The Effects of Recombinant pBD2 on the Growth Performance, Antioxidant Capacity, Immune Function, Intestinal Barrier, and Microbiota of Weaned Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Feeding
2.2. Sample Collection
2.3. Growth Performance Measurement
2.4. Antioxidant Indicator Measurement
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
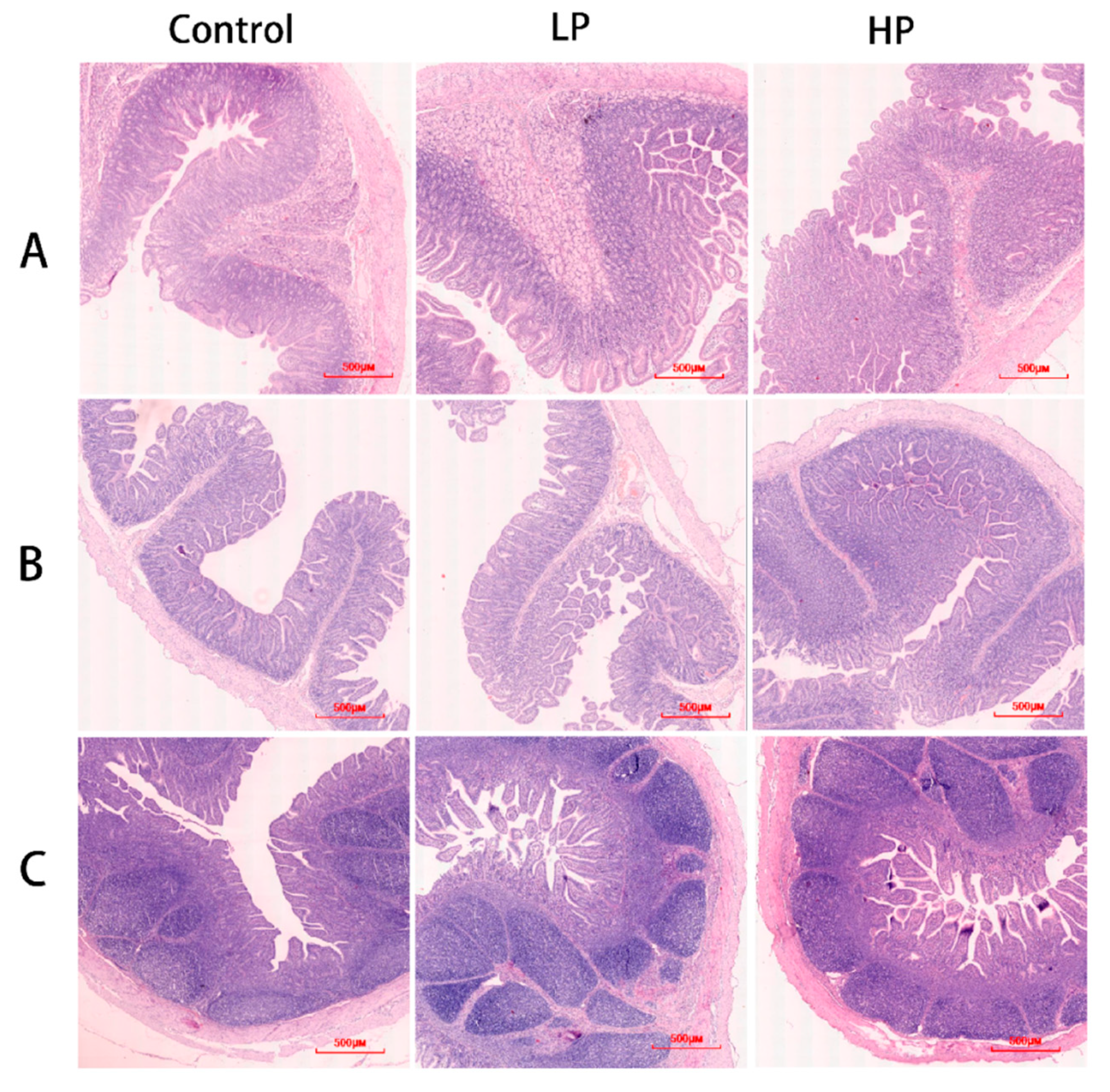
2.6. Morphology of Intestinal Tissues
2.7. RNA Extract
2.8. qPCR
2.9. Microbiome Diversity Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of pBD2 on Growth Performance of Weaned Piglets
3.2. Effects of pBD2 on Antioxidant Capacity in Weaned Piglets
3.3. Effects of pBD2 on Immune Performance in Weaned Piglets
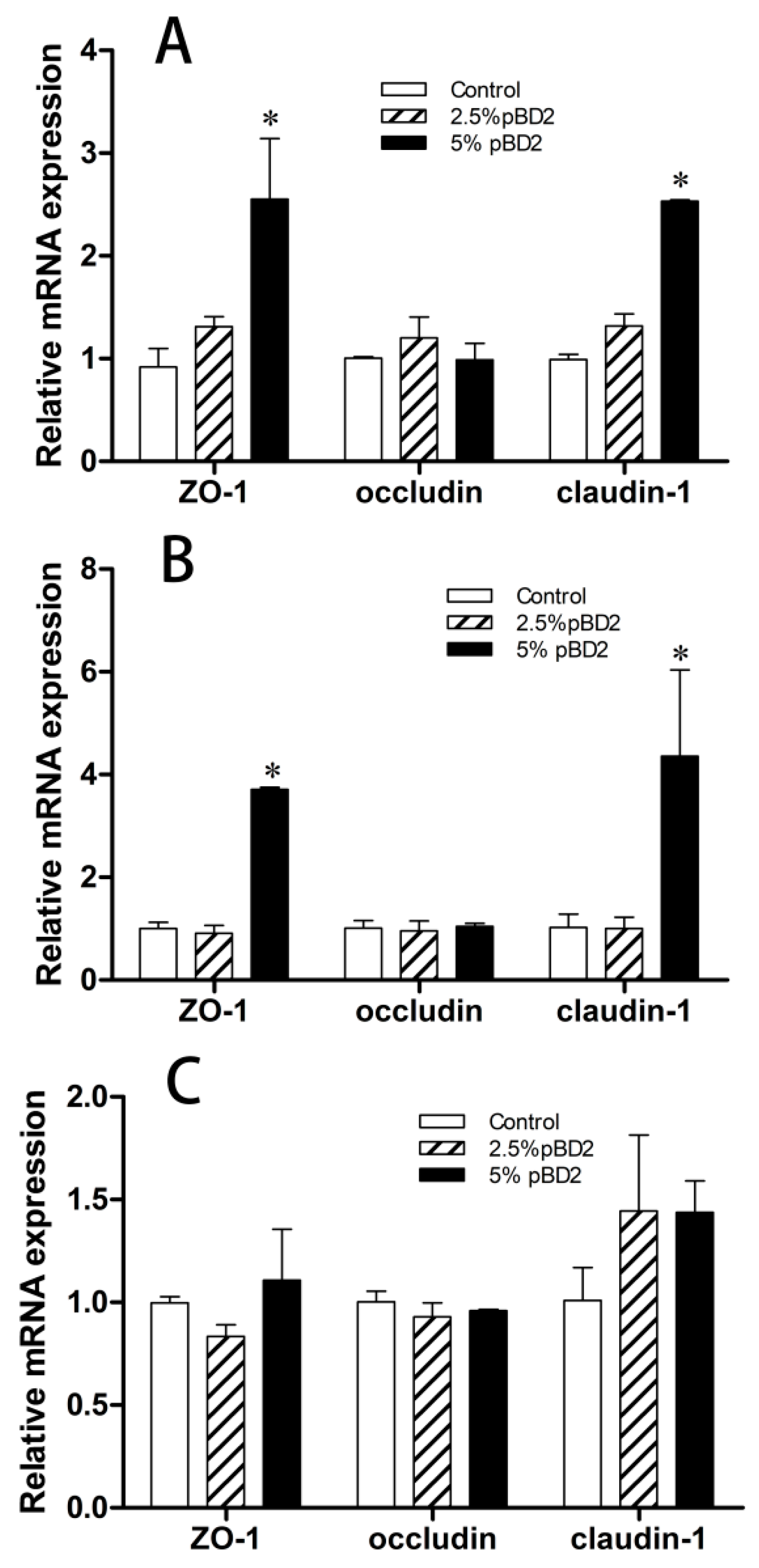
3.4. Effect of pBD2 on Intestinal Barrier Function in Weaned Piglets
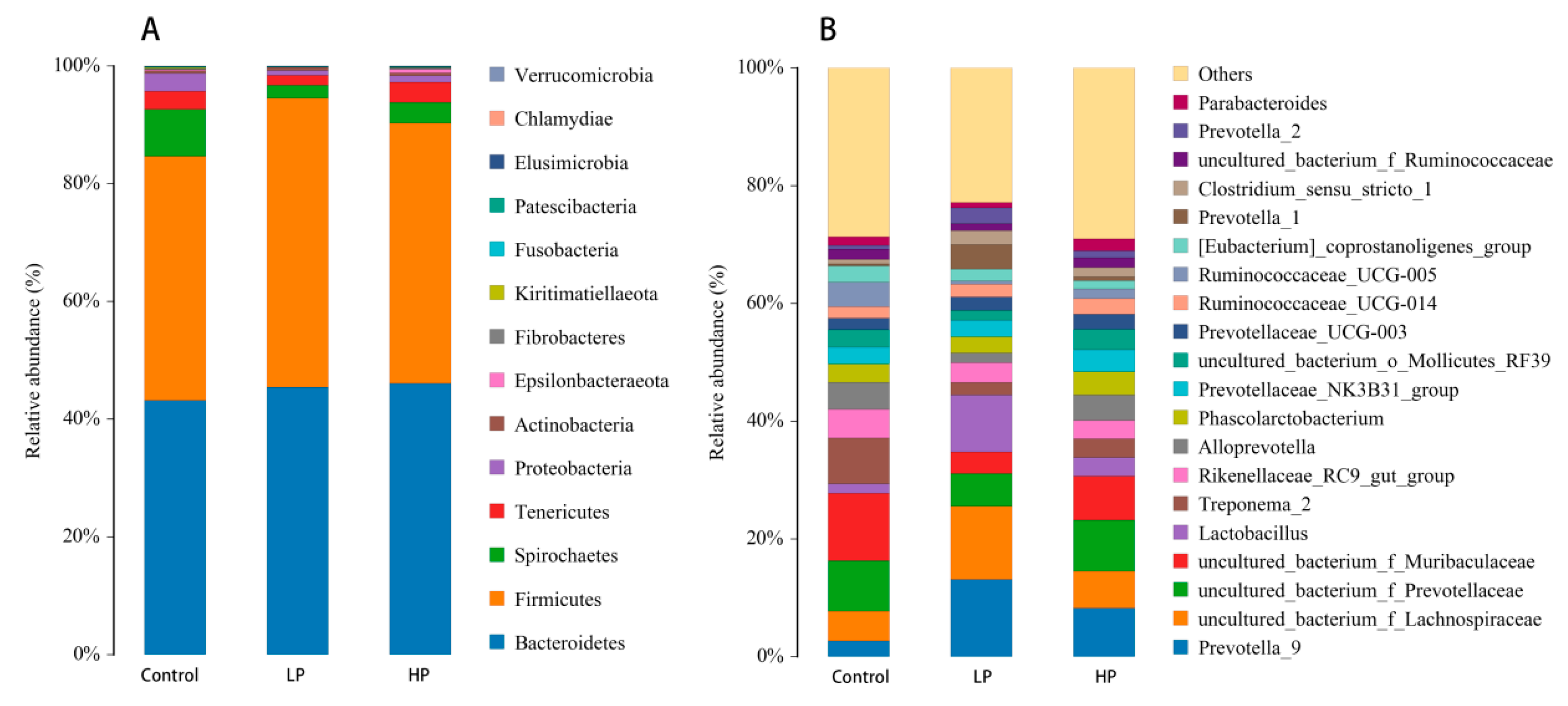
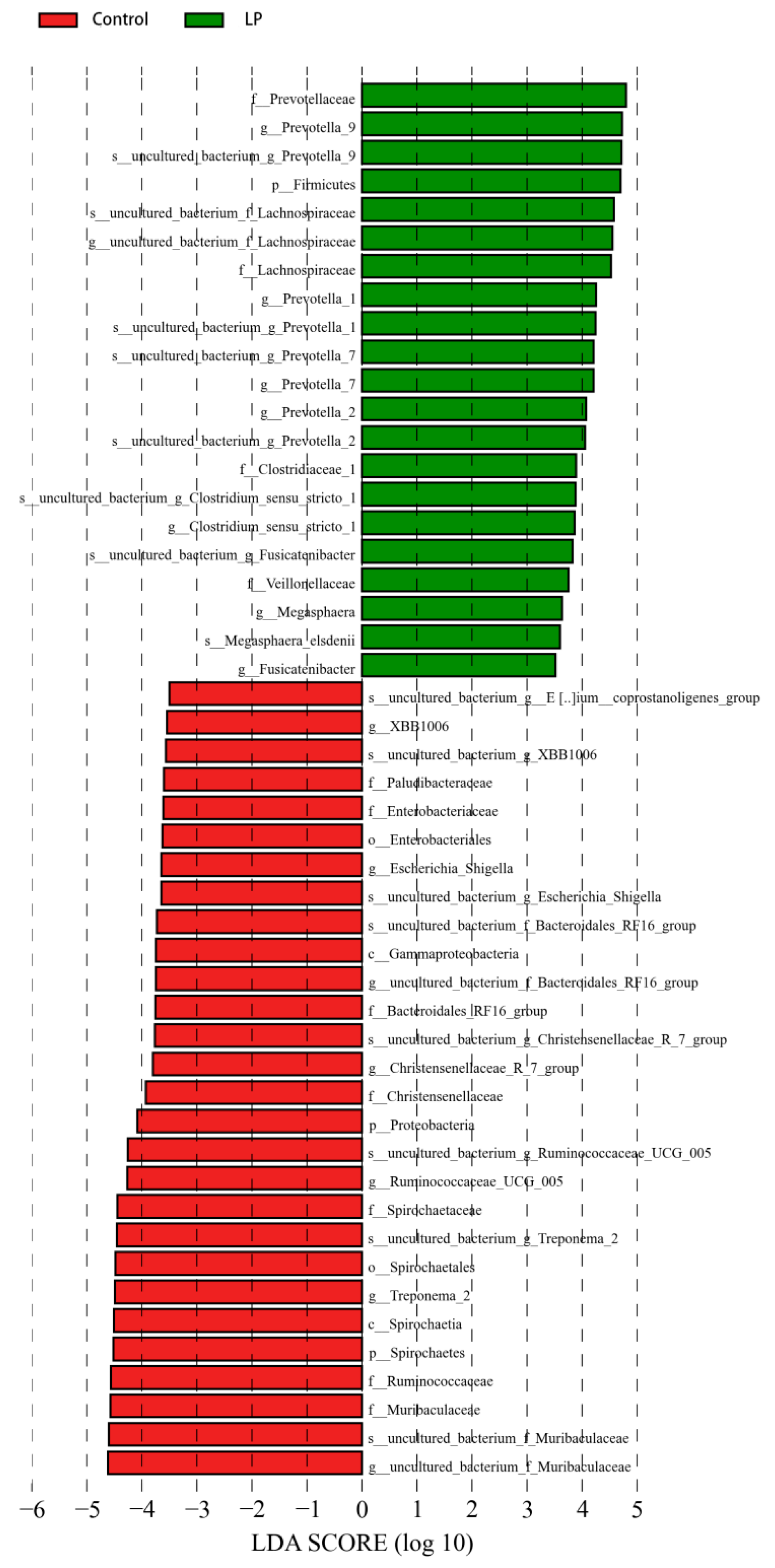
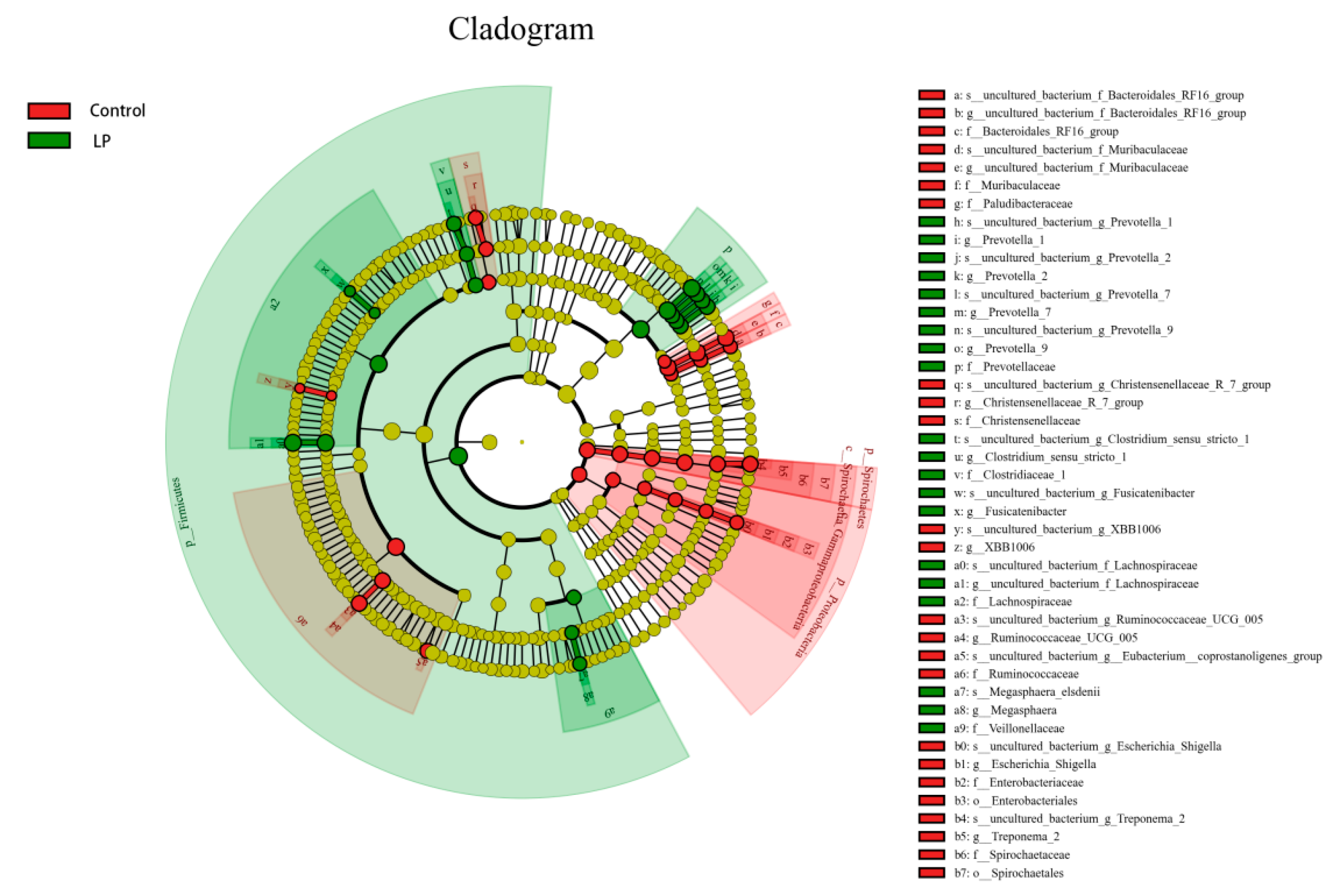
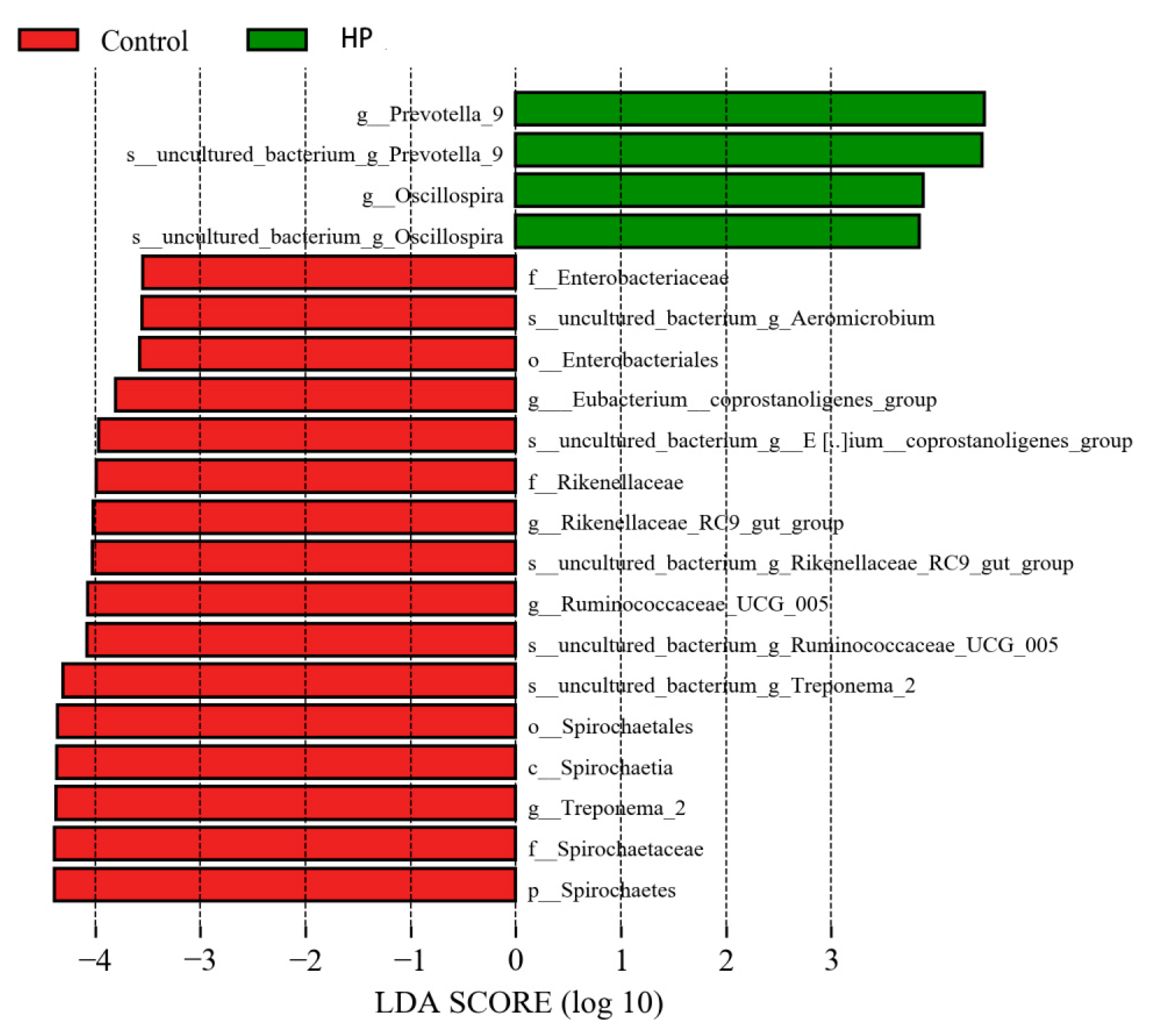
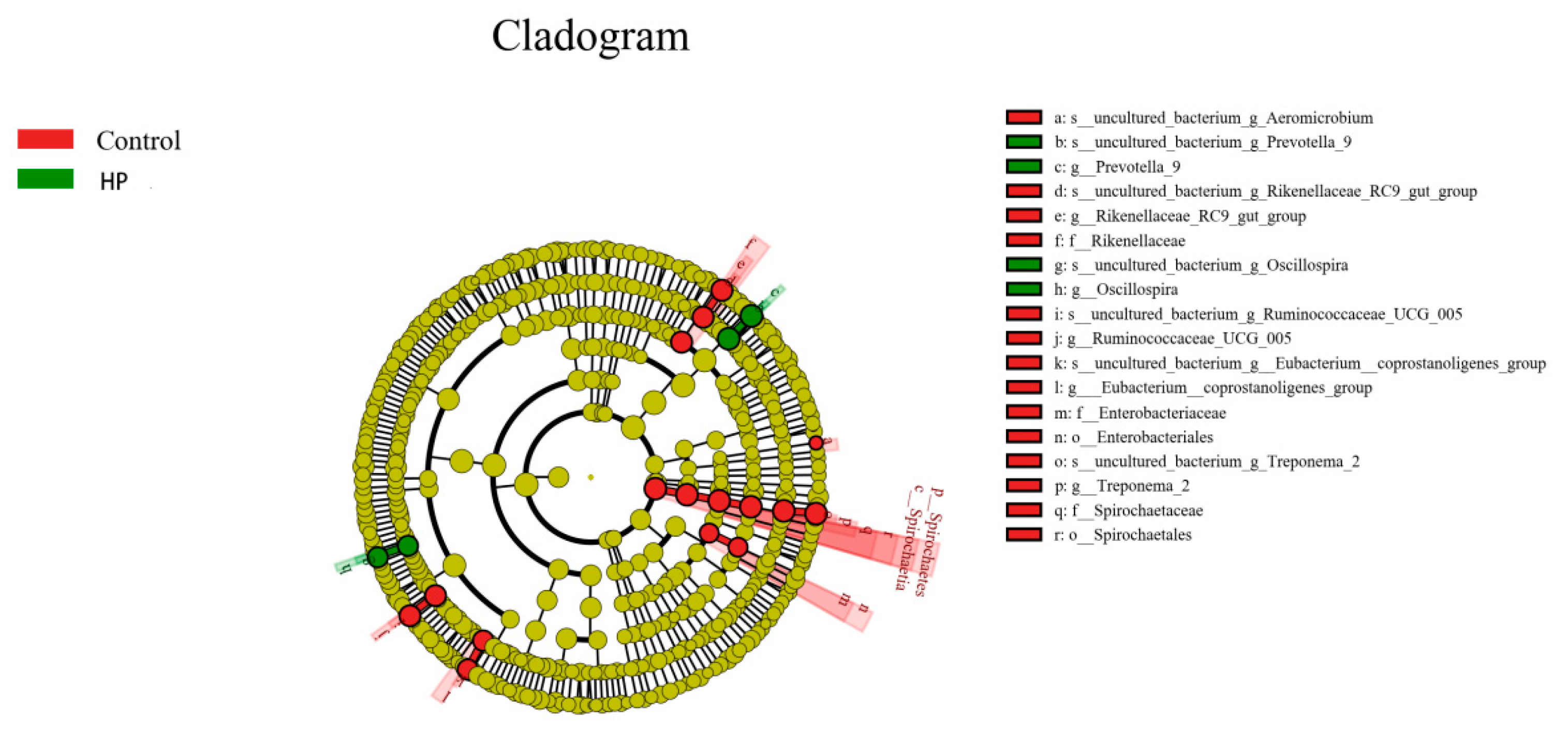
3.5. Effects of pBD2 on Microbial Composition
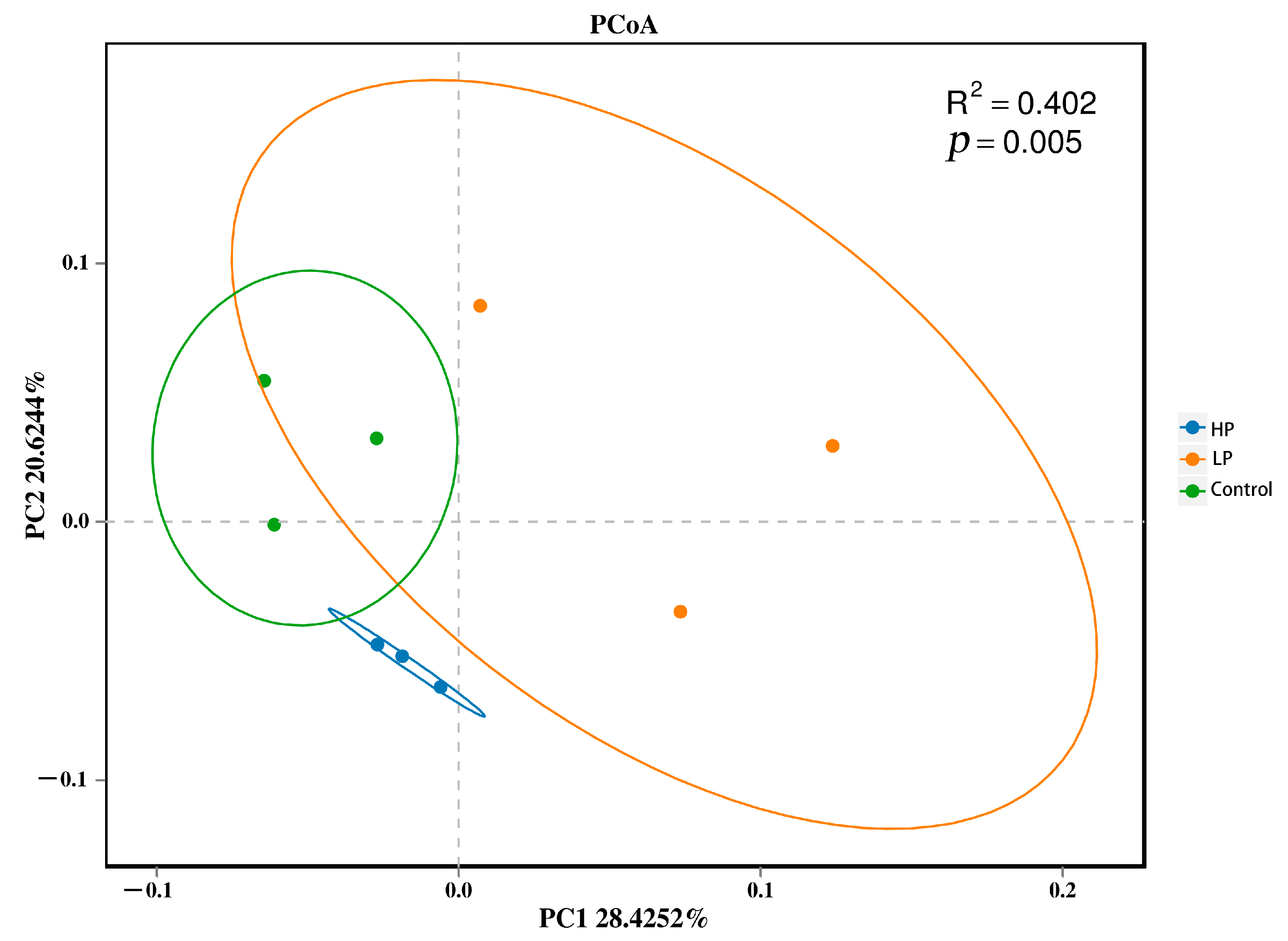
3.6. Effects of pBD2 on Alpha and Beta Diversity
3.7. Effect of pBD2 on Microbial Abundance
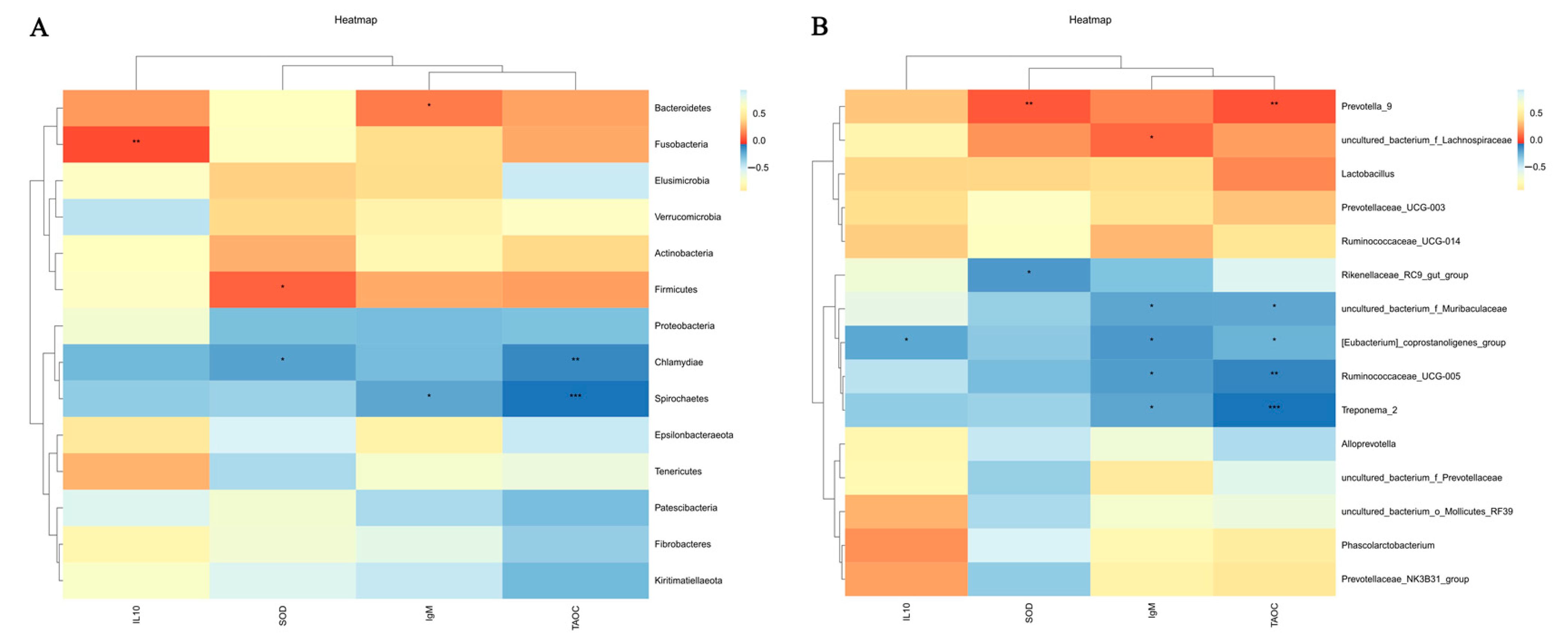
3.8. Correlation Analysis of Microorganisms with Immune Performance and Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Montout, L.; Poullet, N.; Bambou, J.C. Systematic Review of the Interaction between Nutrition and Immunity in Livestock: Effect of Dietary Supplementation with Synthetic Amino Acids. Animals 2021, 11, 2813. [Google Scholar] [CrossRef] [PubMed]
- Kober, A.; Riaz Rajoka, M.S.; Mehwish, H.M.; Villena, J.; Kitazawa, H. Immunomodulation Potential of Probiotics: A Novel Strategy for Improving Livestock Health, Immunity, and Productivity. Microorganisms 2022, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.P.; Fleming, S.A.; Abdul Rasheed, M.S.; Jha, R.; Dilger, R.N. The role of oligosaccharides and polysaccharides of xylan and mannan in gut health of monogastric animals. J. Nutr. Sci. 2020, 9, e21. [Google Scholar] [CrossRef]
- Vanrolleghem, W.; Tanghe, S.; Verstringe, S.; Bruggeman, G.; Papadopoulos, D.; Trevisi, P.; Zentek, J.; Sarrazin, S.; Dewulf, J. Potential dietary feed additives with antibacterial effects and their impact on performance of weaned piglets: A meta-analysis. Vet. J. 2019, 249, 24–32. [Google Scholar] [CrossRef]
- Gao, X.; Ding, J.; Liao, C.; Xu, J.; Liu, X.; Lu, W. Defensins: The natural peptide antibiotic. Adv. Drug Deliv. Rev. 2021, 179, 114008. [Google Scholar] [CrossRef]
- Contreras, G.; Shirdel, I.; Braun, M.S.; Wink, M. Defensins: Transcriptional regulation and function beyond antimicrobial activity. Dev. Comp. Immunol. 2020, 104, 103556. [Google Scholar] [CrossRef]
- Van Dijk, A.; Hedegaard, C.J.; Haagsman, H.P.; Heegaard, P.M.H. The potential for immunoglobulins and host defense peptides (HDPs) to reduce the use of antibiotics in animal production. Vet. Res. 2018, 49, 68. [Google Scholar] [CrossRef]
- Choi, M.K.; Le, M.T.; Nguyen, D.T.; Choi, H.; Kim, W.; Kim, J.H.; Chun, J.; Hyeon, J.; Seo, K.; Park, C. Genome-level identification, gene expression, and comparative analysis of porcine ß-defensin genes. BMC Genet. 2012, 13, 98. [Google Scholar] [CrossRef]
- Finatto, A.N.; Meurens, F.; de Oliveira Costa, M. Piggybacking on nature: Exploring the multifaceted world of porcine β-defensins. Vet. Res. 2025, 56, 47. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, Q.H.; Sun, J.; Zhang, M.; Xiang, Y.Q. Porcine β-defensin-2 alleviates AFB1-induced intestinal mucosal injury by inhibiting oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2023, 262, 115161. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qi, Y.; Wang, A.; Huang, C.; Liu, X.; Yang, X.; Li, L.; Zhou, R. Porcine β-defensin 2 inhibits proliferation of pseudorabies virus in vitro and in transgenic mice. Virol. J. 2020, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lian, S.; Shen, X.; Zhao, X.; Zhao, W.; Li, C. Recombinant porcine beta defensin 2 alleviates inflammatory responses induced by Escherichia coli in IPEC-J2 cells. Int. J. Biol. Macromol. 2022, 208, 890–900. [Google Scholar] [CrossRef]
- Li, C.L.; Zhao, Y.C.; Song, X.Y.; Huang, X.X.; Zhao, W.D. Molecular cloning, expression and characterization of the porcine β defensin 2 in E. coli. Protein Pept. Lett. 2013, 20, 715–723. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Li, L.; Zhang, H.; Gao, C.Y.; Xiao, C.B.; Li, C.L. Preparation of polyclonal antibody against porcine beta defensin 2 and identification of its distribution in tissues of pig. Genet. Mol. Res. 2015, 14, 18863–18871. [Google Scholar] [CrossRef]
- Gao, C.Y.; Xu, T.T.; Zhao, Q.J.; Li, C.L. Codon optimization enhances the expression of porcine β-defensin-2 in Escherichia coli. Genet. Mol. Res. 2015, 14, 4978–4988. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, X.; Han, L.; Wang, M.; Lian, S.; Wang, K.; Li, C. Effects on the intestinal morphology, inflammatory response and microflora in piglets challenged with enterotoxigenic Escherichia coli K88. Res. Vet. Sci. 2023, 157, 50–61. [Google Scholar] [CrossRef]
- Shen, X.; Gu, M.; Zhan, F.; Cai, H.; Zhang, K.; Wang, K.; Li, C. Porcine beta defensin 2 attenuates inflammatory responses in IPEC-J2 cells against Escherichia coli via TLRs-NF-κB/MAPK signaling pathway. BMC Vet. Res. 2024, 20, 357. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.; Huang, J.; Liu, X.; Yang, X.; Jin, H.; Huang, Q.; Li, L.; Zhou, R. Porcine Beta-Defensin 2 Provides Protection Against Bacterial Infection by a Direct Bactericidal Activity and Alleviates Inflammation via Interference with the TLR4/NF-κB Pathway. Front. Immunol. 2019, 10, 1673. [Google Scholar] [CrossRef]
- Huang, J.; Yang, X.; Wang, A.; Huang, C.; Tang, H.; Zhang, Q.; Fang, Q.; Yu, Z.; Liu, X.; Huang, Q.; et al. Pigs Overexpressing Porcine β-Defensin 2 Display Increased Resilience to Glaesserella parasuis Infection. Antibiotics 2020, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, Y.T.; Tan, M.F.; Zhang, H.W.; Liu, W.Q.; Zou, G.; Zhang, L.S.; Zhang, C.Y.; Deng, S.M.; Yu, L.; et al. Overexpression of Porcine Beta-Defensin 2 Enhances Resistance to Actinobacillus pleuropneumoniae Infection in Pigs. Infect. Immun. 2015, 83, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, L.; Shi, B.; Deng, H.; Lai, X.; Liu, J.; Sun, Z. Oral administration of synthetic porcine beta-defensin-2 improves growth performance and cecal microbial flora and down-regulates the expression of intestinal toll-like receptor-4 and inflammatory cytokines in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim. Sci. J. 2016, 87, 1258–1266. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Han, X.; Hu, X.; Jin, W.; Liu, G. Dietary nutrition, intestinal microbiota dysbiosis and post-weaning diarrhea in piglets. Anim. Nutr. 2024, 17, 188–207. [Google Scholar] [CrossRef]
- Ji, S.; An, F.; Zhang, T.; Lou, M.; Guo, J.; Liu, K.; Zhu, Y.; Wu, J.; Wu, R. Antimicrobial peptides: An alternative to traditional antibiotics. Eur. J. Med. Chem. 2024, 265, 116072. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lohakare, J.; Park, Y.K.; Park, J.C.; Kwon, I.K.; Chae, B.J. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J. Sci. Food Agric. 2013, 93, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, A.; Xie, L.; Song, W.; Wang, J.; Yin, Z.; Zhou, D.; Li, F. Use of recombinant porcine β-defensin 2 as a medicated feed additive for weaned piglets. Sci. Rep. 2016, 6, 26790. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sang, Z.; Zhuo, Y.; Wang, X.; Guo, Z.; He, L.; Zeng, C.; Dai, H. Transport stress induces pig jejunum tissue oxidative damage and results in autophagy/mitophagy activation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef]
- Yu, W.; Cai, X.; Wang, C.; Peng, X.; Xu, L.; Gao, Y.; Tian, T.; Zhu, G.; Pan, Y.; Chu, H.; et al. FOXM1 affects oxidative stress, mitochondrial function, and the DNA damage response by regulating p21 in aging oocytes. Theriogenology 2024, 229, 66–74. [Google Scholar] [CrossRef]
- Xie, Y.; Gu, Y.; Li, Z.; Zhang, L.; Hei, Y. Effects of exercise on different antioxidant enzymes and related indicators: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2025, 15, 12518. [Google Scholar] [CrossRef]
- Liu, N.; Ma, X.; Jiang, X. Effects of Immobilized Antimicrobial Peptides on Growth Performance, Serum Biochemical Index, Inflammatory Factors, Intestinal Morphology, and Microbial Community in Weaning Pigs. Front. Immunol. 2022, 13, 872990. [Google Scholar] [CrossRef]
- Wang, J.; Wilson, A.E.; Su, B.; Dunham, R.A. Functionality of dietary antimicrobial peptides in aquatic animal health: Multiple meta-analyses. Anim. Nutr. 2023, 12, 200–214. [Google Scholar] [CrossRef]
- Doron, I.; Kusakabe, T.; Iliev, I.D. Immunoglobulins at the interface of the gut mycobiota and anti-fungal immunity. Semin. Immunol. 2023, 67, 101757. [Google Scholar] [CrossRef]
- Fossum, C. Cytokines as markers for infections and their effect on growth performance and well-being in the pig. Domest. Anim. Endocrinol. 1998, 15, 439–444. [Google Scholar] [CrossRef]
- Šerstņova, K.; Pilmane, M.; Vitenberga-Verza, Z.; Melderis, I.; Gontar, Ł.; Kochański, M.; Drutowska, A.; Maróti, G.; Prieto-Simón, B. Expression of anti-inflammatory markers IL-2, IL-10, TGF-β1, βDEF-2, βDEF-3 and Cathelicidin LL37 in dairy cattle milk with different health status of the udder. Pol. J. Vet. Sci. 2022, 25, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.X.; Wang, B.; Li, B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front. Immunol. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Yurong, Y.; Yibao, J.; Ruiping, S.; Qingqiang, Y.; Kaisong, P.; Huihui, B.; Decheng, W.; Tianlong, L.; Xuemei, Z. Effects of chicken intestinal antimicrobial peptides on humoral immunity of chickens and antibody titres after vaccination with infectious bursal disease virus vaccine in chicken. Arch. Anim. Nutr. 2006, 60, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, H.; Xia, X.; Xiong, H.; Song, D.; Zong, X.; Wang, Y. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 2015, 194, 1882–1893. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H. Tight Junction Proteins in the Weaned Piglet Intestine: Roles and Regulation. Curr. Protein. Pept. Sci. 2019, 20, 652–660. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, L.; Dong, C. Antimicrobial Peptides: An Overview of their Structure, Function and Mechanism of Action. Protein Pept. Lett. 2022, 29, 641–650. [Google Scholar] [CrossRef]
- Robinson, K.; Deng, Z.; Hou, Y.; Zhang, G. Regulation of the Intestinal Barrier Function by Host Defense Peptides. Front. Vet. Sci. 2015, 2, 57. [Google Scholar] [CrossRef]
- Tang, Z.R.; Deng, H.; Zhang, X.L.; Zen, Y.; Xiao, D.F.; Sun, W.Z.; Zhang, Z. Effects of orally administering the antimicrobial peptide buforin II on small intestinal mucosal membrane integrity, the expression of tight junction proteins and protective factors in weaned piglets challenged by enterotoxigenic Escherichia coli. Anim. Feed Sci. Technol. 2013, 186, 177–185. [Google Scholar] [CrossRef]
- Song, D.; Zong, X.; Zhang, H.; Wang, T.; Yi, H.; Luan, C.; Wang, Y. Antimicrobial peptide Cathelicidin-BF prevents intestinal barrier dysfunction in a mouse model of endotoxemia. Int. Immunopharmacol. 2015, 25, 141–147. [Google Scholar] [CrossRef]
- Knecht, D.; Cholewińska, P.; Jankowska-Mąkosa, A.; Czyż, K. Development of Swine’s Digestive Tract Microbiota and Its Relation to Production Indices-A Review. Animals 2020, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.H.; Wright, D.P.; Christie, D.L.; Clinch, K.; Furneaux, R.H.; Roberton, A.M. A novel mechanism for desulfation of mucin: Identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J. Bacteriol. 2005, 187, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Liu, L.; Huang, F.; Dong, B. Effects of Three-Layer Encapsulated Tea Tree Oil on Growth Performance, Antioxidant Capacity, and Intestinal Microbiota of Weaned Pigs. Front. Vet. Sci. 2021, 8, 789225. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhou, X.; Ye, H.; Wu, Y.; Wang, Z.; Lu, D.; Wang, J.; Han, D. The High Level of Xylooligosaccharides Improves Growth Performance in Weaned Piglets by Increasing Antioxidant Activity, Enhancing Immune Function, and Modulating Gut Microbiota. Front. Nutr. 2021, 8, 764556. [Google Scholar] [CrossRef]
- Rhouma, M.; Braley, C.; Thériault, W.; Thibodeau, A.; Quessy, S.; Fravalo, P. Evolution of Pig Fecal Microbiota Composition and Diversity in Response to Enterotoxigenic Escherichia coli Infection and Colistin Treatment in Weaned Piglets. Microorganisms 2021, 9, 1459. [Google Scholar] [CrossRef]
- Liu, G.; Li, P.; Hou, L.; Niu, Q.; Pu, G.; Wang, B.; Du, T.; Kim, S.W.; Niu, P.; Li, Q.; et al. Metagenomic Analysis Reveals New Microbiota Related to Fiber Digestion in Pigs. Front. Microbiol. 2021, 12, 746717. [Google Scholar] [CrossRef]
- Long, C.; Rösch, C.; de Vries, S.; Schols, H.; Venema, K. Cellulase and Alkaline Treatment Improve Intestinal Microbial Degradation of Recalcitrant Fibers of Rapeseed Meal in Pigs. J. Agric. Food. Chem. 2020, 68, 11011–11025. [Google Scholar] [CrossRef]
- Suh, M.J.; Kuntumalla, S.; Yu, Y.; Pieper, R. Proteomes of pathogenic Escherichia coli/Shigella group surveyed in their host environments. Expert Rev. Proteom. 2014, 11, 593–609. [Google Scholar] [CrossRef]
- Gryaznova, M.V.; Dvoretskaya, Y.D.; Syromyatnikov, M.Y.; Shabunin, S.V.; Parshin, P.A.; Mikhaylov, E.V.; Strelnikov, N.A.; Popov, V.N. Changes in the Microbiome Profile in Different Parts of the Intestine in Piglets with Diarrhea. Animals 2022, 12, 320. [Google Scholar] [CrossRef]
- Råsbäck, T.; Jansson, D.S.; Johansson, K.E.; Fellström, C. A novel enteropathogenic, strongly haemolytic spirochaete isolated from pig and mallard, provisionally designated ‘Brachyspira suanatina’ sp. nov. Environ. Microbiol. 2007, 9, 983–991. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Meneguetti, B.T.; Oliveira-Júnior, N.G.; Macedo, M.L.R.; Franco, O.L. Antimicrobial peptide production in response to gut microbiota imbalance. Peptides 2022, 157, 170865. [Google Scholar] [CrossRef]
| Feed Composition | Content (%) |
|---|---|
| Corn | 22.78 |
| Broken rice | 20.00 |
| Expanded corn | 12.0 |
| Expanded soybean | 9.00 |
| Soybean meal | 13.00 |
| Soybean protein concentrate | 2.00 |
| Whey powder | 4.00 |
| Fish meal | 4.00 |
| Fermented soybean meal | 6.00 |
| Soybean oil | 2.00 |
| White sugar | 2.00 |
| Salt | 0.27 |
| DL methionine | 0.17 |
| L-lysine (98%) | 0.20 |
| Threonine (98.5%) | 0.09 |
| Tryptophan (98%) | 0.02 |
| Calcium hydrogen phosphate | 0.92 |
| Stone powder | 0.55 |
| Premix 1 | 1.00 |
| Total | 100 |
| Nutritional level | |
| (Kcal/kg) | 2660 |
| Crude protein (%) | 20.79 |
| Calcium (%) | 0.62 |
| Available phosphorus (%) | 0.37 |
| Lysine (%) | 1.32 |
| Threonine (%) | 0.88 |
| Methionine + cysteine (%) | 0.84 |
| Gene Name | Primer Sequence (5′→3′) | GenBank Number | Product Length |
|---|---|---|---|
| ZO-1 | Forward: AGCCCGAGGCGTGTTT Reverse: GGTGGGAGGATGCTGTTG | XM_021098896.1 | 147 bp |
| Claudin-1 | Forward: ATTTCAGGTCTGGCTATCTTAGTTGC Reverse: AGGGCCTTGGTGTTGGGTAA | NM_001244539.1 | 214 bp |
| Occludin | Forward: ATCAACAAAGGCAACTCT Reverse: GCAGCAGCCATGTACTCT | NM_001163647.2 | 157 bp |
| GAPDH | Forward: ATGGTGAAGGTCGGAGTGAA Reverse: CGTGGGTGGAATCATACTGG | NM_001206359.1 | 154 bp |
| Index | Control | LP | HP | SEM | p-Value |
|---|---|---|---|---|---|
| Initial weight (kg) | 6.18 | 6.38 | 6.21 | 0.11 | 0.79 |
| Total weight gain (kg) | 1.45 b | 1.95 a | 2.24 a | 0.13 | 0.02 |
| ADG (kg) | 0.10 b | 0.14 a | 0.16 a | 0.01 | 0.02 |
| ADFI (g) | 198.58 | 256.04 | 285.91 | 17.14 | 0.11 |
| FCR | 0.52 | 0.54 | 0.59 | 0.02 | 0.42 |
| Index | Control | LP | HP | SEM | p-Value |
|---|---|---|---|---|---|
| T-AOC (μmol/mL) | 2.44 b | 3.67 a | 3.31 a | 0.20 | 0.04 |
| SOD (U/mL) | 78.74 b | 212.37 a | 167.38 b | 24.45 | 0.05 |
| MDA (nmol/mL) | 8.16 | 8.89 | 6.88 | 0.57 | 0.45 |
| Index | Control | LP | HP | SEM | p-Value |
|---|---|---|---|---|---|
| IgA (μg/mL) | 639.02 | 654.47 | 646.64.97 | 7.76 | 0.27 |
| IgM (mg/mL) | 14.00 b | 15.26 a | 15.37 a | 0.27 | 0.033 |
| IgG (mg/mL) | 18.72 | 18.47 | 19.84 | 0.33 | 0.214 |
| IL-2 (pg/mL) | 265.08 | 298.21 | 294.33 | 6.77 | 0.066 |
| IL-10 (pg/mL) | 356.16 b | 375.28 ab | 400.09 a | 8.00 | 0.05 |
| IL-6 (pg/mL) | 80.22 | 82.88 | 88.39 | 1.80 | 0.17 |
| TNF-α (pg/mL) | 494.48 | 498.87 | 510.34 | 8.78 | 0.784 |
| Intestinal Tissue | Index | Control | LP | HP | SEM | p-Value |
|---|---|---|---|---|---|---|
| Duodenum | Villus (μm) | 314.15 | 330.30 | 336.86 | 3.01 | 0.18 |
| Crypt (μm) | 151.46 | 144.05 | 137.69 | 6.92 | 0.41 | |
| C/V | 2.08 b | 2.30 a | 2.46 a | 0.059 | 0.023 | |
| Jejunum | Villus (μm) | 232.39 c | 309.75 b | 368.31 a | 16.10 | 0.00 |
| Crypt (μm) | 151.26 | 151.84 | 141.42 | 4.76 | 0.63 | |
| C/V | 1.59 b | 2.05 a | 2.60 a | 0.12 | 0.00 | |
| Ileum | Villus (μm) | 278.26 | 286.89 | 317.22 | 11.00 | 0.34 |
| Crypt (μm) | 132.81 | 123.36 | 118.51 | 3.41 | 0.23 | |
| C/V | 2.10 c | 2.34 b | 2.67 a | 0.085 | 0.013 |
| Index | Control | LP | HP | SEM | p-Value |
|---|---|---|---|---|---|
| ACE | 596.94 a | 580.69 ab | 550.7 b | 8.08 | 0.026 |
| Chao1 | 605.29 | 584.39 | 571.18 | 7.19 | 0.143 |
| Simpson | 0.98 a | 0.98 a | 0.96 b | 0.004 | 0.023 |
| Shannon | 6.9 a | 7.01 a | 6.05 b | 0.16 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Z.; Meng, Q.; Ren, M.; Lv, B.; Yuan, L.; Zhang, N.; Wang, Q.; Zhang, K.; Li, C. The Effects of Recombinant pBD2 on the Growth Performance, Antioxidant Capacity, Immune Function, Intestinal Barrier, and Microbiota of Weaned Piglets. Microorganisms 2025, 13, 1443. https://doi.org/10.3390/microorganisms13071443
Teng Z, Meng Q, Ren M, Lv B, Yuan L, Zhang N, Wang Q, Zhang K, Li C. The Effects of Recombinant pBD2 on the Growth Performance, Antioxidant Capacity, Immune Function, Intestinal Barrier, and Microbiota of Weaned Piglets. Microorganisms. 2025; 13(7):1443. https://doi.org/10.3390/microorganisms13071443
Chicago/Turabian StyleTeng, Zhanwei, Qing Meng, Mengting Ren, Bingke Lv, Liping Yuan, Ningning Zhang, Qinghua Wang, Kun Zhang, and Chunli Li. 2025. "The Effects of Recombinant pBD2 on the Growth Performance, Antioxidant Capacity, Immune Function, Intestinal Barrier, and Microbiota of Weaned Piglets" Microorganisms 13, no. 7: 1443. https://doi.org/10.3390/microorganisms13071443
APA StyleTeng, Z., Meng, Q., Ren, M., Lv, B., Yuan, L., Zhang, N., Wang, Q., Zhang, K., & Li, C. (2025). The Effects of Recombinant pBD2 on the Growth Performance, Antioxidant Capacity, Immune Function, Intestinal Barrier, and Microbiota of Weaned Piglets. Microorganisms, 13(7), 1443. https://doi.org/10.3390/microorganisms13071443







