection. Redman et al. [251] also pointed out that slight genetic differences between plant cultivars of a species may significantly alter the outcome of fungal-plant symbiosis. Based on these findings, Colletotrichum infections have several possible outcomes; however, predicting lifestyle switching based on fungal-plant interactions remains difficult.
The switch from endophyte to pathogen involves significant changes in fungal gene expression, protein synthesis, and metabolic functions, which can be uncovered through comparative multi-omics approaches [285]. By identifying the genes and pathways that influence host interaction, nutrient uptake, and stress responses at various stages, multi-omics approaches facilitate the development of more focused and sustainable strategies for disease management. Multi-omic studies have been utilized to elucidate the mechanisms underlying endophytic and pathogenic states of Diaporthe [30] which are relevant to C. fructicola. Hilario and Goncalve [30] outlined the molecular mechanisms involved in the transition from endophyte to pathogen, including MAPK pathways, small RNAs, and systems for effector secretion.
Both transcriptomic and genomic approaches can yield valuable insights into the fungal switching from an endophytic to a pathogenic state. The authors of [206] conducted a comparative genomics and transcriptomics study that revealed that effectors and enzymes related to secondary metabolism are activated during the endophytic state, while hydrolases and transporters are upregulated during the transition to necrotrophy or the pathogenic state. Given the dual lifestyle of C. fructicola, omics technologies could be employed in the future to uncover candidate genes essential for sustaining endophytic relationships with plants and to elucidate the mechanisms that drive transitions to pathogenicity.
Relatedness Between Endophytic and Pathogenic Lifestyles
A phylogenetic approach was used to analyze the general relationship between fungal lifestyles. From the phylogenetic analysis, fungal endophytes were generally found to be closely related to plant pathogens, either as biotrophic or necrotrophic fungi, which was also applicable to C. fructicola; as with any other plant pathogenic Colletotrichum species, the fungus showed biotrophic and necrotrophic stages.
The switching to endophytic, biotrophic, and necrotrophic lifestyles in various fungal strains was analyzed by Delaye et al. [286] using a phylogenetic analysis of 5.8S rRNA gene sequences combined with ancestral character mapping. Based on the evolutionary scale, switching from an endophytic lifestyle to necrotrophy, and conversely, occurred multiple times at an equal frequency, whereas switching from an endophyte to a biotroph was infrequent. These findings imply that the disruption of mutualistic interactions resulted in a pathogenic lifestyle [287,288]. The phylogenetic analysis also showed that the endophytic stage is evolutionarily labile, with frequent switching occurring between endophytic and pathogenic states [275,289].
Clustering of the tested fungal species in the phylogenetic tree showed that five clusters contained only biotrophs, suggesting that once biotrophy had developed, reversal to an endophytic state or necrotrophy would not occur. The clustering of biotrophs also indicated that they were often depicted as having derived and evolutionarily stable traits. Endophytes and necrotrophs clustered together in most of the major clades, which indicated lifestyle switching at a very short evolutionary timescale [286]. Both endophytic and necrotrophic lifestyles have been reported for species within the gloeosporioides complex, such as C. musae and C. gloeosporioides [254,271,290].
Phylogenetic analysis based on multiple markers was performed on neotropical strains of endophytic and pathogenic Colletotrichum isolated from the leaves of cacao (T. cacao) and other plant species. Phylogenetic analysis revealed that the endophytic and pathogenic strains were clustered into two separate clades. The endophyte clade consisted of strains from cacao and other plants, whereas the pathogenic clades contained strains from a single host plant [19]. The two species, C. tropicale and C. ignotum (syn. C. fructicola), were the most common endophytes associated with cacao and other plants, whereas C. theobromicola was associated with anthracnose in cacao fruit and leaves. These findings suggest that endophytes do not show host-specificity, as they occur in various host plants, and that pathogens may have specialized relationships with host plants [19]. Host-specificity was also not detected among endophytic Colletotrichum isolated from the leaves of 12 tree species [291].
9. Bioactivity of Colletotrichum fructicola
Plant pathogenic and endophytic Colletotrichum spp. produce a variety of secondary metabolites with extensive biological activities. Secondary metabolites produced by plant pathogenic Colletotrichum play a role as pathogenicity and virulence factors during pathogenesis. Secondary metabolites produced by endophytic fungi may intensify host plant resistance against diseases, abiotic stress, insect infestation, and herbivory [292].
According to Moraga et al. [293], approximately 189 secondary metabolites have been recovered from Colletotrichum spp. and were biologically and chemically characterized. The most common secondary metabolites produced are sterols, terpenes, phenolics, pyrones, fatty acids, and nitrogen-containing compounds [294]. These secondary metabolites have potential applications in the agriculture and pharmaceutics industries as antimicrobial compounds, antiherbicides, and drugs to treat maladies.
To date, only two reports on the secondary metabolite compounds produced by C. fructicola have been made (Table 19). Endophytic C. fructicola recovered from Nothapodytes nimmoniana could synthesize camptothecin, an anticancer compound, under submerged conditions. The presence of camptothecin was detected using TLC and HPLC [295]. In another study, the endophytic C. fructicola isolated from the leaves of Co. arabica could produce indole-3-acetic acid (IAA) in vitro. A crude extract of the IAA was tested for plant growth ability in corn (Zea mays L.), rice (Oryza sativa L.), and rye (Secale cereale L.). Application of the IAA crude extract resulted in the elongation of the coleoptile segment of the tested plants, indicating that IAA can stimulate plant growth [296].

Table 19.
Biocompounds and bioactivity of Colletotrichum fructicola.
Based on the whole-genome sequence data of C. fructicola N425 causing anthracnose in tea plants and C. fructicola causing circular leaf spot in rubber trees, gene clusters related to secondary metabolites were detected. These secondary metabolite gene clusters may be involved in host-specific interactions and the pathogenicity of C. fructicola [232,298]. The findings demonstrated that C. fructicola has the capability to produce diverse groups of secondary metabolites.
In addition to the gene clusters related to secondary metabolites, numerous unique pectinases were detected in the genomes of dicot plants infected by C. fructicola. The genome of the pathogen encoded more than 100 pectinases [257]. Among the Colletotrichum spp. genome sequences, that of C. fructicola contained the largest number of cell wall-degrading enzymes [299]. The expression of a large number of extracellular enzymes might reflect the ability of C. fructicola to colonize a wide host range and adapt to various environments [257].
Cell wall-degrading enzymes, including pectinases, cutinases, hemicellulases, and cellulases, are involved in the penetration and colonization of host plants [300,301,302]. The production of extracellular enzymes, namely pectin lyase, polygalacturonase, and laccase, has been reported for C. fructicola causing apple bitter rot and Glomerella leaf spot. The production of different extracellular enzymes by the pathogen could also be related to the different symptoms observed in apple fruits and leaves [297].
Plant pathogens produce toxic metabolites that induce diseases and are involved in the development of disease symptoms [303]. Toxic metabolites can also act as virulence factors involved in pathogenicity [304,305]. Thus far, no reports on C. fructicola-produced toxic metabolites have been made. However, several phytotoxins have been detected from several Colletotrichum spp. that are involved in disease development. Colletotrichins A, B, and C were recovered from C. nicotianae cultures, and when applied to tobacco, lettuce, and rice seedlings, symptoms similar to those of anthracnose appeared [306,307]. From the culture filtrates of C. higginsianum, colletophyrandione was isolated, which was found to affect Sonchus arvensis, Helianthus annuus, Convolvulus arvensis, and Ambrosia artemisiifolia [308]. Chlororesorcinol recovered from C. higginsianum and produced necrosis on the leaves of S. arvensis and tomato plants. In addition, this compound showed potential herbicidal activity [308].
10. Management of Plant Diseases Caused by Colletotrichum fructicola
Chemical control is the main method used by many growers, which relies on the use of different classes of fungicides. However, the improper use of fungicides has resulted in toxic effects in humans and the generation of resistant species [309,310]. This has led to the development of more environmentally friendly methods, including biocontrol, the use of essential oils, and heat treatment.
10.1. Chemical Control
Chemical control using fungicides with different modes of action is frequently applied to control anthracnose in various crops. The fungicide classes commonly used to control Colletotrichum spp. associated with anthracnose was previously reviewed by Dowling et al. [311]. The most frequently used fungicide classes for controlling anthracnose are methyl benzimidazole carbamates (MBCs), dithiocarbamates, and quinone outside inhibitors (QoIs); however, due to increased fungal resistance, QoIs and MBCs are no longer effective [312,313,314].
For the proper management of anthracnose caused by C. fructicola, several screening studies of fungicide sensitivity and efficacy, as well as field trials, have been conducted on strawberries, pears, apples, peaches, and chili fruit (Table 20). The fungicide sensitivity study results provide information on the application and implementation of suitable fungicides for their effective usage and effective control of anthracnose. Fungicide sensitivity also provides information on the risk of resistance and status of the tested fungicides.

Table 20.
Fungicide sensitivity testing to control Colletotrichum fructicola infections in various crops.
10.2. Essential Oils
Essential oils are used as an alternative method to control postharvest diseases, particularly for pathogen-infected fruit crops. Essential oils are commonly extracted from plants and contain various types of bioactive compounds. These bioactive compounds act synergistically to exhibit antioxidant, antimicrobial, and insecticidal properties [317,318,319]. The use of essential oils to control postharvest diseases is often combined with edible coatings, such as chitosan, which act as carriers of the bioactive compounds to increase their antimicrobial properties and contribute to a longer shelf life [320]. The use of essential oils to control postharvest diseases caused by Colletotrichum spp. in fruit crops was previously reviewed by da Costa Goncalves et al. [320].
Studies on the use of essential oils to control anthracnose and leaf spot caused by C. fructicola in several fruit crops are presented in Table 21. Several essential oils from lemongrass and mint, such as carvacrol, honokiol, magnolol, thymol, and magnolol, have the potential to inhibit C. fructicola growth and reduce the development of anthracnose lesions (Table 21). Although the use of essential oils is promising, further studies on their toxicity, safety levels, storage conditions, antifungal mechanisms, and cost-effectiveness are needed [300].

Table 21.
Essential oil testing against Colletotrichum fructicola infections in various crops.
10.3. Biological and Other Control Methods
Many biocontrol agents, including avirulent Colletotrichum, bacteria, filamentous fungi, and yeast, have been tested against Colletotrichum spp. that cause anthracnose in postharvest fruit crops [322]. For the biocontrol of C. fructicola, the Lysobacter enzymogenes OH11 and Bacillus tequilensis YYC 155 strains showed the potential to control anthracnose in pears and the leaves of Ca. oleifera, respectively (Table 22).
Similar to essential oils, the use of biocontrol agents against Colletotrichum spp. still lacks information regarding the mechanism underlying antimicrobial inhibition, microbial toxicity and viability, storage conditions, and cost-effectiveness [322]. Other methods tested to control C. fructicola in fruit crops are neutral electrolyzed water, hot water and chitosan, and natamycin (Table 22). These methods have shown the potential to control anthracnose formation on strawberries, papaya, and apples [323,324,325].

Table 22.
Biocontrol and other methods tested to control Colletotrichum fructicola infections in various crops.
Table 22.
Biocontrol and other methods tested to control Colletotrichum fructicola infections in various crops.
| Diseases/Crops | Results | References | |
|---|---|---|---|
| Biocontrol Methods | |||
Lysobacter enzymogenes OH11
| Pear anthracnose | Potential biocontrol agent against C. fructicola causing pear anthracnose and could help reduce the use of fungicides | [326] |
Bacillus tequilensis strain YYC 155
| Camellia oleifera leaf anthracnose | Potential biocontrol agent against C. fructicola causing leaf anthracnose of Camellia oleifera | [327] |
| Neutral electrolyzed water (pH 6.5–7.5): Applied through an overhead irrigation system | Strawberry anthracnose | Effective to control strawberry anthracnose | [323] |
| Hot water and chitosan Hot water dip at 49 °C for 20 min combined with 1% and 2% chitosan | Papaya anthracnose | Possibly used to control papaya anthracnose during postharvest storage without exerting negative effects on fruit physicochemical quality | [324] |
Natamycin
| Apple postharvest rot | Natamycin has the potential to be used as a biopreservative against C. fructicola infection in apples | [325] |
Similar to the other infections caused by Colletotrichum species, control measures used to manage infections by C. fructicola can be specific depending on the host plant; however, some measures are generally applied to most host plants. To control anthracnose and leaf spot across a range of plants, an integrated approach is typically applied, which includes cultural practices, fungicide use, and biocontrol. Cultural practices have been employed to reduce inoculum production. The use of appropriate cultural practices and suitable fungicides could help improve disease management. Many biocontrol agents have been tested in vitro; however, their field efficacy has not been encouraging thus far, although they have been combined with chemical control methods in some trials.
In general, formulating effective control methods to manage anthracnose caused by Colletotrichum spp. requires relevant data and an understanding of the host plant, species of the causal pathogen, and the environment. According to Dowling et al. [311], these three factors are not easy to establish, and they had proposed three essential aspects that can enhance the understanding of plant pathogenic Colletotrichum spp. that could eventually lead to better disease management strategies. The first aspect involves identification of the causal pathogen, which is essential for establishing strategic disease management. As the genus Colletotrichum consists of several species’ complexes, distinguishing individual species is important due to differences in species-specific sensitivity profiles. The second aspect involves the extensive or large-scale field sampling of Colletotrichum isolates. A larger collection of isolates would provide precise data for developing suitable plant disease management strategies. The third aspect comprises fungicide sensitivity testing, which should include fungicides of different classes to provide a better formulation of disease management strategies. Fungicide sensitivity testing would also uncover potential threats of resistance or reduced sensitivity to a particular fungicide, as well as the inherent resistances of individual species Dowling et al. [311].
11. Conclusions and Future Directions
This review highlighted the widespread occurrence of C. fructicola in tropical, subtropical, and temperate regions, which is likely attributable to the increasing trade and movement of agricultural crops and plant materials. From current studies available, C. fructicola was demonstrated to adopt endophytic and pathogenic lifestyles that contribute to its adaptation and distribution. Many disease notes, new disease reports, and publications have reported new plant host infected by C. fructicola in several regions worldwide, indicating the expansion of its host and geographical areas. Similar to other plant pathogens, the host plant expansion of C. fructicola could be associated with the availability of a suitable host and climatic conditions that are favorable for its growth and establishment. Control measures used to manage C. fructicola infections still rely on fungicides; nevertheless, for effective disease management, an integrated approach is required to reduce disease incidence and severity.
The transition from endophytic to pathogenic behavior in C. fructicola presents an opportunity to exploit its endophytic form for biological control and disease suppression. In plants that are nonhosts or less susceptible to infection, the fungal endophyte survives in an endophytic manner and offer defense against various pathogens [256]. Additionally, the ability of symbiotic plasticity enables certain strains to switch between endophytic to pathogenic behavior depending on environmental factors and host plant growth stages [256].
Studies on closely related Colletotrichum species offer promising insights into the possibility of applying endophytic C. fructicola for disease suppression and biocontrol. For example, endophytic C. gloeosporioides showed antagonistic activity against fungal pathogens of Camellia sinensis, which may play a role in plant defense mechanisms against the pathogen [328]. In a study by da Silva Santos et al. [329], strains of endophytic C. siamense demonstrated growth promoting traits and suppressed the growth of Fusarium oxysporum in tomato plants. The main challenge in utilizing endophytic C. fructicola for disease suppression and biological control arise from its potential to act as a pathogen. The transition from being an endophyte to pathogen can frequently be initiated by changes in environmental conditions or the health status of the host plant. Thus, any use of endophytic C. fructicola as a biological control agent would necessitate a comprehensive understanding of the genetic and environmental factors that influence its lifestyle [284].
RNA interference (RNAi), a method for silencing genes, offers potential for the management of diseases caused by C. fructicola. The application of RNAi in managing Colletotrichum spp. infections has been reported by Gu et al. [330], Mahto et al. [331], Qiao et al. [332], and Goulin et al. [333]. The results from these studies are pertinent to C. fructicola, as RNAi can be utilized to target and silence essential genes that hinder the pathogen growth and ability to cause disease. Among the essential target genes for the pathogen’s development and pathogenicity are those involved in the formation of conidia and appressoria, cell wall biosynthesis, and pathogenicity factors [242,331]. Despite the promising application in disease management, several challenges must be resolved for RNAi to be widely adopted, including ensuring effective uptake by fungal cells and accurately targeting genes to prevent silencing of nontarget genes. Additionally, protocols for large-scale application and the necessary regulatory approvals are still being developed [334]. Future studies should focus on enhancing delivery mechanisms, such as through nanoparticle carriers, identifying specific target genes, and integrating RNAi with other strategies for integrated disease management [334].
Genomic comparisons of C. fructicola with other species of the gloeosporioides complex and other omics data, such as transcriptomics, provide a more comprehensive understanding of the ability of C. fructicola to infect a large number of hosts, the infection process, adaptation patterns, and insight into its transformation from endophytic to pathogenic lifestyles. To date, four genomes of C. fructicola have been sequenced, including C. fructicola Nara-gc5 causing strawberry crown rot [207], C. fructicola N425 that can infect tea plants [219], C. fructicola 1104–7 isolated from lesions of 320 leaf spot [232], and C. fructicola causing circular leaf spot in rubber trees [335]. In addition, three draft genomes of C. fructicola have been reported, namely of isolates associated with mango anthracnose [336], strawberry anthracnose [337], and tea leaf spot [298]. Thus far, no data exists on the genome sequence of endophytic C. fructicola. Nevertheless, the genomic and transcriptomic data of pathogenic C. fructicola provides notable information on the genes encoding secondary metabolites, effectors, and pectin-degrading enzymes that are associated with the transformation from the endophytic to pathogenic stages.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum-current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and necrotrophic lifestyle choice during postharvest disease development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef]
- Hacquard, T.; Kracher, B.; Hiruma, K.; Munch, P.C.; Garrido-Oter, G.; Thon, M.R.; Weimann, A.; Damm, U.; Dallery, J.F.; Hainaut, M.; et al. Survival trade-offs in plant roots during colonization by closely related beneficial and pathogenic fungi. Nat. Commun. 2016, 7, e11362. [Google Scholar] [CrossRef] [PubMed]
- Dini-Andreote, F. Endophytes: The second layer of plant defense. Trends Plant Sci. 2020, 25, 319–322. [Google Scholar] [CrossRef]
- Liu, F.; Weir, B.S.; Damm, U.; Crous, P.W.; Wang, Y.; Liu, B.; Wang, M.; Zhang, M.; Cai, L. Unravelling Colletotrichum species associated with Camellia: Employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Pers. Mol. Phylogeny Evol. Fungi 2015, 35, 63–86. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.S.; Hyde, K.D.; Damm, U.; Cai, L.; Liu, M.; Li, X.H.; Zhang, W.; Zhao, W.S.; Yan, J.Y. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef]
- Damm, U.; Sato, T.; Alizadeh, A.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud. Mycol. 2019, 92, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.S.; Hyde, K.D.; Chen, Y.J.; Papp, V.; Palla, B.; Papp, D.; Bhunjun, C.S.; Hurdeal, V.G.; Senwanna, C.; Manawasinghe, I.S.; et al. One stop shop IV: Taxonomic update with molecular phylogeny for important phytopathogenic genera: 76–100. Fungal Divers. 2020, 103, 87–218. [Google Scholar] [CrossRef]
- Liu, F.; Ma, Z.Y.; Hou, L.W.; Diao, Y.Z.; Wu, W.P.; Damm, U.; Song, S.; Cai, L. Updating species diversity of Colletotrichum, with a phylogenomic overview. Stud. Mycol. 2022, 101, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Doyle, V.P.; Oudemans, P.V.; Rehner, S.A.; Litt, A. Habitat and host indicate lineage identity in Colletotrichum gloeosporioides s. l. from wild and agricultural landscapes in North America. PLoS ONE 2013, 8, e62394. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, N.; Weir, B.S.; Hyde, K.D.; Shenoy, B.D. The ApMat marker can resolve Colletotrichum species: A case study with Mangifera indica. Fungal Divers. 2013, 61, 117–138. [Google Scholar] [CrossRef]
- Vieira, W.A.S.; Lima, W.G.; Nascimento, E.S.; Michereff, S.J.; Câmara, M.P.S.; Doyle, V.P. The impact of phenotypic and molecular data on the inference of Colletotrichum diversity associated with Musa. Mycologia 2017, 109, 912–934. [Google Scholar] [CrossRef]
- dos Santos Vieira, W.A.; Bezerra, P.A.; da Silva, A.C.; Veloso, J.S.; Câmara, M.P.S.; Doyle, V.P. Optimal markers for the identification of Colletotrichum species. Mol. Phylogenet. Evol. 2020, 143, 106694. [Google Scholar] [CrossRef]
- Prihastuti, H.; Cai, L.; Chen, H.; McKenzie, E.H.C.; Hyde, K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009, 39, 89–109. [Google Scholar]
- Rojas, E.I.; Rehner, S.A.; Samuels, G.J.; Van Bael, S.A.; Herre, E.A.; Cannon, P.; Chen, R.; Pang, J.; Wang, R.; Zhang, Y.; et al. Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panama: Multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia 2010, 102, 1318–1338. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Downer, A.J.; Swain, S.V.; Crump, A. UC IPM Pest Notes: Anthracnose (No. 7420); University of California Agriculture and Natural Resources: Davis, CA, USA, 2020; p. 16. [Google Scholar]
- Nicholson, R.L.; Moraes, W.B.C. Survival of Colletotrichum graminicola: Importance of the spore matrix. Phytopathology 1980, 70, 255–261. [Google Scholar] [CrossRef]
- Chung, P.C.; Wu, H.Y.; Chen, Y.C.; Hung, T.H.; Chung, C.L. Development of a nested PCR assay for detecting Colletotrichum siamense and Colletotrichum fructicola on symptomless strawberry plants. PLoS ONE 2022, 17, e0270687. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Sun, X.; Dong, W.; Ma, L.; Li, H. Detection and quantification of anthracnose pathogen Colletotrichum fructicola in cultivated tea-oil Camellia species from southern China using a DNA-based qPCR assay. Plant Dis. 2023, 107, 363–371. [Google Scholar] [CrossRef] [PubMed]
- McHenry, D.J.; Aćimović, S.G. New species-specific real-time PCR assays for Colletotrichum species causing bitter rot of apple. Microorganisms 2024, 12, 878. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Courtecuisse, R.; Robin, C.; Husson, C.; Moreau, P.A.; Blancard, D.; Selosse, M.A.; Lung-Escarmant, B.; Piou, D.; Sache, I. Species diversity and drivers of spread of alien fungi (sensu lato) in Europe with a particular focus on France. Biol. Invasions 2010, 12, 157–172. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Garrett, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Gladieux, P.; Feurtey, A.; Hood, M.E.; Snirc, A.; Clavel, J.; Dutech, C.; Roy, M.; Giraud, T. The population biology of fungal invasions. In Invasion Genetics: The Baker and Stebbins Legacy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 81–100. [Google Scholar]
- Hilário, S.; Gonçalves, M.F. Mechanisms underlying the pathogenic and endophytic lifestyles in Diaporthe: An omics-based approach. Horticulturae 2023, 9, 423. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Pest categorisation of Colletotrichum fructicola. EFSA J. 2021, 19, e06803. [Google Scholar] [PubMed]
- Burgess, T.I.; Crous, C.J.; Slippers, B.; Hantula, J.; Wingfield, M.J. Tree invasions and biosecurity: Eco-evolutionary dynamics of hitchhiking fungi. AoB Plants 2016, 8, plw076. [Google Scholar] [CrossRef]
- Jeffries, P.; Dodd, J.C.; Jeger, M.J.; Plumbley, R.A. The biology and control of Colletotrichum species on tropical fruit crops. Plant Pathol. 1990, 39, 343–366. [Google Scholar] [CrossRef]
- Velho, A.C.; Stadnik, M.J.; Wallhead, M. Unraveling Colletotrichum species associated with Glomerella leaf spot of apple. Trop. Plant Pathol. 2019, 44, 197–204. [Google Scholar] [CrossRef]
- Smith, C.; Oten, K.; Anthracnose Diseases of Trees. NC Extension. 2022. Available online: https://content.ces.ncsu.edu/anthracnose#section_heading_17027 (accessed on 23 May 2025).
- Downer, A.J.; Swain, S.S. Pest Notes: Anthracnose UC ANR Publication 7420. 2020. Available online: https://ipm.ucanr.edu/home-and-landscape/anthracnose/pest-notes/#gsc.tab=0 (accessed on 23 May 2025).
- Velho, A.C.; Alaniz, S.; Casanova, L.; Mondino, P.; Stadnik, M.J. New insights into the characterization of Colletotrichum species associated with apple diseases in southern Brazil and Uruguay. Fungal Biol. 2015, 119, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, M.F.; Velho, A.C.; Gonçalves, A.E.; Mondino, P.E.; Alaniz, S.M.; Stadnik, M.J. Genetic structure of Colletotrichum fructicola associated to apple bitter rot and Glomerella leaf spot in southern Brazil and Uruguay. Phytopathology 2016, 106, 774–781. [Google Scholar] [CrossRef]
- Casanova, L.; Hernández, L.; Martínez, E.; Velho, A.C.; Rockenbach, M.F.; Stadnik, M.J.; Alaniz, S.; Mondino, P. First report of Glomerella leaf spot of apple caused by Colletotrichum fructicola in Uruguay. Plant Dis. 2017, 101, 834. [Google Scholar] [CrossRef]
- Denardi, F.; Berton, O.; Spengler, M.M. Resistência genética à podridão amarga em maçãs, determinadas pela taxa de desenvolvimento da doença em frutos com e sem. Rev. Bras. Frutic. 2003, 25, 494–497. [Google Scholar] [CrossRef]
- Nodet, P.; Chalopin, M.; Crété, X.; Baroncelli, R.; Le Floch, G. First report of Colletotrichum fructicola causing apple bitter rot in Europe. Plant Dis. 2019, 103, 1767. [Google Scholar] [CrossRef]
- Sutton, B.C. The genus Glomerella and its anamorph Colletotrichum. In Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CAB International: Wallingford, UK, 1992; pp. 1–26. [Google Scholar]
- Alaniz, S.; Hernández, L.; Mondino, P. Colletotrichum fructicola is the dominant and one of the most aggressive species causing bitter rot of apple in Uruguay. Trop. Plant Pathol. 2015, 40, 265–274. [Google Scholar] [CrossRef]
- Kim, C.; Hassan, O.; Lee, D.; Chang, T. First report of anthracnose of apple caused by Colletotrichum fructicola in Korea. Plant Dis. 2018, 102, 2653. [Google Scholar] [CrossRef]
- Kim, C.H.; Hassan, O.; Chang, T. Diversity, pathogenicity, and fungicide sensitivity of Colletotrichum species associated with apple anthracnose in South Korea. Plant Dis. 2020, 104, 2866–2874. [Google Scholar] [CrossRef]
- Wenneker, M.; Pham, K.T.K.; Kerkhof, E.; Harteveld, D.O. First report of preharvest fruit rot of ‘Pink Lady’apples caused by Colletotrichum fructicola in Italy. Plant Dis. 2021, 105, 1561. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, D.; Wang, W.; Gleason, M.L.; Zhang, R.; Liang, X.; Sun, G. Diversity of Colletotrichum species causing apple bitter rot and Glomerella leaf spot in China. J. Fungi 2022, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.D.; Wang, W.; Qin, R.F.; Zhang, R.; Sunb, G.Y.; Gleason, M.L. Colletotrichum fructicola, first record of bitter rot of apple in China. Mycotaxon 2014, 126, 23–30. [Google Scholar] [CrossRef]
- Munir, M.; Amsden, B.; Dixon, E.; Vaillancourt, L.; Gauthier, N.W. Characterization of Colletotrichum species causing bitter rot of apple in Kentucky orchards. Plant Dis. 2016, 100, 2194–2203. [Google Scholar] [CrossRef]
- Yokosawa, S.; Eguchi, N.; Kondo, K.I.; Sato, T. Phylogenetic relationship and fungicide sensitivity of members of the Colletotrichum gloeosporioides species complex from apple. J. Gen. Plant Pathol. 2017, 83, 291–298. [Google Scholar] [CrossRef]
- Oo, M.M.; Yoon, H.Y.; Jang, H.A.; Oh, S.K. Identification and characterization of Colletotrichum species associated with bitter rot disease of apple in South Korea. Plant Patho. J. 2018, 34, 480. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, B.R.; Park, I.H.; Hahm, S.S. First report of two Colletotrichum species associated with bitter rot on apple fruit in Korea. C. fructicola and C. siamense. Mycobiology 2018, 46, 154–158. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.T.; Jeon, Y. Research to fungicide sensitivity of Colletotrichum spp. isolated from apple fruits in Cheongsong, Korea. Res. Plant Dis. 2023, 29, 145–157. [Google Scholar] [CrossRef]
- Chowdappa, P.; Reddy, G.S.; Kumar, A.; Rao, B.M.; Rawal, R.D. Morphological and molecular characterization of Colletotrichum species causing anthracnose of grape in India. Asian Australas. J. Plant Sci. Biotechnol. 2009, 3, 71–77. [Google Scholar]
- Sawant, I.S.; Narkar, S.P.; Shetty, D.S.; Upadhyay, A.; Sawant, S.D. Emergence of Colletotrichum gloeosporioides sensu lato as the dominant pathogen of anthracnose disease of grapes in India as evidenced by cultural, morphological and molecular data. Australas. Plant Pathol. 2012, 41, 493–504. [Google Scholar] [CrossRef]
- Wilcox, W.F.; Gubler, W.D.; Uyemoto, J.K. Compendium of Grape Diseases, Disorders, and Pests, 2nd ed.; The American Phytopathological Society: St. Paul, MN, USA, 2015; p. 232. [Google Scholar]
- Ye, B.; Zhang, J.; Chen, X.; Xiao, W.; Wu, J.; Yu, H.; Zhang, C. Genetic Diversity of Colletotrichum spp. causing grape anthracnose in Zhejiang, China. Agronomy 2023, 13, 952. [Google Scholar] [CrossRef]
- Daykin, M.E.; Milholland, R.D. Ripe rot of Muscadine grape caused by Colletotrichum gloeosporioides and its control. Phytopathology 1984, 74, 710–714. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Scariot, F.J.; Fontanella, G.; Favaron, F.; Sella, L.; Santos, M.C.; Schwambach, J.; Pedrotti, C.; Delamare, A.P.L. Colletotrichum species causing grape ripe rot disease in Vitis labrusca and V. vinifera varieties in the highlands of southern Brazil. Plant Pathol. 2020, 69, 1504–1512. [Google Scholar] [CrossRef]
- Peng, L.J.; Sun, T.; Yang, Y.L.; Cai, L.; Hyde, K.D.; Bahkali, A.H.; Liu, Z.Y. Colletotrichum species on grape in Guizhou and Yunnan provinces, China. Mycoscience 2012, 54, 29–41. [Google Scholar] [CrossRef]
- Guginski-Piva, C.A.; Bogo, A.; Gomes, B.R.; Menon, J.K.; Nodari, R.O.; Welter, L.J. Morphological and molecular characterization of Colletotrichum nymphaeae and C. fructicola associated with anthracnose symptoms of grape in Santa Catarina State, southern Brazil. J. Plant Dis. Prot. 2018, 125, 405–413. [Google Scholar] [CrossRef]
- Lim, Y.S.; Hassan, O.; Chang, T. First report of anthracnose of Shine muscat caused by Colletotrichum fructicola in Korea. Mycobiology 2020, 48, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yokosawa, S.; Eguchi, N.; Sato, T. Characterization of the Colletotrichum gloeosporioides species complex causing grape ripe rot in Nagano Prefecture, Japan. J. Gen. Plant Path. 2020, 86, 163–172. [Google Scholar] [CrossRef]
- Fu, M.; Crous, P.W.; Bai, Q.; Zhang, P.F.; Xiang, J.; Guo, Y.S.; Zhao, F.F.; Yang, M.M.; Hong, N.; Xu, W.X.; et al. Colletotrichum species associated with anthracnose of Pyrus spp. in China. Pers. Mol. Phylogeny Evol. Fungi 2019, 42, 1–35. [Google Scholar] [CrossRef]
- Jiang, J.; Zhai, H.; Li, H.; Wang, Z.; Chen, Y.; Hong, N.; Wang, G.; Chofong, G.N.; Xu, W. Identification and characterization of Colletotrichum fructicola causing black spots on young fruits related to bitter rot of pear (Pyrus bretschneideri Rehd.) in China. Crop Prot. 2014, 58, 41–48. [Google Scholar] [CrossRef]
- Choi, E.D.; Park, S.Y. First report of anthracnose caused by Colletotrichum fructicola on hybrid pear fruit in Korea. Plant Dis. 2021, 105, 3291. [Google Scholar] [CrossRef]
- Li, H.N.; Jiang, J.J.; Hong, N.; Wang, G.P.; Xu, W.X. First report of Colletotrichum fructicola causing bitter rot of pear (Pyrus bretschneideri) in China. Plant Dis. 2013, 97, 1000. [Google Scholar] [CrossRef]
- Zhang, P.F.; Zhai, L.F.; Zhang, X.K.; Huang, X.Z.; Hong, N.; Xu, W.; Wang, G. Characterization of Colletotrichum fructicola, a new causal agent of leaf black spot disease of sandy pear (Pyrus pyrifolia). Eur. J. Plant Pathol. 2015, 143, 651–662. [Google Scholar] [CrossRef]
- Peng, L.; Yang, Y.; Hyde, K.D.; Bahkali, A.H.; Liu, Z. Colletotrichum species on Citrus leaves in Guizhou and Yunnan provinces, China. Cryptogam. Mycol. 2012, 33, 267–283. [Google Scholar]
- Huang, F.; Chen, G.Q.; Hou, X.; Fu, Y.S.; Cai, L.; Hyde, K.D.; Li, H.Y. Colletotrichum species associated with cultivated citrus in China. Fungal Divers. 2013, 61, 61–74. [Google Scholar] [CrossRef]
- Hu, W.L.; Ma, Y.Z.; Chen, J.Z. First report of Citrus sinensis anthracnose caused by Colletotrichum fructicola in China. Plant Dis. 2019, 103, 1018. [Google Scholar] [CrossRef]
- Uysal, A.; Kurt, Ş.; Guarnaccia, V. Distribution and characterization of Colletotrichum species associated with citrus anthracnose in eastern Mediterranean region of Turkey. Eur. J. Plant Pathol. 2022, 163, 125–141. [Google Scholar] [CrossRef]
- Taheri, H.; Javan-Nikkhah, M.; Elahinia, S.A.; Khodaparast, S.A.; Golmohammadi, M. Species of Colletotrichum associated with citrus trees in Iran. Mycol. Iran. 2016, 3, 1–14. [Google Scholar]
- Ben Hadj Daoud, H.; Ben Salem, I.; Sánchez, J.; Gallego, E.; Boughalleb-M’Hamdi, N. Occurrence of Colletotrichum fruticola along with C. gloeosporioides in causing anthracnose disease on Citrus sinensis in Tunisia. Indian Phytopathol. 2019, 72, 409–419. [Google Scholar] [CrossRef]
- Wang, W.; de Silva, D.D.; Moslemi, A.; Edwards, J.; Ades, P.K.; Crous, P.W.; Taylor, P.W. Colletotrichum species causing anthracnose of citrus in Australia. J. Fungi 2021, 7, 47. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Y.; Gao, X.Y.; Zhang, Z.H. Study on the identification techniques for determining strawberry cultivars resistance to anthracnose. J. Fruit. Sci. 2008, 25, 542–547. [Google Scholar]
- Zhang, L.; Song, L.; Xu, X.; Zou, X.; Duan, K.; Gao, Q. Characterization and fungicide sensitivity of Colletotrichum species causing strawberry anthracnose in eastern China. Plant Dis. 2020, 104, 1960–1968. [Google Scholar] [CrossRef]
- Jian, Y.; Li, Y.; Tang, G.; Zheng, X.; Khaskheli, M.I.; Gong, G. Identification of Colletotrichum species associated with anthracnose disease of strawberry in Sichuan Province, China. Plant Dis. 2021, 105, 3025–3036. [Google Scholar] [CrossRef]
- Gan, P.; Nakata, N.; Suzuki, T.; Shirasu, K. Markers to differentiate species of anthracnose fungi identify Colletotrichum fructicola as the predominant virulent species in strawberry plants in Chiba Prefecture of Japan. J. Gen. Plant Pathol. 2017, 83, 14–22. [Google Scholar] [CrossRef]
- Li, Y. Anthracnose of Strawberry. 2023. The Connecticut Agricultural Experiment Station. Available online: http://portal.ct.gov/caes (accessed on 20 January 2025).
- Zhang, Y.; Yu, H.; Hu, M.; Wu, J.; Zhang, C. Fungal pathogens associated with strawberry crown rot disease in China. J. Fungi 2022, 8, 1161. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Wang, N.Y.; Peres, N.A. Multilocus phylogenetic analyses of Colletotrichum gloeosporioides species complex causing crown rot on strawberry in Florida. Phytopathology 2022, 112, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.S.; Huang, J.K.; Jin, B.C.; Yan, J.Y.; Li, X.H.; Hyde, K.D.; Bahkali, A.H.; Yin, S.L.; Zhang, G.Z. An account of Colletotrichum species associated with strawberry anthracnose in China based on morphology and molecular data. Mycosphere 2016, 7, 1147–1191. [Google Scholar] [CrossRef]
- Han, Y.C.; Zeng, X.G.; Xiang, F.Y.; Ren, L.; Chen, F.Y.; Gu, Y.C. Distribution and characteristics of Colletotrichum spp. associated with anthracnose of strawberry in Hubei, China. Plant Dis. 2016, 100, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, X.; Gao, Y.; Li, B.; Mu, W.; Liu, F. Characterization and fungicide sensitivity of Colletotrichum spp. from different hosts in Shandong, China. Plant Dis. 2019, 103, 34–43. [Google Scholar] [CrossRef]
- Chen, X.Y.; Dai, D.J.; Zhao, S.F.; Shen, Y.; Wang, H.D.; Zhang, C.Q. Genetic diversity of Colletotrichum spp. causing strawberry anthracnose in Zhejiang, China. Plant Dis. 2020, 104, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.C.; Wu, H.Y.; Wang, Y.W.; Ariyawansa, H.A.; Hu, H.P.; Hung, T.H.; Tzean, S.S.; Chung, C.L. Diversity and pathogenicity of Colletotrichum species causing strawberry anthracnose in Taiwan and description of a new species, Colletotrichum miaoliense sp. nov. Sci. Rep. 2020, 10, 14664. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Y.; Yu, H.; Zhou, J.; Hu, M.; Liu, A.; Wu, J.; Wang, H.; Zhang, C. Colletotrichum spp. diversity between leaf anthracnose and crown rot from the same strawberry plant. Front. Microbiol. 2022, 13, 860694. [Google Scholar] [CrossRef]
- Han, S.; Xu, X.; Jiang, Y.; Zheng, Z.; Yuan, H.; Li, S.; Liu, Y.; Lin, T.; Qiao, T.; Yang, C.; et al. Colletotrichum fructicola, causal agent of shot-hole symptoms on leaves of Prunus sibirica in China. Plant Dis. 2023, 107, 2530. [Google Scholar] [CrossRef]
- Saini, T.J.; Gupta, S.G.; Anandalakshmi, R. First report of papaya anthracnose caused by Colletotrichum fructicola in India. New Dis. Rep. 2016, 34, 27. [Google Scholar] [CrossRef]
- Marquez-Zequera, I.; Cruz-Lachica, I.; Ley-Lopez, N.; Carrillo-Facio, J.A.; Osuna-Garcia, L.A.; Garcia-Estrada, R.S. First report of Carica papaya fruit anthracnose caused by Colletotrichum fructicola in Mexico. Plant Dis. 2018, 102, 2649. [Google Scholar] [CrossRef]
- dos Santos Vieira, W.A.; Veloso, J.S.; da Silva, A.C.; dos Santos Nunes, A.; Doyle, V.P.; Castlebury, L.A.; Câmara, M.P.S. Elucidating the Colletotrichum spp. diversity responsible for papaya anthracnose in Brazil. Fungal Biol. 2022, 126, 623. [Google Scholar]
- Lima, N.B.; de, A. Batista, M.V.; Morais, M.A., Jr.; Barbosa, M.A.G.; Michereff, S.J.; Hyde, K.D.; Câmara, M.P.S. Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 2013, 61, 75–88. [Google Scholar] [CrossRef]
- Mo, J.; Zhao, G.; Li, Q.; Soalngi, G.S.; Tang, L.; Huang, S.; Guo, T.; Hsiang, T. Identification and characterization of Colletotrichum species associated with mango anthracnose in Guangxi, China. Plant Dis. 2018, 102, 283–1289. [Google Scholar] [CrossRef]
- Joa, J.H.; Lim, C.K.; Choi, I.Y.; Park, M.J.; Shin, H.D. First report of Colletotrichum fructicola causing anthracnose on mango in Korea. Plant Dis. 2016, 100, 1793. [Google Scholar] [CrossRef]
- Tovar-Pedraza, J.M.; Mora-Aguilera, J.A.; Nava-Diaz, C.; Lima, N.B.; Michereff, S.J.; Sandoval-Islas, J.S.; Camara, M.P.S.; Téliz-Ortiz, D.; Leyva-Mir, S.G. Distribution and pathogenicity of Colletotrichum species associated with mango anthracnose in Mexico. Plant Dis. 2020, 104, 137–146. [Google Scholar] [CrossRef]
- Dela Cueva, F.M.; Laurel, N.R.; Dalisay, T.U.; Sison, M.L.J. Identification and characterisation of Colletotrichum fructicola, C. tropicale and C. theobromicola causing mango anthracnose in the Philippines. Arch. Phytopathol. Plant Prot. 2021, 54, 1989–2006. [Google Scholar] [CrossRef]
- Ismail, A.M.; El-Ganainy, S.M. Characterization of Colletotrichum species associating with anthracnose disease of mango in Egypt. J. Plant Dis. Prot. 2022, 129, 449–454. [Google Scholar] [CrossRef]
- Lin, W.L.; Duan, C.H.; Wang, C.L. Identification and virulence of Colletotrichum species causing anthracnose on mango. Plant Pathol. 2023, 72, 623–635. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Rivera-Vargas, L.I.; Goenaga, R.; Navarro, E.D.; French-Monar, R.D. First report of Colletotrichum fructicola and C. queenslandicum causing fruit rot of rambutan (Nephelium lappaceum). Plant Dis. 2017, 101, 1043. [Google Scholar] [CrossRef]
- Armand, A.; Hyde, K.D.; Jayawardena, R.S. First report of Colletotrichum fructicola causing fruit rot and leaf-tip dieback on pineapple in Northern Thailand. Plants 2023, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Luo, C.X.; Wu, H.J.; Peng, B.; Kang, B.S.; Liu, L.M.; Zhang, M.; Gu, Q.S. Colletotrichum species associated with anthracnose disease of watermelon (Citrullus lanatus) in China. J. Fungi 2022, 8, 790. [Google Scholar] [CrossRef]
- Li, W.; Ran, F.; Long, Y.; Mo, F.; Shu, R.; Yin, X. Evidence of Colletotrichum fructicola causing anthracnose on Passiflora edulis Sims in China. Pathogens 2021, 11, 6. [Google Scholar] [CrossRef]
- Evallo, E.; Taguiam, J.D.; Bengoa, J.; Maghirang, R.; Balendres, M.A. First report of Colletotrichum fructicola, causing anthracnose of Hylocereus plants, in the Philippines. Czech Mycol. 2021, 73, 79–90. [Google Scholar] [CrossRef]
- Nur-Shakirah, A.O.; Khadijah, M.S.; Kee, Y.J.; Chew, B.L.; Zakaria, L.; Nor, N.M.I.M.; Subramaniam, S.; Leong, Y.H.; Mohd, M.H. Colletotrichum species associated with fig (Ficus carica L.) in Malaysia. Crop Prot. 2023, 169, 106256. [Google Scholar] [CrossRef]
- Tang, Z.; Lou, J.; He, L.; Wang, Q.; Chen, L.; Zhong, X.; Wu, C.; Zhang, L.; Wang, Z.Q. First report of Colletotrichum fructicola causing anthracnose on cherry (Prunus avium) in China. Plant Dis. 2022, 106, 317. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Li, Y.; Ji, S.; Li, X.; Hyde, K.D.; Zhang, K.; Phillips, A.J.; Manawasinghe, I.S.; Yan, J. Identification and characterization of Colletotrichum species associated with cherry leaf spot disease in China. Plant Dis. 2023, 107, 500–513. [Google Scholar] [CrossRef]
- Carraro, T.A.; Lichtemberg, P.S.F.; Michailides, T.J.; Pereira, W.V.; Figueiredo, J.A.G.; May-De Mio, L.L. First report of Colletotrichum fructicola, C. nymphaeae, and C. melonis causing persimmon anthracnose in Brazil. Plant Dis. 2019, 103, 2692. [Google Scholar] [CrossRef]
- Evallo, E.; Taguiam, J.D.; Balendres, M.A. Colletotrichum fructicola associated with fruit anthracnose of persimmon. J. Phytopathol. 2022, 170, 194–201. [Google Scholar] [CrossRef]
- Wu, C.J.; Lin, M.C.; Ni, H.F. Colletotrichum species causing anthracnose disease on avocado fruit in Taiwan. Eur. J. Plant Pathol. 2023, 165, 629–647. [Google Scholar] [CrossRef]
- Armand, A.; Jayawardena, R.S. Morphomolecular identification and pathogenicity of Colletotrichum species associated with avocado anthracnose in northern Thailand. Plant Pathol. 2024, 73, 186–197. [Google Scholar] [CrossRef]
- Shu, J.; Guo, T.; Li, Q.; Tang, L.; Huang, S.; Mo, J.; Yu, Z.; Forte-Perri, V. First report of leaf spot caused by Colletotrichum fructicola and C. siamense on Ziziphus mauritiana in Guangxi, China. Plant Dis. 2021, 105, 2021. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Fang, L.; Xie, X.; Wang, L. First report of Colletotrichum fructicola and Colletotrichum nymphaeae causing leaf spot on Rubus corchorifolius in Zhejiang province, China. Plant Dis. 2021, 105, 3746. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mo, J.; Guo, T.; Li, Q.; Tang, L.; Huang, S.; Wei, J.; Hsiang, T. First report of Colletotrichum fructicola causing anthracnose on Pouteria campechiana in China. Plant Dis. 2021, 105, 708. [Google Scholar] [CrossRef]
- Li, S.C.; Xiao, L.H.; Wu, F.; Wang, Y.B.; Jia, M.S.; Chen, M.; Chen, J.Y.; Xiang, M.L. First report of leaf spot caused by Colletotrichum fructicola on Myrica rubra in China. Plant Dis. 2022, 106, 1993. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W. Postharvest anthracnose of carambola (Averrhoa carambola) caused by Colletotrichum fructicola in China. Plant Dis. 2023, 107, 1234. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, X.; Xu, Z.; Zhang, L.; Wang, Y.; Cui, R.; Kuang, W.; Xia, Y.; Ma, J. First Report of Colletotrichum fructicola causing anthracnose on Punica granatum in China. Plant Dis. 2023, 107, 2246. [Google Scholar] [CrossRef]
- Huang, R.; Sun, W.; Guo, T.; Huang, S.; Tang, L.; Chen, X.; Li, Q. Morphological and pathological characterization of Colletotrichum species causing anthracnose of litchi leaves in Guangxi, China. J. Phytopathol. 2023, 171, 609–619. [Google Scholar] [CrossRef]
- Poti, T.; Kisaki, G.; Arita, K.; Akimitsu, K. Identification and characterization of Colletotrichum species causing kiwifruit anthracnose in Kagawa Prefecture, Japan. J. Gen. Plant Pathol. 2023, 89, 84–90. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Mariño, Y.A.; Bayman, P. Pathogens causing anthracnose and fruit rots of coffee associated with the coffee berry borer and the entomopathogenic fungus Beauveria bassiana in Puerto Rico. Phytopathology 2020, 110, 1541–1552. [Google Scholar] [CrossRef]
- Gaitán, A.L.; Cristancho, M.A.; Caicedo, B.C.; Rivillas, C.A.; Gómez, G.C. Compendium of Coffee Diseases and Pests; American Phytopathological Society: St. Paul, MN, USA, 2015. [Google Scholar]
- Shi, N.N.; Du, Y.X.; Ruan, H.C.; Yang, X.J.; Dai, Y.L.; Gan, L.; Chen, F.R. First report of Colletotrichum fructicola causing anthracnose on Camellia sinensis in Guangdong Province, China. Plant Dis. 2018, 102, 241. [Google Scholar] [CrossRef]
- Lin, S.R.; Yu, S.Y.; Chang, T.D.; Lin, Y.J.; Wen, C.J.; Lin, Y.H. First report of anthracnose caused by Colletotrichum fructicola on tea in Taiwan. Plant Dis. 2021, 105, 710. [Google Scholar] [CrossRef]
- Chen, X.G.; Liu, C.L.; Liu, J.A.; Zhou, G.Y. First report of Colletotrichum fructicola causing anthracnose on Camellia yuhsienensis in China. Plant Dis. 2022, 106, 321. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ye, N.; Chen, Y.; Lian, L.; Jin, S.; Lai, J.; Xie, Y. Identification and phylogenetic analysis of anthracnose pathogen Colletotrichum fructicola isolated from Camellia sinensis. J. Tea Sci. 2014, 34, 95–104. [Google Scholar]
- Wang, Y.C.; Hao, X.Y.; Wang, L.; Xiao, B.; Wang, X.C.; Yang, Y.J. Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci. Rep. 2016, 6, 35287. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wang, Y.; Li, N.; Ni, D.; Yang, Y.; Wang, X. Differences in the characteristics and pathogenicity of Colletotrichum camelliae and C. fructicola isolated from the tea plant [Camellia sinensis (L.) O. Kuntze]. Front. Microbiol. 2018, 9, 3060. [Google Scholar] [CrossRef]
- Lin, S.R.; Lin, Y.H.; Ariyawansa, H.A.; Chang, Y.C.; Yu, S.Y.; Tsai, I.; Chung, C.L.; Hung, T.H. Analysis of the pathogenicity and phylogeny of Colletotrichum species associated with brown blight of tea (Camellia sinensis) in Taiwan. Plant Dis. 2023, 107, 97–106. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.Y.; Xu, X.; Cheng, J.; Zheng, L.; Huang, J.; Li, D.W. Identification and characterization of Colletotrichum species associated with anthracnose disease of Camellia oleifera in China. Plant Dis. 2020, 104, 474–482. [Google Scholar] [CrossRef]
- Zhu, H.; He, C. Identification and characterization of Colletotrichum species causing tea-oil Camellia (Camellia oleifera C. Abel) anthracnose in Hainan, China. Forests 2023, 14, 1030. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Zhang, D.P.; Wu, H.L.; Pan, L.Q.; Liao, N.Y.; Liu, W.C. First report of anthracnose caused by Colletotrichum siamense and C. fructicola of Camellia chrysantha in China. Plant Dis. 2021, 105, 2020. [Google Scholar] [CrossRef]
- Peng, X.J.; Wang, Q.C.; Zhang, S.K.; Guo, K.; Zhou, X.D. Colletotrichum species associated with Camellia anthracnose in China. Mycosphere 2023, 14, 130–157. [Google Scholar] [CrossRef]
- Sharma, G.; Shenoy, B.D. Colletotrichum fructicola and C. siamense are involved in chilli anthracnose in India. Arch. Phytopathol. Plant Prot. 2014, 47, 1179–1194. [Google Scholar] [CrossRef]
- Chethana, C.S.; Chowdappa, P.; Pavani, K.V. Colletotrichum truncatum and C. fructicola causing anthracnose on chilli in Karnataka state of India. Indian Phytopathol. 2015, 68, 270–278. [Google Scholar]
- Liu, F.; Tang, G.; Zheng, X.; Li, Y.; Sun, X.; Qi, X.; Zhou, Y.; Xu, J.; Chen, H.; Chang, X.; et al. Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province, China. Sci. Rep. 2016, 6, 32761. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.Z.; Zhang, C.; Liu, F.; Wang, W.Z.; Liu, L.; Cai, L.; Liu, X.L. Colletotrichum species causing anthracnose disease of chilli in China. Pers. Mol. Phylogeny Evol. Fungi 2017, 38, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Katoch, A.; Sharma, P.; Sharma, P.N. Identification of Colletotrichum spp. associated with fruit rot of Capsicum annuum in North Western Himalayan region of India using fungal DNA barcode markers. J.Plant Biochem. Biot. 2017, 26, 216–223. [Google Scholar] [CrossRef]
- Nuraini, M.N.; Latiffah, Z. Identification and characterization of Colletotrichum spp. associated with chili anthracnose in peninsular Malaysia. Eur. J. Plant Pathol. 2018, 151, 961–973. [Google Scholar]
- Shi, N.N.; Ruan, H.C.; Jie, Y.L.; Chen, F.R.; Du, Y.X. Characterization, fungicide sensitivity and efficacy of Colletotrichum spp. from chili in Fujian, China. Crop Prot. 2021, 143, 105572. [Google Scholar] [CrossRef]
- Nguyen, V.Q.H.; Tran, T.T.N.; Tran, L.T.; Nguyen, T.T.T.; Pham, T.T.T.; Hoang, Q.T.; Pham, T.T.D. Identification of fungal species associated with chilli fruit disease in North-Central Vietnam. J. Plant Pathol. 2023, 106, 507–526. [Google Scholar] [CrossRef]
- Narmadhavathy, S.; Nayar, K.A.; Gokulapalan, C.; Geetha, D. First report of leaf spot disease of culinary melon caused by Colletotrichum fructicola in India. Indian Phytopathol. 2016, 69, 318–319. [Google Scholar]
- Jiang, D.L.; Harata, K.; Ogawa, M.; Shirota, K.; Sasaki, A.; Nakamura, T.; Okamoto, S.; Park, E.Y.; Sato, K.; Nakamura, Y.; et al. Multiple Colletotrichum species cause anthracnose disease on Japanese pickling melon var. Katsura-uri (Cucumis melo var. conomon). J. Gen. Plant Pathol. 2023, 89, 249–259. [Google Scholar] [CrossRef]
- Li, P.; Zhu, J.Z.; Li, X.G.; Zhong, J. Identification and characterization of Colletotrichum fructicola and Colletotrichum siamense causing anthracnose on luffa sponge gourd in China. Plants 2022, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lan, G.; Yang, Y.; Tang, Y.; Li, Z.; She, X.; He, Z. First report of anthracnose caused by Colletotrichum fructicola on Brassica parachinensis in China. Crop Prot. 2022, 154, 105842. [Google Scholar] [CrossRef]
- Silva-Cabral, J.R.A.; da Silva, J.L.; Soares, L.D.S.; Costa, J.F.O.; Amorim, E.D.R.; Lima, G.D.A.; Assunção, I.P. First report of Colletotrichum fructicola and C. tropicale causing anthracnose on orchids in Brazil. Plant Dis. 2019, 103, 2672. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Q.; Liang, S.; Li, C.; Chen, X.; Li, Z. Colletotrichum species causing Bletilla striata anthracnose in the Guizhou Province, China. Crop Prot. 2023, 170, 106277. [Google Scholar] [CrossRef]
- Wang, S.; Huang, J.; Zheng, M.; Wang, Y.; Yuan, Q.; Gao, Q.; Zhou, H. First report of anthracnose on Bletilla striata caused by Colletotrichum fructicola in China. Plant Dis. 2022, 106(2), 756. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Guo, M. First report of anthracnose on Dendrobium officinale caused by Colletotrichum fructicola in Anhui Province, China. Plant Dis. 2020, 104, 574. [Google Scholar] [CrossRef]
- Qing, Z.; Xiao, D.; Chen, H.; Shen, Y.; Pan, L.; Wen, R. First report of Colletotrichum fructicola causing anthracnose on Crinum asiaticum in Guangxi province, China. J. Plant Pathol. 2020, 102, 971. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Gilardi, G.; Martino, I.; Garibaldi, A.; Gullino, M.L. Species diversity in Colletotrichum causing anthracnose of aromatic and ornamental Lamiaceae in Italy. Agronomy 2019, 9, 613. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, J.; Li, Q.; Forte-Perri, V.; Bu, Z.; La, Y.; Tao, D. First report of anthracnose of Mandevilla× amabilis caused by Colletotrichum fructicola in Guangxi, Southern China. Plant Dis. 2020, 104, 3258. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Martino, I.; Gilardi, G.; Garibaldi, A.; Gullino, M.L. High fungal species diversity in association with woody ornamental plants: Colletotrichum as a case study. Acta Hortic. 2021, 1331, 293–302. [Google Scholar] [CrossRef]
- Wan, Y.; Jin, G.Q.; Li, D.W.; Wu, S.; Zhu, L.H. First report of Colletotrichum fructicola causing leaf spots on Liriodendron chinense × tulipifera in China. For. Pathol. 2022, 52, e12779. [Google Scholar] [CrossRef]
- Yin, Q.; Shi, X.; Zhu, Z.; Wang, Y.; Tian, L.; Sang, Z.; Jia, Z.; Ma, L. First report of leaf spot caused by Colletotrichum fructicola on Magnolia wufengensis in Hubei, China. Plant Dis. 2022, 106, 2987. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, Y.; Li, Q.; Huang, S.; Chen, X.; Guo, T.; Tang, L. Leaf spot caused by Colletotrichum siamense, C. fructicola, and C. aeschynomenes on Ixora chinensis in Guangxi, China. Plant Dis. 2024, 108, 225. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Du, C.; Ding, C. First report of Colletotrichum fructicola causing anthracnose on Rosa chinensis in China. Plant Dis. 2023, 107, 3316. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Xiang, M.; Xiao, L.; Zheng, Z.; Yang, H.; Zeng, J.; Jiang, H.; Chen, J.; Chen, M. First report of Colletotrichum fructicola causing anthracnose on Paeonia lactiflora in China. Plant Dis. 2023, 107, 4022. [Google Scholar] [CrossRef]
- He, J.; Li, D.W.; Bian, J.Y.; Zhu, L.H.; Huang, L. Unravelling species diversity and pathogenicity of Colletotrichum associated with anthracnose on Osmanthus fragrans in Quanjiao, China. Plant Dis. 2023, 107, 350–362. [Google Scholar] [CrossRef]
- Zhang, M.; Li, D.; Si, Y.; Ju, Y.; Zhu, L. Colletotrichum species associated with anthracnose in Salix babylonica in China. Plants 2023, 12, 1679. [Google Scholar] [CrossRef]
- Wang, Q.H.; Li, D.W.; Duan, C.H.; Liu, X.H.; Niu, S.G.; Hou, L.Q.; Wu, X.Q. First report of walnut anthracnose caused by Colletotrichum fructicola in China. Plant Dis. 2018, 102, 247. [Google Scholar] [CrossRef]
- Chang, J.; Zhai, F.; Zhang, Y.; Wang, D.; Shu, J.; Yao, X. Identification and characterization of Colletotrichum fioriniae and C. fructicola that cause anthracnose in pecan. Front. Plant Sci. 2022, 13, 1043750. [Google Scholar] [CrossRef]
- Gong, J.; Sun, D.; Bian, N.; Wang, R.; Wang, X.; Wang, X. First report of Colletotrichum fructicola causing anthracnose on peanut (Arachis hypogaea) in China. Plant Dis. 2023, 107, 2879. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, F.; Xie, C.P.; Zhang, C.; Li, X. First report of Colletotrichum fructicola causing anthracnose on macadamia in China. Plant Dis. 2023, 107, 1230. [Google Scholar] [CrossRef]
- Ma, J.; Xue, Q.; Min, L.J.; Zhang, L.Q. First report of Colletotrichum fructicola causing anthracnose on Carya cathayensis Sarg. in China. Plant Dis. 2023, 107, 2253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, M.; Rao, B.; Chen, Y.; Cai, C.; Gao, H. First report of anthracnose on Paris. polyphylla var. chinensis caused by Colletotrichum fructicola in Northern Fujian, China. Plant Dis. 2020, 104, 2728. [Google Scholar]
- Song, L.; Lin, W.; Jiang, N.; Zhang, Z.; Tan, G.; Wei, S. Anthracnose disease of Amomum villosum caused by Colletotrichum fructicola in China. J. Gen. Plant Pathol. 2021, 87, 259–263. [Google Scholar] [CrossRef]
- Jiang, G.H.; Jiang, A.M.; Fan, C.L.; Wei, J.G.; Ren, L.Y.; Luo, J.T. First report of anthracnose on Kadsura coccinea Caused by Colletotrichum fructicola in China. Plant Dis. 2022, 106, 1757. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, J.; Wang, A.; Zhang, Y.; Song, Y.; Lin, Z.; Jia, X.; Feng, Z.; Zeng, C.; Zhang, W. Occurrence of anthracnose caused by Colletotrichum fructicola on Ficus hirta in Southern China. Plant Dis. 2023, 108, 205. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Lin, S.; Guo, J.; Shi, Y.P.; Gao, Q.; Zhou, H. First report of Colletotrichum fructicola causing leaf spot on Smilax glabra Roxb in China. Plant Dis. 2024, 104, 1112. [Google Scholar] [CrossRef]
- Shu, J.; Ning, P.; Guo, T.; Tang, L.; Huang, S.; Li, Q.; Mo, J.; Yu, Z.; Hsiang, T. First report of leaf spot caused by Colletotrichum fructicola on Callerya speciosa (Millettia speciosa) in Guangxi, China. Plant Dis. 2020, 104, 1–6. [Google Scholar] [CrossRef]
- Hou, W.; Chu, L.; Yang, L.; Dong, N.; Dan, Z.; Zhong, H.; Dong, C. First report of Colletotrichum fructicola causing anthracnose on Epimedium sagittatum (Sieb. et Zucc.) Maxim. in China. Plant Dis. 2024, 108, 813. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lin, Z.; Zhang, Z.; Chen, J. First report of Colletotrichum fructicola causing anthracnose on water hyacinth in China. Plant Dis. 2021, 105, 2246. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Asano, S.; Okayama, K.O.; Ohki, S.T.; Tojo, M. Weeds as the potential inoculum source of Colletotrichum fructicola responsible for strawberry anthracnose in Nara, Japan. J. Gen. Plant Pathol. 2018, 84, 12–19. [Google Scholar] [CrossRef]
- Li, Q.; Yu, J.; Liu, Y.; Zhang, W.; Liu, Y.; Guo, W.; Huo, J. First report of Colletotrichum fructicola causing anthracnose of Japanese brome (Bromus japonicus Thunb.) in China. J. Plant Pathol. 2023, 105, 1167. [Google Scholar] [CrossRef]
- Atghia, O.; Alizadeh, A.; Fotouhifar, K.B.; Damm, U.; Stukenbrock, E.H.; Javan-Nikkhah, M. First report of Colletotrichum fructicola as the causal agent of anthracnose on common bean and cowpea. Mycol. Iran. 2015, 2, 139–140. [Google Scholar]
- Wang, H.C.; Huang, Y.F.; Chen, Q.; Wang, M.S.; Xia, H.Q.; Shang, S.H.; Zhang, C.Q. Anthracnose caused by Colletotrichum fructicola on tobacco (Nicotiana tabacum) in China. Plant Dis. 2016, 100, 1235. [Google Scholar] [CrossRef]
- Niu, X.P.; Gao, H.; Chen, Y.; Qi, J.M. First report of anthracnose on white jute (Corchorus capsularis) caused by Colletotrichum fructicola and C. siamense in China. Plant Dis. 2016, 100, 1243. [Google Scholar] [CrossRef]
- Shi, N.N.; Du, Y.X.; Chen, F.R.; Ruan, H.C.; Yang, X.J. First report of leaf spot caused by Colletotrichum fructicola on Japanese fatsia (Fatsia japonica) in Fujian Province in China. Plant Dis. 2017, 101, 1552. [Google Scholar] [CrossRef]
- Conforto, C.; Lima, N.B.; Garcete-Gómez, J.M.; Câmara, M.P.S.; Michereff, S.J. First report of Colletotrichum siamense and C. fructicola causing cladode brown spot in Nopalea cochenillifera in Brazil. J. Plant Pathol. 2017, 99, 812. [Google Scholar]
- Sun, J.W.; Si, Y.Z.; Li, D.W.; Jin, G.Q.; Zhu, L.H. First report of leaf blotch of Aesculus chinensis caused by Colletotrichum gloeosporioides and Colletotrichum fructicola in China. Plant Dis. 2020, 104, 3065. [Google Scholar] [CrossRef]
- Mangwende, E.; Truter, M.; Aveling, T.A.S.; Chirwa, P.W. Anthracnose leaf spot pathogens, Colletotrichum fructicola and Colletotrichum cigarro, associated with Eucalyptus seed produced in South Africa. Australas. Plant Pathol. 2021, 50, 533–543. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, R.; Ying, Q.; Zhang, Y.; Zhang, L. First report of leaf spot caused by Colletotrichum fructicola on Dalbergia hupeana in China. Plant Dis. 2022, 106, 1526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yu, Z.; Li, Q.; Tang, L.; Guo, T.; Huang, S.; Mo, J.; Hsiang, T. Leaf spot caused by Colletotrichum fructicola on star anise (Illicium verum) in China. Plant Dis. 2022, 106, 1060. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.; Shin, Y.U.; Lee, K.S.; Lee, D.W.; Chang, T. First report of anthracnose on spotted laurel caused by Colletotrichum fructicola in South Korea. Plant Dis. 2023, 107, 2522. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhuo, D.; Yang, T.; Dai, L.; Li, L.; Zhao, H.; Liu, X.; Cai, Z. Characterization of Colletotrichum causing anthracnose on rubber trees in Yunnan: Two new records and two new species from China. Plant Dis. 2023, 107, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, P.; Meng, H.; Liu, J.; Wu, X.; Gong, G.; Chen, H.; Shang, J.; Yang, W.; Chang, X. First report of Colletotrichum fructicola causing anthracnose on Glycine max in China. Plant Dis. 2023, 107, 2240. [Google Scholar] [CrossRef]
- Dos Santos, G.C.; Lima Horn, L.M.; Trezzi Casa, R.; Gorayeb, E.S.; Nascimento da Silva, F.; Bogo, A.; Nascimento, S.C.D.; Soardi, K.; Sartori Pereira, F.; Gonçalves, M.J. First Report of Colletotrichum fructicola causing anthracnosis on Glycine max in Brazil. Plant Dis. 2024, 108, 814. [Google Scholar] [CrossRef]
- Norphanphoun, C.; Hyde, K.D. First report of Colletotrichum fructicola, C. rhizophorae sp. nov. and C. thailandica sp. nov. on mangrove in Thailand. Pathogens 2023, 12, 1436. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, W.; Yang, G.; Liu, J.; Xu, L.; Li, G.; Wang, J. First report of anthracnose on Paeonia delavayi caused by Colletotrichum fructicola in China. Plant Dis. 2023, 107, 3309. [Google Scholar] [CrossRef]
- Wang, C.B.; Li, Y.; Xue, H.; Piao, C.G.; Jiang, N. Diversity of Colletotrichum species causing anthracnose on three oak species (Quercus acutissima, Q. mongolica and Q. variabilis) in China. Plant Pathol. 2023, 72, 1699–1715. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, K.; Li, C.; Wei, C.; Luan, F.; Li, D.; Song, Q. First Report of anthracnose leaf spot caused by Colletotrichum fructicola on Manglietia decidua (Magnoliaceae) in China. Plant Dis. 2023, 107, 1950. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, A.; Ren, M.; Wei, G.; Xu, H. First report on Colletotrichum fructicola causing anthracnose in Chinese sorghum and its management using phytochemicals. J. Fungi 2023, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.G.; Amaral, A.G.G.; Vieira, W.A.D.S.; Veloso, J.S.; Silva, A.C.D.; Silva, C.D.F.B.D.; Balbino, V.D.Q.; Castlebury, L.A.; Câmara, M.P.S. Diversity of Colletotrichum species associated with torch ginger anthracnose. Mycologia 2023, 115, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Wang, B.; Zhang, S.; Liu, G.; Liang, X.; Zhang, R.; Gleason, M.L.; Sun, G. A novel effector CfEC92 of Colletotrichum fructicola contributes to Glomerella leaf spot virulence by suppressing plant defences at the early infection phase. Mol. Plant Pathol. 2020, 21, 936–950. [Google Scholar] [CrossRef]
- Li, J.; Xiong, C.; Ruan, D.; Du, W.; Li, H.; Ruan, C. Identification of Camellia oleifera WRKY transcription factor genes and functional characterization of CoWRKY78. Front. Plant Sci. 2023, 14, 1110366. [Google Scholar] [CrossRef] [PubMed]
- Perfect, S.E.; Hughes, H.B.; O’Connell, R.J.; Green, J.R. Colletotrichum: A model genus for studies on pathology and fungal plant interactions. Fungal Genet. Biol. 1999, 27, 186–198. [Google Scholar] [CrossRef]
- Wharton, P.S.; Schilder, A.C. Novel infection strategies of Colletotrichum acutatum on ripe blueberry fruit. Plant Pathol. 2008, 57, 122–134. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M. Plant infection and the establishment of fungal biotrophy. Trends Plant Sci. 2002, 7, 352–356. [Google Scholar] [CrossRef]
- Cannon, P.F.; Simmons, C.M. Diversity and host preference of leaf endophytic fungi in the Iwokrama Forest Reserve, Guyana. Mycologia 2002, 94, 210–220. [Google Scholar] [CrossRef]
- Prusky, D. Pathogen quiescence in postharvest diseases. Annu. Rev. Phytopathol. 1996, 34, 413. [Google Scholar] [CrossRef] [PubMed]
- Páez-Redondo, A.; Prusky, D.; Hoyos-Carvajal, L. Quiescence as a strategic stage for the infective process of Colletotrichum species. Rev. U.D.C.A Act. Div. Cient. 2022, 25, e2073. [Google Scholar] [CrossRef]
- Sharma, M.; Kulshrestha, S. Colletotrichum gloeosporioides: An anthracnose causing pathogen of fruits and vegetables. Biosci. Biotechnol. Res. Asia 2015, 12, 1233–1246. [Google Scholar] [CrossRef]
- de Silva, D.D.; Crous, P.W.; Ades, P.K.; Hyde, K.D.; Taylor, P.W. Lifestyles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017, 31, 155–168. [Google Scholar] [CrossRef]
- Latunde-Dada, A.O. Colletotrichum: Tales of forcible entry, stealth, transient confinement and breakout. Mol. Plant Pathol. 2001, 2, 187–198. [Google Scholar] [CrossRef]
- Liang, X.; Wang, B.; Dong, Q.; Li, L.; Rollins, J.A.; Zhang, R.; Sun, G. Pathogenic adaptations of Colletotrichum fungi revealed by genome wide gene family evolutionary analyses. PLoS ONE 2018, 13, e0196303. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; He, C.; Zhang, Q.-Y.; Zou, X.; Duan, K.; Gao, Q. Novel fungal pathogenicity and leaf defense strategies are revealed by simultaneous transcriptome analysis of Colletotrichum fructicola and strawberry infected by this fungus. Front. Plant Sci. 2018, 9, 434. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.; Li, S.; Zhou, G.; Liu, J.; Xu, J.; Li, H. Functional analysis of CfSnf1 in the development and pathogenicity of anthracnose fungus Colletotrichum fructicola on tea-oil tree. BMC Genet. 2019, 20, 94. [Google Scholar] [CrossRef]
- Liu, W.; Liang, X.; Gleason, M.L.; Cao, M.; Zhang, R.; Sun, G. Transcription factor CfSte12 of Colletotrichum fructicola is a key regulator of early apple Glomerella leaf spot pathogenesis. Appl. Environ. Microbiol. 2020, 87, e02212-20. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Li, B.; Li, H. The SNARE protein CfVam7 is required for growth, endoplasmic reticulum stress response, and pathogenicity of Colletotrichum fructicola. Front. Microbiol. 2021, 12, 736066. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, S.; Li, H. H3K4 methyltransferase CfSet1 is required for development and pathogenesis in Colletotrichum fructicola. J. Fungi 2022, 8, 363. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.; Chen, S.; Li, H. The histone acetyltransferase CfGcn5 regulates growth, development, and pathogenicity in the anthracnose fungus Colletotrichum fructicola on the tea-oil tree. Front. Microbiol. 2021, 12, 680415. [Google Scholar] [CrossRef]
- Liu, W.; Han, L.; Chen, J.; Liang, X.; Wang, B.; Gleason, M.L.; Zhang, R.; Sun, G. The CfMcm1 regulates pathogenicity, conidium germination, and sexual development in Colletotrichum fructicola. Phytopathology® 2022, 112, 2159–2173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, J.; Li, Y.; Jiao, H.; Luo, L.; Chen, Q.; Li, H.; Zhang, S. The CfAtg5 regulates the autophagy and pathogenicity of Colletotrichum fructicola on Camellia oleifera. Agronomy 2023, 13, 1237. [Google Scholar] [CrossRef]
- Kong, Y.; Yuan, Y.; Yang, M.; Lu, Y.; Liang, X.; Gleason, M.L.; Zhang, R.; Sun, G. CfCpmd1 regulates pathogenicity and sexual development of plus and minus strains in Colletotrichum fructicola causing Glomerella leaf spot on apple in China. Phytopathology 2023, 113, 1985–1993. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Bruno, J.C.; Alonzo, F., III; Xayarath, B.; Cianciotto, N.P.; Freitag, N.E. Contribution of chitinases to Listeria monocytogenes pathogenesis. Appl. Environ. Microbiol. 2010, 76, 7302–7305. [Google Scholar] [CrossRef]
- Dou, D.; Zhou, J.M. Phytopathogen effectors subverting host immunity: Different foes, similar battleground. Cell Host Microbe 2012, 12, 484–495. [Google Scholar] [CrossRef]
- Bozkurt, T.O.; Schornack, S.; Banfield, M.J.; Kamoun, S. Oomycetes, effectors, and all that jazz. Curr. Opin. Plant Biol. 2012, 15, 483–492. [Google Scholar] [CrossRef]
- Gan, P.; Ikeda, K.; Irieda, H.; Narusaka, M.; O’Connell, R.J.; Narusaka, Y. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 2013, 197, 1236–1249. [Google Scholar] [CrossRef]
- Lelwala, R.V.; Korhonen, P.K.; Young, N.D.; Scott, J.B.; Ades, P.K.; Gasser, R.B.; Taylor, P.W.J.; Coleman, C.E. Comparative genome analysis indicates high evolutionary potential of pathogenicity genes in Colletotrichum tanaceti. PLoS ONE 2019, 14, e0212248. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Miao, J.; Shen, D.; Dou, D. Proteinaceous effector discovery and characterization in plant pathogenic Colletotrichum fungi. Front. Microbiol. 2022, 13, 914035. [Google Scholar] [CrossRef]
- da Silva, L.L.; Correia, H.L.N.; Gonçalves, O.S.; Vidigal, P.M.P.; Rosa, R.O.; Santana, M.F.; de Queiroz, M.V. What lies behind the large genome of Colletotrichum lindemuthianum. Front. Fungal Biol. 2024, 5, 1459229. [Google Scholar] [CrossRef]
- Tsushima, A.; Narusaka, M.; Gan, P.; Kumakura, N.; Hiroyama, R.; Kato, N.; Takahashi, S.; Takano, Y.; Narusaka, Y.; Shirasu, K. The conserved Colletotrichum spp. effector candidate CEC3 induces nuclear expansion and cell death in plants. Front. Microbiol. 2021, 12, 682155. [Google Scholar] [CrossRef]
- Wang, C.; Han, M.; Min, Y.; Hu, J.; Pan, Y.; Huang, L.; Nie, J. Colletotrichum fructicola co-opts cytotoxic ribonucleases that antagonize host competitive microorganisms to promote infection. Mbio 2024, 15, e01053-24. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- He, C.; Duan, K.; Zhang, L.; Zhang, L.; Song, L.; Yang, J.; Zou, X.; Wang, Y.; Gao, Q. Fast quenching the burst of host salicylic acid is common in early strawberry/Colletotrichum fructicola interaction. Phytopathology 2019, 109, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Bai, Q.; Zhang, H.; Guo, Y.; Peng, Y.; Zhang, P.; Shen, L.; Hong, N.; Xu, W.; Wang, G. Transcriptome analysis of the molecular patterns of pear plants infected by two Colletotrichum fructicola pathogenic strains causing contrasting sets of leaf symptoms. Front. Plant Sci. 2022, 13, 761133. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, X.; Lu, Q.; Wang, L.; Qian, W.; Li, N.; Ding, C.; Wang, X.; Yang, Y.W. Transcriptional analysis and histochemistry reveal that hypersensitive cell death and H2O2 have crucial roles in the resistance of tea plant (Camellia sinensis (L.) O. Kuntze) to anthracnose. Hortic. Res. 2018, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.K.; Zhou, Z.W.; Guo, T.L.; Peng, C.; Chen, M.; Liu, W. Sequence and Annotation of Colletotrichum fructicola N425 Genome on Tea Plant. Fujian J. Agric. Sci. 2023, 38, 1437–1444. [Google Scholar]
- Eulgem, T.; Somssich, I.E. Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 2007, 10, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Dis. 1998, 82, 596–605. [Google Scholar] [CrossRef]
- Giblin, F.R.; Coates, L.M.; Irwin, J.A.G. Pathogenic diversity of avocado and mango isolates of Colletotrichum gloeosporioides causing anthracnose and pepper spot in Australia. Australas. Plant Path. 2010, 39, 50–62. [Google Scholar] [CrossRef]
- Phoulivong, S.; McKenzie, E.; Hyde, K. Cross infection of Colletotrichum species; a case study with tropical fruits. Curr. Res. Environ. Appl. Mycol. 2012, 2, 99–111. [Google Scholar] [CrossRef]
- de Silva, D.D.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Colletotrichum species associated with chili anthracnose in Australia. Plant Pathol. 2016, 66, 254–267. [Google Scholar] [CrossRef]
- Lakshmi, B.K.M.; Reddy, P.N.; Prasad, R.D. Cross-infection potential of Colletotrichum gloeosporioides Penz. isolates causing anthracnose in subtropical fruit crops. Trop. Agric. Res. 2011, 22, 183–193. [Google Scholar] [CrossRef]
- Freeman, S.; Horowitz-Brown, S.; Afanador-Kafuri, L.; Maymon, M.; Minz, D. Colletotrichum: Host specificity and pathogenicity on selected tropical and subtropical crops. Acta Hortic. 2013, 975, 209–216. [Google Scholar] [CrossRef]
- Eaton, M.J.; Edwards, S.; Inocencio, H.A.; Machado, F.J.; Nuckles, E.M.; Farman, M.; Gauthier, N.A.; Vaillancourt, L.J. Diversity and cross-infection potential of Colletotrichum causing fruit rots in mixed-fruit orchards in Kentucky. Plant Dis. 2021, 105, 115–1128. [Google Scholar] [CrossRef]
- Alahakoon, P.W.; Sreenivasaprasad, S.; Brown, A.E.; Mills, P.R. Selection of a genetic variant within Colletotrichum gloeosporioides isolates pathogenic on mango by passaging through wounded tomato fruits. Physiol. Mol. Plant Pathol. 1992, 41, 227–240. [Google Scholar] [CrossRef]
- Sanders, G.M.; Korsten, L. Comparison of cross inoculation potential of South African avocado and mango isolates of Colletotrichum gloeosporioides. Microbiol. Res. 2003, 158, 143–150. [Google Scholar] [CrossRef]
- Freeman, S.; Shabi, E. Cross-infection of subtropical and temperate fruits by Colletotrichum species from various hosts. Physiol. Mol. Plant Pathol. 1996, 49, 395–404. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; McKenzie, E.H.C.; Hyde, K.D. Are some endophytes of Musa acuminata latent pathogens? Fungal Divers. 2004, 16, 131–140. [Google Scholar]
- Alahakoon, P.W.; Brown, A.E.; Sreenivasaprasad, S. Cross infection potential of genetic groups of Colletotrichum gloeosporioides on tropical fruits. Physiol. Mol. Plant. Pathol. 1994, 44, 93–103. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cai, L.; Cannon, P.F.; Crouch, J.A.; Crous, P.W.; Damm, U.; Zhang, J.Z. Colletotrichum—Names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Vega, F.E.; Simpkins, A.; Aime, M.C.; Posada, F.; Peterson, S.W.; Rehner, S.A.; Arnold, A.E. Fungal endophyte diversity in coffee plants from Colombia, Hawai’i, Mexico and Puerto Rico. Fungal Ecol. 2010, 3, 122–138. [Google Scholar] [CrossRef]
- Gazis, R.; Rehner, S.; Chaverri, P. Species delimitation in fungal endophyte diversity studies and its implications in ecological and biogeographic inferences. Mol. Ecol. 2011, 20, 3001–3013. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Prusky, D.; Freeman, S.; Rodriguez, R.J.; Keen, N.T. A nonpathogenic mutant strain of Colletotrichum magna induces resistance to C. gloeosporioides in avocado fruits. MPMI-Mol. Plant-Microbe Interact. 1994, 7, 326–333. [Google Scholar] [CrossRef]
- Redman, R.S.; Dunigan, D.D.; Rodriguez, R.J. Fungal symbiosis from mutualism to parasitism: Who controls the outcome, host or invader? New Phytol. 2001, 151, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Horowitz, S.; Sharon, A. Pathogenic and nonpathogenic lifestyles in Colletotrichum acutatum from strawberry and other plants. Phytopathology 2001, 91, 986–992. [Google Scholar] [CrossRef]
- Promputtha, I.; Lumyong, S.; Dhanasekaran, V.; McKenzie, E.H.C.; Hyde, K.D.; Jeewon, R. A phylogenetic evaluation of whether endophytes become saprotroph at host senescence. Microb. Ecol. 2007, 53, 579–590. [Google Scholar] [CrossRef]
- Promputtha, I.; Hyde, K.D.; McKenzie, E.H.; Peberdy, J.F.; Lumyong, S. Can leaf degrading enzymes provide evidence that endophytic fungi becoming saprobes? Fungal Divers. 2010, 41, 89–99. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Crouch, J.; O’Connell, R.; Gan, P.; Buiate, E.; Torres, M.F.; Beirn, L.; Shirasu, K.; Vaillancourt, L. The genomics of Colletotrichum. In Genomics of Plant-Associated Fungi: Monocot Pathogens; Dean, R.A., Lichens-Park, A., Kole, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–102. [Google Scholar]
- Manamgoda, D.S.; Udayanga, D.; Cai, L.; Chukeatirote, E.; Hyde, K.D. Endophytic Colletotrichum from tropical grasses with a new species C. endophytica. Fungal Divers. 2013, 61, 107–115. [Google Scholar] [CrossRef]
- Azuddin, N.F.; Mohamad Noor Azmy, M.S.; Zakaria, L. Molecular identification of endophytic fungi in lawn grass (Axonopus compressus) and their pathogenic ability. Sci. Rep. 2023, 13, 4239. [Google Scholar] [CrossRef]
- Vieira, W.A.; Michereff, S.J.; de Morais, M.A.; Hyde, K.D.; Câmara, M.P. Endophytic species of Colletotrichum associated with mango in northeastern Brazil. Fungal Divers. 2014, 67, 181–202. [Google Scholar] [CrossRef]
- Ma, X.; Nontachaiyapoom, S.; Jayawardena, R.S.; Hyde, K.D.; Gentekaki, E.; Zhou, S.; Qian, Y.; Wen, T.; Kang, J. Endophytic Colletotrichum species from Dendrobium spp. in China and Northern Thailand. MycoKeys 2018, 43, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, D.O.; Silva, M.A.; Pinho, D.B.; Pereira, O.L.; Furtado, G.Q. Diversity of pathogenic and endophytic Colletotrichum isolates from Licania tomentosa in Brazil. For. Pathol. 2018, 48, e12448. [Google Scholar] [CrossRef]
- Hanin, N.A.; Fitriasari, P.D. Identification of endophytic fungi from fruits and seeds of jambolana (Syzygium cumini L.) skeels. In IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012060. [Google Scholar] [CrossRef]
- Bhavana, N.S.; Prakash, H.S.; Nalini, M.S. Fungal endophytes from Tabernaemontana heyneana Wall. (Apocynaceae), their molecular characterization, L-asparaginase and Antioxidant Activities. Jordan. J. Biol. Sci. 2020, 13, 543–550. [Google Scholar]
- de Silva, N.I.; Maharachchikumbura, S.S.N.; Thambugala, K.M.; Bhat, D.J.; Karunarathna, S.C.; Tennakoon, D.S.; Phookamsak, R.; Jayawardena, R.S.; Lumyong, S.; Hyde, K.D. Morpho-molecular taxonomic studies reveal a high number of endophytic fungi from Magnolia candolli and M. garrettii in China and Thailand. Mycosphere 2021, 12, 163–237. [Google Scholar] [CrossRef]
- Zheng, H.; Yu, Z.; Jiang, X.; Fang, L.; Qiao, M. Endophytic Colletotrichum species from aquatic plants in southwest China. J. Fungi 2022, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.C.; Lee, H.B.; Lee, J.H.; Shin, K.S.; Ryu, T.H.; Kwon, H.R.; Kim, Y.K.; Youn, Y.N.; Yu, S.H. Endophytic fungi from Lycium chinense Mill and characterization of two new Korean records of Colletotrichum. Int.J. Mol. Sci. 2014, 15, 15272–15286. [Google Scholar] [CrossRef]
- Ernst, M.; Mendgen, K.W.; Wirsel, S.G.R. Endophytic fungal mutualists: Seed-borne Stagonospora spp. Enhance reed biomass production in axenic microcosms. Mol. Plant–Microb. Interact. 2003, 16, 580–587. [Google Scholar] [CrossRef]
- Gao, K.X.; Mendgen, K. Seed-transmitted beneficial endophytic Stagonospora sp. can penetrate the walls of the root epidermis, but does not proliferate in the cortex, of Phragmites australis. Botany 2006, 84, 981–988. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Stenger, E.; Meffert, A.; Hahn, M. Brefeldin a production by Phoma medicaginis in dead pre-colonized plant tissue: A strategy for habitat conquest? Mycol. Res. 2004, 108, 662–671. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; Hyde, K.D. Endophytic fungi of wild banana (Musa acuminata) at doi Suthep Pui National Park, Thailand. Mycol. Res. 2001, 105, 1508–1513. [Google Scholar] [CrossRef]
- Carroll, G. Fungal endophytes in stems and leaves—From latent pathogen to mutualistic symbiont. Ecology 1988, 69, 2–9. [Google Scholar] [CrossRef]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.L.; Arnold, A.E.; Miadlikowska, J.; Sarvate, S.D.; Lutzoni, F. Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol. Phylogenet. Evol. 2007, 42, 543–555. [Google Scholar] [CrossRef]
- Redman, R.S.; Ranson, J.C.; Rodriguez, R.J. Conversion of the pathogenic fungus Colletotrichum magna to a nonpathogenic, endophytic mutualist by gene disruption. Mol. Plant–Microb. Interact. 1999, 12, 969–975. [Google Scholar] [CrossRef]
- Freeman, S.; Rodrigues, R.J. Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science 1993, 260, 75–78. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic Fungi: A Tool for Plant Growth Promotion and Sustainable Agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jensen, B.; Jørgensen, H.J. Fungal Endophytes in Plants and Their Relationship to Plant Disease. Curr. Opin. Microbiol. 2022, 69, 102177. [Google Scholar] [CrossRef]
- Mengistu, A.A. Endophytes: Colonization, behaviour, and their role in defense mechanism. Int. J. Microbiol. 2020, 2020, 6927219. [Google Scholar] [CrossRef]
- Rai, M.; Agarkar, G. Plant-fungal interactions: What triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 2016, 42, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, K.; Gerlach, N.; Sacristán, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramírez, D.; Bucher, M.; O’Connell, R.J.; et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef]
- Shi, X.; Qin, T.; Gao, Y.; Zhao, N.; Ren, A. Comparative genome and transcriptome analysis of the endophytic fungus Epichloë sibirica reveals biological control mechanism in host Achnatherum sibiricum. Biol. Control 2025, 205, 105778. [Google Scholar] [CrossRef]
- Newfeld, J.; Ujimatsu, R.; Hiruma, K. Uncovering the host range–lifestyle relationship in the endophytic and anthracnose pathogenic genus Colletotrichum. Microorganisms 2025, 13, 428. [Google Scholar] [CrossRef]
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” tools for better understanding the plant–endophyte interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef]
- Delaye, L.; García-Guzmán, G.; Heil, M. Endophytes versus biotrophic and necrotrophic pathogens—Are fungal lifestyles evolutionarily stable traits? Fungal Diver. 2013, 60, 125–135. [Google Scholar] [CrossRef]
- Stukenbrock, E.H.; McDonald, B.A. The origins of plant pathogens in agro-ecosystems. Annu. Rev. Phytopathol. 2008, 46, 75–100. [Google Scholar] [CrossRef]
- Eaton, C.J.; Cox, M.P.; Scott, B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 2011, 180, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kogel, K.H.; Franken, P.; Hückelhoven, R. Endophyte or parasite–what decides? Curr. Opin. Plant Biol. 2006, 9, 358–363. [Google Scholar] [CrossRef]
- Choi, O.; Choi, O.; Kwak, Y.-S.; Kim, J.; Kwon, J.-H. Spot anthracnose disease caused by Colletotrichum gloeosporioides on tulip tree in Korea. Mycobiology 2012, 40, 82–84. [Google Scholar] [CrossRef]
- Guozhong, L.U.; Cannon, P.F.; Alex, R.E.I.D.; Simmons, C.M. Diversity and molecular relationships of endophytic Colletotrichum isolates from the Iwokrama Forest Reserve, Guyana. Mycol. Res. 2004, 108, 53–63. [Google Scholar]
- Torres-Mendoza, D.; Ortega, H.E.; Cubilla-Rios, L. Patents on endophytic fungi related to secondary metabolites and biotransformation applications. J. Fungi 2020, 6, 58. [Google Scholar] [CrossRef]
- Moraga, J.; Gomes, W.; Pinedo, C.; Cantoral, J.M.; Hanson, J.R.; Carbú, M.; Garrido, C.; Durán-Patrón, R.; Collado, I.G. The current status on secondary metabolites produced by plant pathogenic Colletotrichum species. Phytochem. Rev. 2019, 18, 215–239. [Google Scholar] [CrossRef]
- Kim, J.W.; Shim, S.H. The fungus Colletotrichum as a source for bioactive secondary metabolites. Arch. Pharm. Res. 2019, 42, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Bhalkar, B.N.; Patil, S.M.; Govindwar, S.P. Camptothecine production by mixed fermentation of two endophytic fungi from Nothapodytes nimmoniana. Fungal Biol. 2016, 120, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Numponsak, T.; Kumla, J.; Suwannarach, N.; Matsui, K.; Lumyong, S. Biosynthetic pathway and optimal conditions for the production of indole-3-acetic acid by an endophytic fungus, Colletotrichum fructicola CMU-A109. PLoS ONE 2018, 13, e0205070. [Google Scholar] [CrossRef]
- Velho, A.C.; Mondino, P.; Stadnik, M.J. Extracellular enzymes of Colletotrichum fructicola isolates associated to apple bitter rot and Glomerella leaf spot. Mycology 2018, 9, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Jiang, H.; Li, X.; Lu, Q.; Wang, X.; Wang, Y. Draft genome sequence of Colletotrichum fructicola causing leaf spot on tea plants (Camellia sinensis). Plant Pathol. 2024, 73, 144–156. [Google Scholar] [CrossRef]
- Liang, X.; Shang, S.; Dong, Q.; Wang, B.; Zhang, R.; Gleason, M.L.; Sun, G. Transcriptomic analysis reveals candidate genes regulating development and host interactions of Colletotrichum fructicola. BMC Genom. 2018, 19, 1–21. [Google Scholar] [CrossRef]
- Shih, J.; Wei, Y.; Goodwin, P.H. A comparison of the pectate lyase genes, pel-1 and pel-2, of Colletotrichum gloeosporioides f. sp. malvae and the relationship between their expression in culture and during necrotrophic infection. Gene 2000, 243, 139–150. [Google Scholar] [CrossRef]
- Huang, J.S. Chapter 2, Degradation of cell walls by plant pathogens. In Plant Pathogenesis and Resistance: Biochemistry and Physiology of Plant-Microbe Interactions; Springer: Dordrecht, The Netherlands, 2001; pp. 51–130. [Google Scholar]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.K.S.; Balio, A.; Graniti, A. Phytotoxins in Plant Disease; Academic Press: New York, NY, USA, 1972; p. 530. [Google Scholar]
- Evidente, A.; Cimmino, A.; Masi, M. Phytotoxins produced by pathogenic fungi of agrarian plants. Phytochem. Rev. 2019, 18, 843–870. [Google Scholar] [CrossRef]
- Möbius, N.; Hertweck, C. Fungal phytotoxins as mediators of virulence. Curr. Opin. Plant Biol. 2009, 12, 390–398. [Google Scholar] [CrossRef]
- Gohbara, M.; Hyeon, S.B.; Suzuki, A.; Tamura, S. Isolation and structure elucidation of colletopyrone from Colletotrichum nicotianae. Agric. Biol. Chem. 1976, 40, 1453–1455. [Google Scholar] [CrossRef]
- Gohbara, M.; Kosuge, Y.; Yamasaki, S.; Kimura, Y.; Suzuki, A.; Tamura, S. Isolation, structures and biological activities of colletotrichins, phytotoxic substances from Colletotrichum nicotianae. Agric. Biol. Chem. 1978, 42, 1037–1043. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Boari, A.; Zonno, M.C.; Gorecki, M.; Pescitelli, G.; Tuzi, A.; Vurro, M.; Evidente, A. Colletopyrandione, a new phytotoxic tetrasubstituted indolylidenepyran-2,4-dione, and colletochlorins G and H, new tetrasubstituted chroman- and isochroman-3,5-diols isolated from Colletothichum higginsianum. Tetrahedron 2017, 73, 6644–6650. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A.B.; Singh, D.; Singh, K.; Singh, J. Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ. Chem. Lett. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Tian, S.; Torres, R.; Ballester, A.R.; Li, B.; Vilanova, L.; Gonz’alez-Candelas, L. Molecular aspects in pathogen-fruit interactions: Virulence and resistance. Postharvest Biol. Technol. 2016, 122, 11–21. [Google Scholar] [CrossRef]
- Dowling, M.; Peres, N.; Villani, S.; Schnabel, G. Managing Colletotrichum on fruit crops: A complex challenge. Plant Dis. 2020, 104, 2301–2316. [Google Scholar] [CrossRef]
- Han, Y.C.; Zeng, X.G.; Xiang, F.Y.; Zhang, Q.H.; Guo, C.; Chen, F.Y.; Gu, Y.C. Carbendazim sensitivity in populations of Colletotrichum gloeosporioides complex infecting strawberry and yams in Hubei Province of China. J. Integr. Agric. 2018, 17, 1391–1400. [Google Scholar] [CrossRef]
- Hu, M.J.; Grabke, A.; Dowling, M.E.; Holstein, H.J.; Schnabel, G. Resistance in Colletotrichum siamense from peach and blueberry to thiophanate-methyl and azoxystrobin. Plant Dis. 2015, 99, 806–814. [Google Scholar] [CrossRef]
- Ren, L.; Wang, S.F.; Shi, X.J.; Cao, J.Y.; Zhou, J.B.; Zhao, X.J. Characterization of sensitivity of Colletotrichum gloeosporioides and Colletotrichum capsici, causing pepper anthracnose, to picoxystrobin. J. Plant Dis. Prot. 2020, 127, 657–666. [Google Scholar] [CrossRef]
- Jiang, H.; Meng, X.; Ma, J.; Sun, X.; Wang, Y.; Hu, T.; Cao, K.; Wang, S. Control effect of fungicide pyraclostrobin alternately applied with Bordeaux mixture against apple Glomerella leaf spot and its residue after preharvest application in China. Crop Prot. 2021, 142, 105489. [Google Scholar] [CrossRef]
- Usman, H.M.; Tan, Q.; Karim, M.M.; Adnan, M.; Yin, W.X.; Zhu, F.X.; Luo, C.X. Sensitivity of Colletotrichum fructicola and Colletotrichum siamense of peach in China to multiple classes of fungicides and characterization of pyraclostrobin-resistant isolates. Plant Dis. 2021, 105, 3459–3465. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.A.R.; Berger, L.R.R.; de Araújo, S.A.; Câmara, M.P.S.; de Souza, E.L. Synergistic mixtures of chitosan and Mentha piperita L. essential oil to inhibit Colletotrichum species and anthracnose development in mango cultivar Tommy Atkins. Food Microbiol. 2017, 66, 96–103. [Google Scholar] [CrossRef]
- Oliveira, P.D.L.; de Oliveira, K.Á.R.; Vieira, W.A.D.S.; Câmara, M.P.S.; de Souza, E.L. Control of anthracnose caused by Colletotrichum species in guava, mango and papaya using synergistic combinations of chitosan and Cymbopogon citratus (D.C. exNees) Stapf. essential oil. Int. J. Food Microbiol. 2018, 266, 87–94. [Google Scholar] [CrossRef]
- Issouffou, C.; Suwansri, S.; Salaipeth, L.; Domig, K.J.; Hwanhlem, N. Synergistic effect of essential oils and enterocin KT2W2G on the growth of spoilage microorganisms isolated from spoiled banana peel. Food Control 2018, 89, 260–269. [Google Scholar] [CrossRef]
- da Costa Gonçalves, D.; Ribeiro, W.R.; Goncalves, D.C.; Menini, L.; Costa, H. Recent advances and future perspective of essential oils in control Colletotrichum spp.: A sustainable alternative in postharvest treatment of fruits. Food Res. Int. 2021, 150, 110758. [Google Scholar] [CrossRef]
- Pei, S.; Liu, R.; Gao, H.; Chen, H.; Wu, W.; Fang, X.; Han, Y. Inhibitory effect and possible mechanism of carvacrol against Colletotrichum fructicola. Postharvest Biol. Technol. 2020, 163, 111126. [Google Scholar] [CrossRef]
- Shi, X.C.; Wang, S.Y.; Duan, X.C.; Wang, Y.Z.; Liu, F.Q.; Laborda, P. Biocontrol strategies for the management of Colletotrichum species in postharvest fruits. Crop Prot. 2021, 141, 105454. [Google Scholar] [CrossRef]
- Hirayama, Y.; Asano, S.; Watanabe, K.; Sakamoto, Y.; Ozaki, M.; Okayama, K.O.; Ohki, S.T.; Tojo, M. Control of Colletotrichum fructicola on strawberry with a foliar spray of neutral electrolyzed water through an overhead irrigation system. J. Gen. Plant Pathol. 2016, 82, 186–189. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.; Chicaiza, G.; Vilaplana, R. Control of postharvest anthracnose in papayas (Carica papaya L.) by hot water and chitosan. Acta Hortic. 2021, 1325, 141–146. [Google Scholar] [CrossRef]
- Cao, Y.; Song, X.; Xu, G.; Zhang, X.; Yan, H.; Feng, J.; Ma, Z.; Liu, X.; Wang, Y. Study on the antifungal activity and potential mechanism of natamycin against Colletotrichum fructicola. J. Agric. Food Chem. 2023, 71, 17713–17722. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, T.; Xu, H.; Xu, G.; Qian, G.; Liu, F. Characterization of Lysobacter spp. strains and their potential use as biocontrol agents against pear anthracnose. Microbiol. Res. 2021, 242, 126624. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, F.; Yin, J.; Peng, R.; Deng, J.; Shen, D.; Wu, J.; Liu, X.; Ma, H. Antifungal action and induction of resistance by Bacillus sp. strain YYC 155 against Colletotrichum fructicola for control of anthracnose disease in Camellia oleifera. Front. Microbiol. 2022, 13, 956642. [Google Scholar] [CrossRef]
- Rabha, A.J.; Naglot, A.; Sharma, G.D.; Gogoi, H.K.; Veer, V. In vitro evaluation of antagonism of endophytic Colletotrichum gloeosporioides against potent fungal pathogens of Camellia sinensis. Indian J. Microbiol. 2014, 54, 302–309. [Google Scholar] [CrossRef] [PubMed]
- da Silva Santos, S.D.S.; da Silva, A.A.; Polonio, J.C.; Polli, A.D.; Orlandelli, R.C.; Dos Santos Oliveira, J.A.D.S.; Brandão Filho, J.U.T.; Azevedo, J.L.; Pamphile, J.A. Influence of plant growth-promoting endophytes Colletotrichum siamense and Diaporthe masirevici on tomato plants (Lycopersicon esculentum Mill.). Mycology 2022, 13, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.-X.; Song, X.-S.; Xiao, X.-M.; Duan, X.-X.; Wang, J.-X.; Duan, Y.-B.; Hou, Y.-P.; Zhou, M.-G. A β2-tubulin dsRNA derived from Fusarium asiaticum confers plant resistance to multiple phytopathogens and reduces fungicide resistance. Pestic. Biochem. Phys. 2019, 153, 36–46. [Google Scholar] [CrossRef]
- Mahto, B.K.; Singh, A.; Pareek, M.; Rajam, M.V.; Dhar-Ray, S.; Reddy, P.M. Host-induced silencing of the Colletotrichum gloeosporioides conidial morphology 1 gene (CgCOM1) confers resistance against Anthracnose disease in chilli and tomato. Plant Mol. Biol. 2020, 104, 381–395. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Goulin, E.H.; de Lima, T.A.; dos Santos, P.J.C.; Machado, M.A. RNAi-induced silencing of the succinate dehydrogenase subunits gene in Colletotrichum abscissum, the causal agent of postbloom fruit drop (PFD) in citrus. Microbiol. Res. 2022, 260, 126938. [Google Scholar] [CrossRef] [PubMed]
- Ciofini, A.; Negrini, F.; Baroncelli, R.; Baraldi, E. Management of post-harvest anthracnose: Current approaches and future perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Vineeth, V.K.; Reshma, T.R.; Babu, S.; Philip, S.; Mahadevan, C. Comprehensive whole-genome sequencing reveals genetic characteristics of Colletotrichum fructicola (Nara gc5) the causative organism of circular leaf spot disease of rubber (Hevea brasiliensis). J. Plant Pathol. 2024, 106, 579–591. [Google Scholar] [CrossRef]
- Li, Q.; Bu, J.; Yu, Z.; Tang, L.; Huang, S.; Guo, T.; Mo, J.; Hsiang, T. Draft genome sequence of an isolate of Colletotrichum fructicola, a causal agent of mango anthracnose. Genome Announc. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Armitage, A.D.; Nellist, C.F.; Bates, H.J.; Zhang, L.; Zou, X.; Gao, Q.H.; Harrison, R.J. Draft genome sequence of the strawberry anthracnose pathogen Colletotrichum fructicola. Microbiol. Resour. Announc. 2020, 19, 01598-19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


