Abstract
Lactic acid bacteria (LAB) and cellulase have been used as additives to improve the fermentation quality of mulberry silage. This study investigated the dynamics of fermentation characteristics and bacterial communities during 60-day ensiling through three established treatment groups: Control (no inoculation), Lactiplantibacillus plantarum (LP) inoculation as well as combination of L. plantarum and cellulase inoculation group (LPC). The results showed that compared with the Control group, the LP and LPC treatments significantly reduced the loss of dry matter, soluble carbohydrates, and crude protein (p < 0.05), effectively promoted the accumulation of lactic acid and acetic acid (p < 0.05), but significantly elevated ammonia nitrogen (NH3-N) production. Inoculation was beneficial to the stability of the bacterial community in mulberry branch and leaf silage because it can maintain a high level of beneficial bacteria (Lactiplantibacillus) and inhibit the growth of harmful bacteria (Escherichia-Shigella). The combination of the inoculation of L. plantarum and cellulase may improve the quality of mulberry branch silage.
1. Introduction
Escalating demand for livestock forage necessitates alternative feed resources, such as forest and fruit byproducts. Mulberry (Morus alba L.) is a perennial deciduous tree belonging to the Moraceae family. It has high nutritional value and strong environmental adaptability and is widely distributed worldwide [1]. Moreover, mulberry branches and leaves contain substantial crude protein (14.66% DM), minerals, and vitamins [2]. However, high moisture content (50.35% DM) and fiber levels (NDF: 54.30% DM; lignin > 18%) limit its preservation and digestibility [3]. Research has shown that the application of silage fermentation technology to mulberry branches and leaves can transform and decompose macromolecular nutrients in the raw materials, reduce the loss of nutrients, and degrade some crude fiber content [4]. Silage involves anaerobic fermentation by microorganisms, leading to the production of lactic acid, thus inhibiting the growth of pathogenic microorganisms and maintaining the nutritional value of the feed [5]. In addition, the fiber content of mulberry branches and leaves is as high as 60%, and the lignin content of the branches is >18%, leading to poor palatability and digestibility. To improve the quality of mulberry silage, it is necessary to add other feedstuffs that are easily fermented or substances rich in soluble carbohydrates as well as appropriate additives [6]. The co-fermentation and co-storage of enzymes and microorganisms during silage production significantly enhance fermentation [7]. Studies have shown that adding plant lactic acid bacteria and cellulase during silage production can promote the growth of beneficial microorganisms, enhance lactic acid fermentation, and improve the digestibility and nutritional value of silage [8,9].
Lactiplantibacillus plantarum (L. plantarum) effectively lowers the pH of silage and creates an acidic environment that accelerates fermentation [10]. In this process, the type, quantity, activity, and diversity of lactic acid bacteria will affect the fermentation quality of silage [11,12]. These effects reduce nutrient loss, improve silage fermentation efficiency, and enhance palatability and aerobic stability [13]. Cellulase supplementation degrades fiber to release fermentable sugars, accelerating pH reduction and improving silage preservation [14]. Inoculation with L. plantarum further enhances this process by rapidly producing lactic acid, suppressing spoilage microorganisms [10,15]. Combined L. plantarum–cellulase treatments improve fermentation in the silage of Rhodesgrass (Chloris gayana Kunth.) and Italian Ryegrass (Lolium multiflorum Lam.) [16], but the effect of the co-inoculation of L. plantarum and cellulase on mulberry silage—notably on bacterial community dynamics—remains unclear.
Therefore, we aim to evaluate the synergistic effects of L. plantarum and cellulase inoculation on the fermentation quality and nutritional preservation of mulberry branch silage. In addition, during the silage process, changes in the bacteria community structure and abundance of some microbial may greatly impact fermentation quality [17]. Therefore, it is necessary to characterize the dynamics of microbial communities and their correlations with fermentation parameters during 60-day ensiling. The findings presented here will provide methods and ideas on the source and utilization of roughage resources in animal husbandry.
2. Materials and Methods
2.1. Silage Prepartion
The leaves and branches of mulberry trees were collected from the Agricultural Science Research Institute in Turpan, Xinjiang (89°20′46″ N, 42°94′79″ E), a region characterized by an arid climate with sandy loam soil, in May 2023. During the peak vegetative growth stage of mulberry trees (approximately 1.5–2 m in height), mechanized harvesting was implemented to collect both branches and foliage, resulting in a biomass ratio of branches to leaves at approximately 1:2. Raw materials were crushed into 1 to 2 cm pieces. The following treatments were applied to the silage: (1) A quantitative urea solution was sprayed onto the surface of the raw material based on its fresh weight prior to ensiling; (2) inoculation with L. plantarum strain CICC (China Center of Industrial Culture Collection, Beijing) 23120 and cellulase (Cellulase AP3 from Trichoderma reesei, Solarbio Science & Technology Co., Ltd., Beijing, China; Product No. C8140) was performed (LPC group), with L. plantarum performed at a concentration of 5 × 106 colony-forming units of per gram (CFU·g−1) fresh weight, cellulase was inoculated at 0.3% fresh weight (enzyme activity ≥ 5000 U·g−1 defined as the amount of enzyme liberating 1 μmol glucose per minute from carboxymethyl cellulose at pH 4.8 and 50 °C); (3) inoculation with L. plantarum was performed at a concentration of 5 × 106 CFU·g−1 of fresh weight (LP group; based on our previous research that the addition of L. plantarum alone at 5 × 106 CFU·g−1 fresh weight enhanced the quality of paper mulberry silage) [18]; (4) equal volume of sterilized water was added (Control group). Each latic acid bacteria (LAB) strain was enriched in de Man Rogosa Sharpe (MRS) liquid culture medium. After quantifying the LAB strains by plate counting, the bacteria were evenly sprayed over each bag of silage and thoroughly mixed. Vacuum-sealed polyethylene bags measuring 35 × 40 cm were prepared for ensiling, with 20 bags allocated to each group (approximately 1.5 kg each). The bags were stored at ambient temperatures ranging from 24 °C to 40 °C throughout the experiment. The experiment followed a completely randomized design with three treatments and four sampling time points (7, 15, 30, and 60 days). On each sampling day, five bags from each group were opened and samples were collected for parameter measurement.
2.2. Mulberry Silage Chemical Components, Fermentation Characteristics, and Microbiological Analyses
The dry matter (DM) content of raw mulberry branches/leaves and silage was determined with modifications [11]: samples were blanched at 105 °C for 30 min in a forced-air oven (DHG-9620A; Shanghai Heheng Equipment Co., Ltd., Shanghai, China), followed by drying at 65 °C to constant weight. Dried samples were ground (DFY-1000D high-speed grinder; Zhejiang Wenling Linda Machinery Co., Ltd., Wenlin, China) to 0.5 mm particles and stored in desiccators pending further analysis. The content of crude protein (CP) was determined by the Kjeldahl method. The content of neutral detergent fiber (NDF) and acid detergent fiber (ADF) was determined by the Van Soest method [19]. The content of ether extract (EE) was determined by the Soxhlet extraction technique [20]. A pH meter (PHS-3C, China Shanghai Instrument and Electrical Science Instrument Co., Ltd., Shanghai, China) was used to measure the pH. Ammoniacal nitrogen (NH3-N) was analyzed using the phenol-hypochlorite program [21]. Organic acids (lactic acid, LA; acetic acid, AA; butyric acid, BA; propionic acid, PA) were quantified using high-performance liquid chromatography [21] (HPLC, 1260 Infinity II, Agilent Technologies, Waldbronn, Germany) equipped with a C18 column (FMF-5559-EONU, 150 × 4.6 mm, 5 μm; FLM Scientific Instrument Co., Ltd., Guangzhou, China). The separation was achieved with a mobile phase consisting of 20 mM potassium dihydrogen phosphate buffer (pH 2.50) and methanol (97:3, v/v), filtered through a 0.22 μm nylon membrane and degassed by sonication for 15 min. Isocratic elution was performed at 0.6 mL/min with the column oven maintained at 50 °C. Total water-soluble carbohydrates (WSCs) were quantified as glucose equivalents using anthrone-sulfuric acid colorimetry according to AOAC Official Method 977.22 [22]. This measures acid-hydrolyzable saccharides including mono- and disaccharides, with results representing total fermentable carbohydrate potential rather than individual sugar concentrations.
The liquid culture medium and physiological saline were divided into separate conical bottles that were sealed and sterilized under high pressure (121 °C, 15 min). After cooling, the physiological saline solution (90 mL) was mixed with the silage sample (10 g per bag) at a ratio of 9:1, sealed in conical bottles, and incubated in an oscillator at a constant temperature for 30 min. The sample solution was then diluted to a predesigned gradient in a laminar flow cabinet (10 mL culture medium and 1 mL sample solution) and incubated. LAB were cultured in MRS medium at 28 °C for 24 h, while the mold was cultured in high-salt PDA medium at 37 °C for 72 h. The yeast was propagated on malt extract agar medium at 37 °C for 72 h, and the aerobic bacteria was cultured on nutrient agar medium at 28 °C for 24 h. After completing the culture process, the microorganisms were counted [23]. The nutrient composition and microbial counts of the raw silage feedstuffs are listed in Table 1.

Table 1.
Characteristics of the mulberry branches and leaves.
2.3. Bacterial Community Analysis
An E.Z.N.A.® soil kit was used to extract DNA from feed samples, the quality of DNA was detected using 1% agarose gel electrophoresis, concentration and purity of DNA was determined using a microvolume spectrophotometer, V3–V4 variable region of the 16SrRNA gene was amplified using PCR, and DNA extracted from the feed samples was used as a template. Primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACH VGGGTWTCTAAT-3′) were selected, and the PCR products were subsequently recovered and detected the using 2% agarose gel electrophoresis. A library was built for the purified and detected PCR products, and the Illumina platform was used for sequencing, followed by high-throughput sequencing data analysis [24,25].
2.4. Statistical Analysis
Data were collected and analyzed using Microsoft Office Excel 2021. The experiment followed a completely randomized design, and one-way analysis of variance with Duncan’s multiple comparison tests was conducted using IBM SPSS (version 17.0; SPSS Inc., Chicago, IL, USA). Data are presented as the means ± standard error (SE). Where ANOVA indicated significant effects (p < 0.05). Microbial count data (LAB, yeast, mold, aerobic bacteria) were log10-transformed to achieve normality and homogeneity of variance. Sequencing data for the bacterial communities were analyzed using the free online Majorbio Cloud Platform (www.Majorbio.com, accessed on 2 Mar 2025).
3. Results
3.1. Changes in Nutritional Quality of Mulberry Branches and Leaves During Silage Fermentation
The dynamics of the chemical components in the mulberry silage inoculated with L. plantarum and cellulase are presented in Table 2 and Table 3. With the extension of silage time, the DM content of each group decreased; however, at the same fermentation time, the DM content of the inoculated groups was significantly higher than that of the Control group (p < 0.05). On the 7th d of fermentation, the CP content of the LPC group was lower than that of the LP and Control groups (p < 0.05); however, on the 60th d, the CP content of the LPC group was higher than that of the Control and LP groups (p < 0.05). The variation in EE content in each group with fermentation time was not obvious; however, the EE content in the inoculation group was higher than that in the Control group. Before fermentation of the silage for 30 d, the NDF content in the raw materials was significantly reduced by inoculation (p < 0.05). On the 60th d, the NDF and ADF contents of the Control group decreased, whereas those of the LP and LPC groups increased significantly and were higher than those of the Control group (p < 0.05). In addition, the soluble carbohydrate content in each group increased with fermentation time and then decreased.

Table 2.
Effect of L. plantarum and cellulase inoculation on the Chemical Composition of mulberry branch and leaf silage.

Table 3.
Effect of L. plantarum and cellulase inoculation on the fiber degradation of mulberry branch and leaf silage.
3.2. Changes in Fermentation Quality of Mulberry Branches and Leaves During Silage Fermentation
The dynamics of the fermentation characteristics of the mulberry branch silage are presented in Table 4. Throughout the fermentation process, the pH of the Control and LPC and LP groups showed a downward trend. Especially in the late stage of silage fermentation, the pH of the LPC group was significantly lower than that of the Control and LP groups (p < 0.05); however, on the 60th d of fermentation, the pH of the Control increased. In all groups, the LA content increased gradually from the 0th to 30th d, but decreased at the 60th d. On the 0th and 60th d of silage, the LA in the LPC and LP groups was significantly higher than that in the Control group (p < 0.05). On the 15th and 30th d of silage, the concentrations of AA and PA in the LPC group were significantly higher than those in the other groups (p < 0.05). However, PA was not detected in any of the groups on the 60th d of silage. Moreover, BA was not detected throughout the fermentation period. In addition, the NH3-N content of all groups showed an increasing trend throughout the silage process; however, the NH3-N content of the inoculation group was significantly higher than that of the Control group (p < 0.05), and it decreased in the LPC group on the 60th d.

Table 4.
Changes in fermentation quality during mulberry branch and leaf silage.
3.3. Change in Microorganism Number During Silage Fermentation of Mulberry Branch and Leaf
The dynamics of the main microorganism counts in mulberry silage are presented in Table 5. The number of LAB subjected to the Control and inoculation treatments initially increased, then decreased throughout the fermentation period, whereas the yeast, mold, and aerobic bacteria counts continued to decrease. Specifically, on the 30th d of silage, the number of LAB in the Control and inoculation groups increased to the highest value, with the number of LAB in the LP group significantly higher than that in the LPC and Control groups (p < 0.05). On the 60th d of silage, the LAB count decreased. On the 30th d of silage, the yeast and mold counts in the LP and LPC groups were significantly lower than those in the Control group (p < 0.05). In addition, mold growth was significantly inhibited in the LP and LPC groups (p < 0.05).

Table 5.
Changes in microbial quantity during fermentation of mulberry branch and leaf silage.
3.4. Alpha Diversity Analysis of Mulberry Twig and Leaf Silage and Beta Diversity Analysis
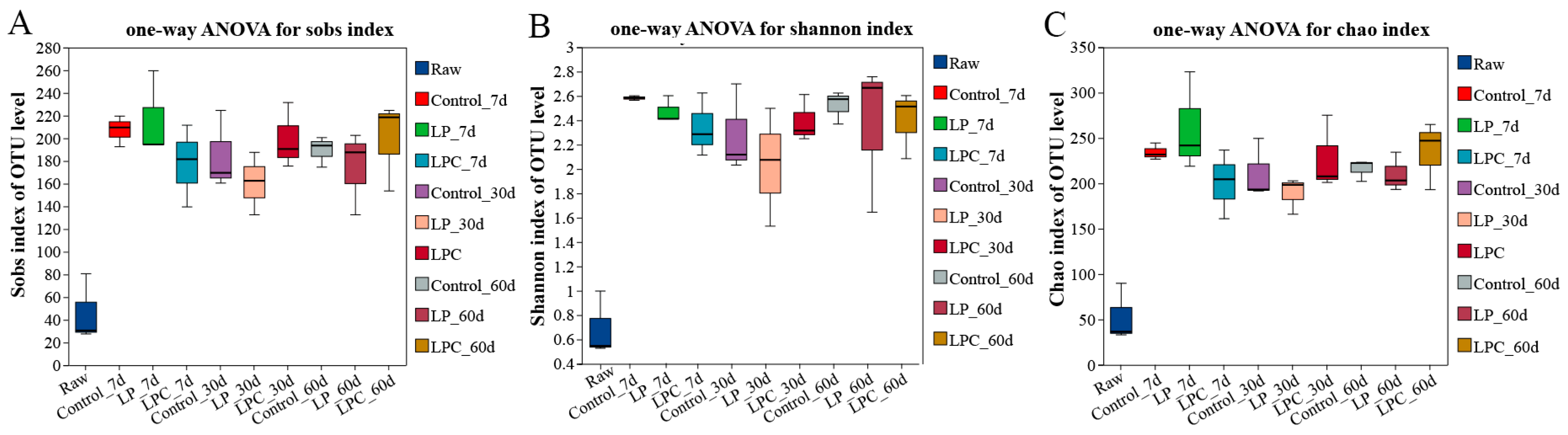
Microbial community data were acquired via high-throughput sequencing of mulberry leaf silage samples fermented for 7, 30, and 60 d. The sequencing depth was adequate, with an average effective sequence length of 424 bp, encompassing most species within the microbial community. At a similarity threshold of 97%, all sequenced reads were clustered into 1,190 operational taxonomic units. Analysis of the microbial diversity revealed notable differences between the groups. Figure 1A–C shows the dynamics of the Shannon and Chao indices. Compared with the raw mulberry branches, the microbial diversity and richness of mulberry branches after fermentation were significantly improved.

Figure 1.
Alpha diversity analysis of mulberry silage. Note: LP denotes the addition of L. plantarum (5 × 106 CFU·g−1 fresh weight), whereas LPC represents the addition of L. plantarum (5 × 106 CFU·g−1 fresh weight) combined with cellulase (0.3% of fresh weight; enzyme activity ≥ 5000 U·g−1); Raw, raw material.
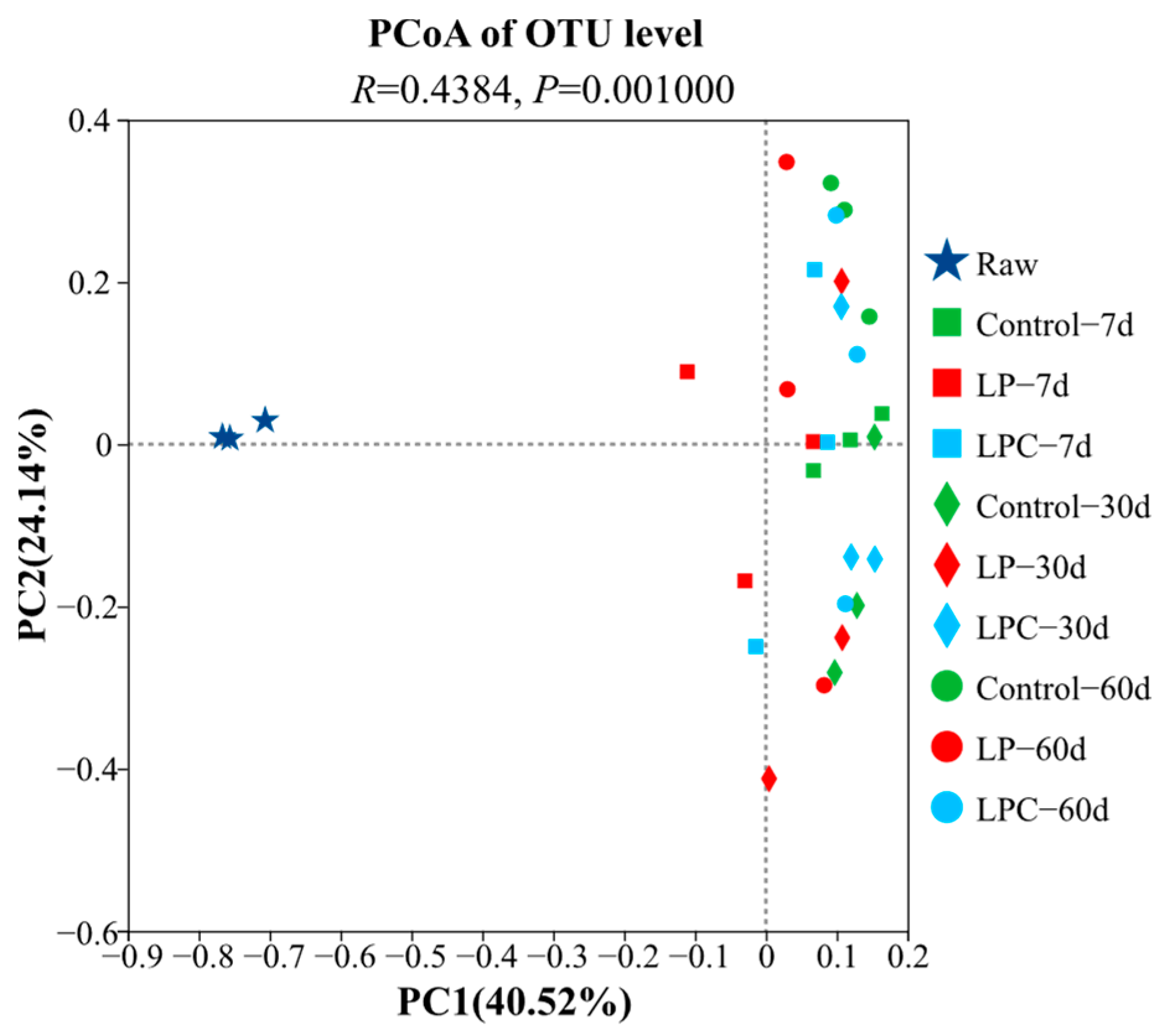
Principal coordinate analysis (Figure 2) was used to evaluate the bacterial communities of the mulberry and leaf silage materials in the Control, LP, and LPC groups. The results showed that the distribution of bacterial communities in the LP and LPC groups changed after 60 d of silage (R = 0.4384, p < 0.01).

Figure 2.
Analysis of the bacterial diversity in mulberry branch silage based on principal coordinate analysis (PCoA). Note: LP denotes the addition of L. plantarum (5 × 106 CFU·g−1 fresh weight), whereas LPC represents the addition of L. plantarum (5 × 106 CFU·g−1 fresh weight) combined with cellulase (0.3% of fresh weight; enzyme activity ≥ 5000 U·g−1). Raw, raw material.
3.5. Analysis of the Bacterial Community Composition of the Branch and Leaf Silage Microbiome
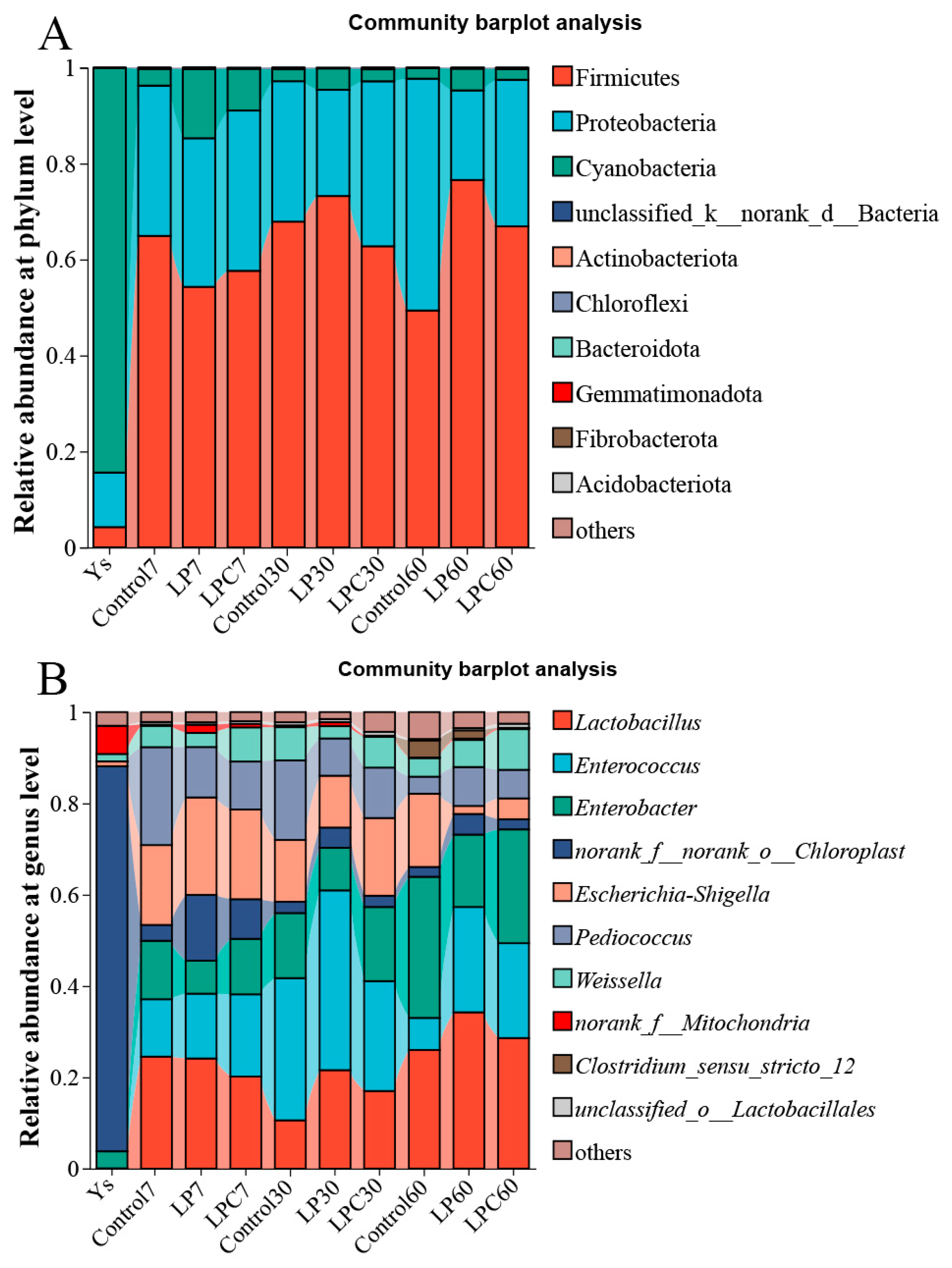
Figure 3A illustrates the relative abundance of raw materials and each group after 7, 30, and 60 d of fermentation. The relative abundance of cyanobacteria was the highest in raw silage (84.36%). In all stages of the silage process, Firmicutes was the dominant phylum in all groups (60.74–68.06%), followed by Proteobacteria (23.91–36.30%). At the genus level (Figure 3B), the relative abundance of Norank_f_norank_o_Chloroplast was the highest (84.36%), followed by Norank_f_mitochondria (6.15%) and Enterobacter (3.74%). On the 7th d, the dominant bacteria in each group were Lactiplantibacillus (20.17–24.55%), followed by Escherichia-Shigella (17.49–21.34%). On the 30th d, the dominant bacteria were Enterococcus (24.08–39.37%), and on the 60th d, the dominant bacteria were Lactiplantibacillus (25.98–34.28%), Enterobacter (15.86–30.90%), and Enterococcus (7.03–23.07%).

Figure 3.
(A) Microbial community structure of mulberry leaf silage at the phylum levels. (B) Microbial community structure of mulberry leaf silage at the genus levels. Note: LP denotes the addition of L. plantarum (5 × 106 CFU·g−1 fresh weight), whereas LPC represents the addition of L. plantarum (5 × 106 CFU·g−1 fresh weight) combined with cellulase (0.3% of fresh weight; enzyme activity ≥ 5000 U·g−1).
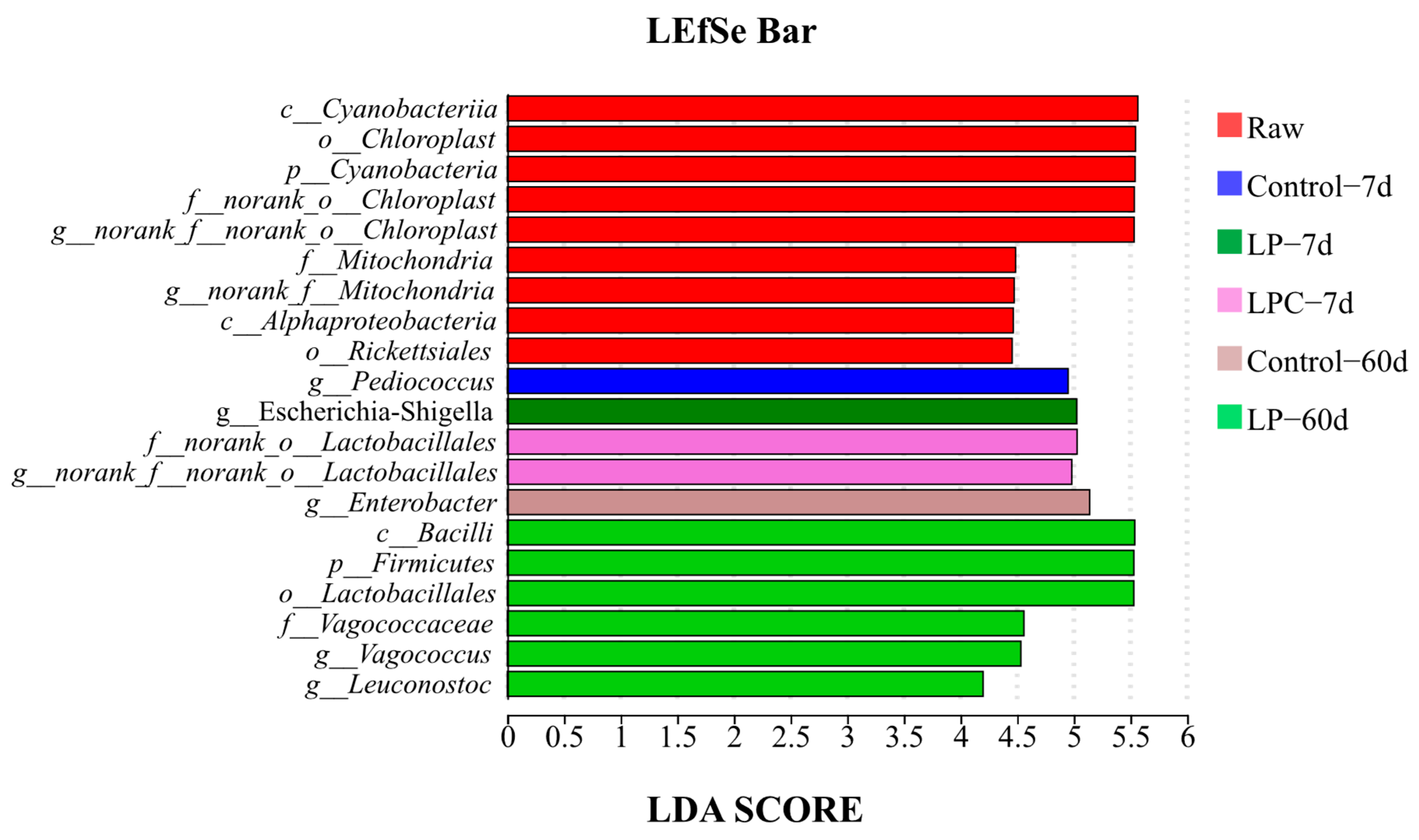
LEfSe was performed to further explore variations in the bacterial communities among the groups (Figure 4). On the 7th d of ensiling, the relative abundance of Pediococcus in the Control group, Escherichia-Shigella in the LP group, and two norank strains of Lactiplantibacillus (norank_o__Lactiplantibacillus and norank_f__norank_o__Lactiplantibacillus) in the LPC group were higher (p < 0.05). On the 60th d of ensiling, the relative abundances of Enterobacter in the Control group and Vagocococcus and leuconostoc in the LP group were significantly higher (p < 0.05).

Figure 4.
LEfSe analysis of mulberry foliage silage. Note: LP denotes the addition of L. plantarum (5 × 106 CFU·g−1 fresh weight), whereas LPC represents the addition of L. plantarum (5 × 106 CFU·g−1 fresh weight) combined with cellulase (0.3% of fresh weight; enzyme activity ≥ 5000 U·g−1); Raw, raw material.
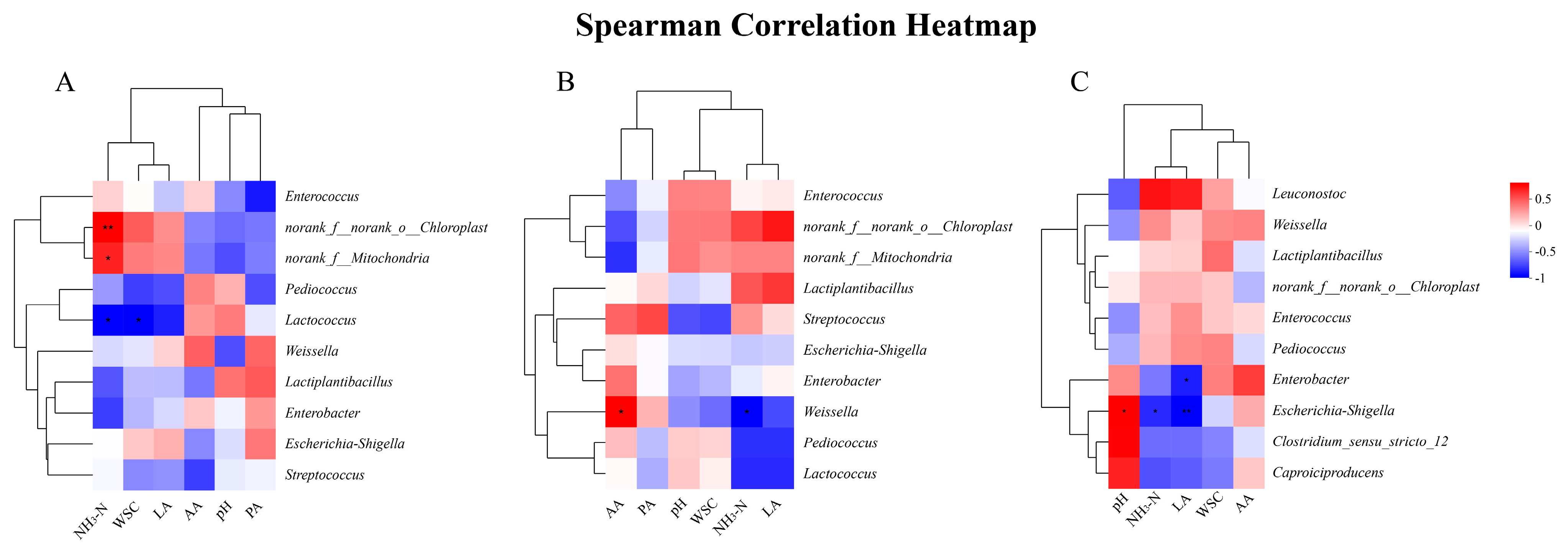
Figure 5 presents Spearman correlation heatmaps between the VIF-screened fermentation parameters (LA, AA, PA, CP, and NH3-N) and top 10 microbial genera during silage fermentation on days 7 (5A), 30 (5B), and 60 (5C). Correlation analysis between the top 10 bacteria with the highest abundance and fermentation index of mulberry branches was carried out according to silage time. On the 7th d of silage, the NH3-N content was correlated with norank_f__norank_o__Chloroplast (R = 0.82, p < 0.01) and norank_f__Mitochondria (R = 0.7, p < 0.01). A significant correlation was noted between the WSC content and Lactococcus (R = −0.7, p < 0.05). On the 30th d of silage, Weissella was significantly correlated with the AA content (R = 0.72, p < 0.05) and NH3-N content (R = −0.78, p < 0.05). On the 60th d of silage, a significant correlation was determined between Enterobacter and LA content (R = −0.72, p < 0.05), between Escherichia-Shigella and the pH value (R = 0.68, p < 0.05), and NH3-N content (R = −0.68, p < 0.05) and LA content.

Figure 5.
(A) Correlation analysis between the microbial community (genus level) and fermentation characteristics of mulberry branch silage on day 7. (B) Correlation analysis between the microbial community (genus level) and fermentation characteristics of mulberry branch silage on day 30. (C) Correlation analysis between the microbial community (genus level) and fermentation characteristics of mulberry branch silage on day 60. Note: NH3-N, ammoniacal nitrogen; WSC, water-soluble carbohydrate; LA, lactic acid; AA, acetic Acid; PA, propanoic acid. “*” indicates significant correlation (p < 0.05); “**” indicates highly significant correlation (p < 0.01).
4. Discussion
4.1. Effect of L. plantarum and Cellulase on the Nutritional Quality, Fermentation Characteristics, and Microorganism Count of Mulberry Silage
The observed reduction in DM loss during the initial phase (before day 30) in L. plantarum inoculated groups (LP and LPC) compared to the Control (Table 2) can be primarily attributed to the rapid establishment of an anaerobic, acidic environment facilitated by the exogenous LAB [26]. Similar findings by Li et al. [14] underscore the role of LAB inoculants in suppressing aerobic respiration and undesirable microbial activity that drive DM loss. Microorganisms attached to silage raw materials need to use carbon sources for their growth activities, leading to a decrease in NDF and ADF in the early stages of silage [15], consistent with the conclusion of this study. The NDF and ADF contents initially decreased, then increased because the LP group effectively alleviated fiber degradation in the later stages of silage fermentation [19], thus maintaining the fiber content. The significant increase in WSC content during the early fermentation phase was primarily driven by the rapid hydrolysis of structural carbohydrates (e.g., cellulose and hemicellulose) by plant-derived enzymes [27]. The comparatively slower rate of WSC decline in the LPC group towards the end of ensiling might indicate either a sustained, albeit slower, release of sugars from ongoing cellulase activity or a more efficient microbial community structure better adapted to utilizing complex substrates, contributing to prolonged fermentation stability [27,28].
The rapid and sustained production of LA and AA, particularly in the inoculated groups (Table 4), is the core driver of pH reduction and the key mechanism for inhibiting some of spoilage microorganisms (yeast, mold, Table 5) and preserving silage quality [29,30]. Moreover, the pH of the LPC group decreased more during the fermentation process, indicating that the combined addition of L. plantarum and cellulase was helpful for maintaining fibrous substances in mulberry leaves, inhibiting miscellaneous bacteria, and reducing CP loss and NH3-N production in silage [5]. On the 7th d of silage fermentation, the LA content rapidly increased, attributed to the increase in LA content during the initial stage caused by inoculation with LAB [31,32]. The sustained dominance of acetic acid production in the LPC group, particularly evident after 15th d, suggests that the combined inoculant promoted a shift towards heterofermentative metabolism within the mulberry silage system [33]. As is well known, the main product of homogeneous fermentation is LA, whereas that of heterogeneous fermentation is AA and LA. This is consistent with the increasing trend in the relative abundance of Enterobacter during the later stages of fermentation in this study. Enterobacter is generally considered to be more inclined towards heterogeneous fermentation [34]. PA also showed a similar trend; however, the undetectable PA at 60 d likely resulted from biological consumption by dominant Lactiplantibacillus utilizing PA as an auxiliary energy source amid declining WSC (WSC% DM: decreased from 6.15 to 3.94 in Control; Table 2) [35]. In addition, the disappearance of PA may involve active reutilization by LAB as an alternative carbon source, consistent with recent studies demonstrating that Lactiplantibacillus spp. can metabolize short-chain fatty acids (including PA) under energy-limited conditions [35]. The LP and LPC groups effectively maintained an acidic anaerobic fermentation environment and inhibited yeast and mold growth. In summary, the inoculation of L. plantarum alone and in combination with cellulase played a key role in supporting the reduction in DM loss, maintenance of fiber content, and improvement of overall feed quality of mulberry branches.
4.2. Effect of L. plantarum and Cellulase on the Bacterial Community Composition of Mulberry Branch Leaf Silage Fermentation
The minimal divergence in bacterial community composition between the Control and inoculated groups throughout fermentation (7–60 d) suggests that the native microbiota of mulberry branches, when subjected to anaerobic conditions, inherently possesses sufficient functional capacity to initiate and sustain ensiling without exogenous augmentation [36]. The predominance of Lactiplantibacillus in LP and LPC treatments during early fermentation drove rapid acidification through efficient lactic acid production, creating a low-pH environment that suppressed spoilage microorganisms [37]. In the Control group, early dominance of facultative anaerobes like Pediococcus likely facilitated initial oxygen scavenging and acid production prior to strict anaerobe establishment [38]. Inoculation with L. plantarum in LP/LPC groups preemptively occupied this niche, suppressing Pediococcus through competitive exclusion. The transient dominance of Enterococcus at mid-ensiling (30th d) across all groups may reflect its tolerance to moderately acidic conditions. The pH of the silage system decreased slowly at this stage, and the slightly acidic environment did not inhibit the growth of Enterococcus, which promoted the dominance of Enterococcus [39]. By day 60, community succession favored acid-tolerant Lactiplantibacillus and versatile Enterobacter, indicating adaptation to stable acidic and nutrient-limited conditions [40]. Certain Enterobacter species are facultative anaerobes capable of utilizing diverse carbon sources, potentially contributing to AA production (Table 4) [41]. Although Enterobacter was highly abundant in the late stage of silage (e.g., 30.90% in Control at day 60). However, their proliferation may also indicate suboptimal fermentation conditions, as Enterobacter can compete with LAB for substrates and produce NH3-N through proteolysis, leading to elevated pH and nutrient loss [42]. The butyric acid (BA) was detected in all groups and the significantly lower NH3-N in LPC (0.82% vs. 0.88% in LP and 0.55% in Control) suggest that spoilage was effectively suppressed. The combined inoculation (LPC) reduced Enterobacter abundance by 49% compared to Control (15.86% vs. 30.90%), likely due to competitive exclusion by dominant Lactiplantibacillus and accelerated acidification (Figure 3B, Table 4). Nevertheless, future studies should monitor Enterobacter dynamics during aerobic exposure to assess its implications for silage stability post-opening.
In addition, with increasing fermentation time, the acidic environment of the silage system tended to be stable, and Lactiplantibacillus was still the dominant flora in the silage fermentation systems. Thus, it played an important role and continued to produce lactic acid to maintain the acidic environment, thus ensuring fermentation quality [39]. The metabolism of the bacterial community greatly influenced the fermentation index during silage fermentation. Despite low abundance, Lactococcus showed significant negative correlation with NH3-N accumulation (Figure 5), suggesting synergistic interactions with dominant Lactiplantibacillus in suppressing proteolysis, possibly through competitive exclusion of ammonifying bacteria [43]. In this study, after 60 d of silage, the pH and NH3-N content were negatively correlated with Enterobacter. In addition, the LA content was significantly negatively correlated with Escherichia-Shigella, especially in the late stage of fermentation, and the inoculation of L. plantarum significantly inhibited the activities of harmful microorganisms such as Escherichia-Shigella in the mulberry silage system.
5. Conclusions
This study investigated the synergistic effects of L. plantarum (LP) and cellulase on mulberry silage quality and microbial dynamics over 60-day ensiling. Key findings demonstrate that combined inoculation (LPC group) significantly enhanced nutritional preservation (reduced DM loss by 6.3% vs. Control; increased CP by 7.1% at day 60), accelerated acidification (pH 4.64 vs. 5.44 in Control; elevated LA by 29.4%), and stabilized microbial ecology (the relative abundance of Lactiplantibacillus (71.63–83.28%) remained at a high level, effectively inhibiting the growth of mold, aerobic bacteria, and Enterobacteria (0.08–1.14%)). Methodological limitations include the absence of a cellulase-only group, which precludes precise quantification of its individual contribution to fiber degradation. Nevertheless, our results robustly demonstrate that combining L. plantarum with cellulase (LPC) maximizes fermentation efficiency, nutritional preservation, and microbial stability in mulberry silage.
Author Contributions
Data curation and writing—original draft preparation, Y.S.; writing—review and editing, Y.C. and Z.H.; resources and investigation, Z.H. and X.Z.; methodology and supervision, G.L. and Y.Q.; visualization, validation, and supervision, C.M. and F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System of the Ministry of Finance (MOF) and the Ministry of Agriculture and Rural Affairs(MARA): CARS; the Support Plan for Innovation and Development of Key Industries in South of Xinjiang (2022DB017); the Key research and development project of Turpan City: “Research on feed micro-storage technology of characteristic forest fruit by-products in Turpan City”; and the Ruminant feed key technology research innovation team (2024D14009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the first author. Data supporting the bacterial community in the Sequence Read Archive (SRA) can be found at the National Center for Biotechnology Information (NCBI). SRA number: PRJNA1266670.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADF | Acid detergent fiber |
| CP | Crude protein |
| EE | Ether extract |
| DM | Dry matter |
| LAB | Lactic acid bacteria |
| LP | Lactiplantibacillus plantarum |
| LPC | Lactiplantibacillus plantarum and cellulase |
| NDF | Neutral detergent fiber |
| NH3-N | Ammoniacal nitrogen |
| WSC | Water-soluble carbohydrate |
References
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and Proteomic Responses of Mulberry Trees (Morus alba. L.) to Combined Salt and Drought Stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef]
- Tian, L.; Fan, Y.; Li, H.; Zhang, Y.; Wang, Y.; Lei, J.; Liu, F.; He, W.; Jiao, Z.; Wang, C. Analysis and comprehensive evaluation of nutritional quality of different mulberry varieties in Xinjiang. J. Food Saf. Qual. 2024, 15, 149–159. [Google Scholar] [CrossRef]
- Linna, G.; Xuekai, W.; Yanli, L.; Xueping, Y.; Kuikui, N.; Fuyu, Y. Microorganisms that are critical for the fermentation quality of paper mulberry silage. Food Energy Secur. 2021, 10, e304. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, Y.; Wang, X.; Guo, L.; Lin, Y.; Ni, K.; Yang, F. Effects of Lactobacillus plantarum on fermentation quality and anti-nutritional factors of paper mulberry silage. Fermentation 2022, 8, 144. [Google Scholar] [CrossRef]
- David, R.; Krogh, J.S.; Morten, A.; Steffen, A.; Paolo, B.; Eric, J.; Stødkilde, L. Nutritional values of forage-legume-based silages and protein concentrates for growing pigs. Animal 2022, 16, 100572. [Google Scholar] [CrossRef]
- Cui, X.; Yang, Y.; Zhang, M.; Jiao, F.; Gan, T.; Lin, Z.; Huang, Y.; Wang, H.; Liu, S.; Bao, L.; et al. Optimized ensiling conditions and microbial community in mulberry leaves silage with Inoculants. Front. Microbiol. 2022, 13, 813363. [Google Scholar] [CrossRef]
- Du, Z.; Yamasaki, S.; Oya, T.; Cai, Y. Cellulase–lactic acid bacteria synergy action regulates silage fermentation of woody plant. Biotechnol. Biofuels Bioprod. 2023, 16, 125. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Wang, X.-K.; Lin, Y.-L.; Zheng, Y.-L.; Ni, K.-K.; Yang, F.-Y. Effects of Microbial Inoculants on Fermentation Quality and Aerobic Stability of Paper Mulberry Silages Prepared with Molasses or Cellulase. Fermentation 2022, 8, 167. [Google Scholar] [CrossRef]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef]
- Kleinschmit, D.H.; Kung, L. A Meta-Analysis of the Effects of Lactobacillus buchneri on the Fermentation and Aerobic Stability of Corn and Grass and Small-Grain Silages. J. Dairy Sci. 2006, 89, 4005–4013. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Lu, W.; Ma, C. Meta-analysis of the effects of combined homo-and hetero-fermentative lactic acid bacteria on the fermentation and aerobic stability of corn silage. Int. J. Agric. Biol. 2018, 20, 1846–1852. Available online: https://www.researchgate.net/publication/326849356_Meta-analysis_of_the_effects_of_combined_homo-_and_heterofermentative_lactic_acid_bacteria_on_the_fermentation_and_aerobic_stability_of_corn_silagel (accessed on 17 June 2025).
- Oliveria, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.; Vyas, D.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Liu, H.; Li, Z.; Nan, W.; Jin, C.; Sui, Y.; Li, G. Effect of Lactobacillus plantarum and Lactobacillus buchneri addition on fermentation, bacterial community and aerobic stability in lucerne silage. Anim. Prod. Sci. 2019, 59, 1528–1536. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Zhang, H.; Yang, T.; Abbas, Z.; Jiang, X.; Zhang, H.; Zhang, R.; Si, D. The Fermentation Quality, Antioxidant Activity, and Bacterial Community of Mulberry Leaf Silage with Pediococcus, Bacillus, and Wheat Bran. Fermentation 2024, 10, 214. [Google Scholar] [CrossRef]
- Alhaag, H.; Yuan, X.; Mala, A.; Bai, J.; Shao, T. Fermentation Characteristics of Lactobacillus plantarum and Pediococcus species Isolated from Sweet Sorghum Silage and Their Application as Silage Inoculants. Appl. Sci. 2019, 9, 1247. [Google Scholar] [CrossRef]
- Ridla, M.; Uchida, S. Comparative Study on the Effects of Combined Treatments of Lactic Acid Bacteria and Cellulases on the Cell Wall Compositions and the Digestibility of Rhodesgrass (Chloris gayana Kunth.) and Italian Ryegrass (Lolium multiflorum Lam.) Silages. Asian-Austral. J. Anim. Sci. 1999, 12, 531–536. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Dong, Z.; Chen, L.; Yuan, X.; Shao, T. The effects of lactic acid bacteria strains isolated from various substrates on the fermentation quality of common vetch (Vicia sativa L.) in tibet. Grass Forage Sci. 2018, 73, 639–647. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Yang, H.J.; Huang, R.Z.; Wang, X.Z.; Ma, C.H.; Zhang, F.F. Effects of Lactiplantibacillus plantarum and Lactiplantibacillus brevis on fermentation, aerobic stability, and the bacterial community of paper mulberry silage. Front. Microbiol. 2022, 13, 1063914. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- AOAC International. Official Method 977.22: Glucose in Plants—Spectrophotometric Method. In Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005; Volume 45, p. 12. [Google Scholar]
- Cheng, Q.; Chen, Y.; Bai, S.; Chen, L.; You, M.; Zhang, K.; Li, P.; Chen, C. Study on the bacterial community structure and fermentation characteristics of fresh and ensiled paper mulberry. Anim. Sci. J. 2021, 92, e13656. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Y.; Liu, B.; Zhao, Y.; Wu, J.; Yuan, X.; Zhu, W.; Cui, Z. Accelerated acidification by inoculation with a microbial consortia in a complex open environment. Bioresour. Technol. 2016, 216, 294–301. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, M.; Fan, X.; Chen, Y.; Sun, H.; Xie, Y.; Zheng, Y.; Chen, C.; Li, P. Effects of epiphytic and exogenous lactic acid bacteria on fermentation quality and microbial community compositions of paper mulberry silage. Front. Microbiol. 2022, 13, 973500. [Google Scholar] [CrossRef]
- Xi, X.J.; Han, L.J.; Yuan, S.Y.L.; Ye, Z.H.J. Effect of adding lactic acid bacteria and cellulase on the quality of corn stalk silage. J. China Agric. Univ. 2003, 2, 21–24. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, Q.Z. Forage Silage Technology; China Agricultural University Press: Beijing, China, 2011. [Google Scholar]
- Liu, W.; Si, Q.; Sun, L.; Wang, Z.; Liu, M.; Du, S.; Ge, G.; Jia, Y. Effects of cellulase and xylanase addition on fermentation quality, aerobic stability, and bacteria composition of low water-soluble carbohydrates oat silage. Fermentation 2023, 9, 638. [Google Scholar] [CrossRef]
- Si, Q.; Wang, Z.; Liu, W.; Liu, M.; Ge, G.; Jia, Y.; Du, S. Influence of cellulase or Lactiplantibacillus plantarum on the ensiling performance and bacterial community in mixed silage of alfalfa and Leymus chinensis. Microorganisms 2023, 11, 426. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Xing, Y.Q.; Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q. Dynamics of proteolysis, protease activity and bacterial community of Neolamarckia cadamba leaves silage and the effects of formic acid and Lactobacillus farciminis. Bioresour. Technol. 2019, 294, 122127. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wang, C.; He, L.; Zhou, W.; Yang, F.; Zhang, Q. The bacterial community and fermentation quality of mulberry (Morus alba) leaf silage with or without Lactobacillus casei and sucrose. Bioresour. Technol. 2019, 293, 122059. [Google Scholar] [CrossRef]
- Jua, K.; Yong-Min, K.; Vincent, R.L.; Wee, Y.-J. Lactic acid for green chemical industry: Recent advances in and future prospects for production technology, recovery, and applications. Fermentation 2022, 8, 609. [Google Scholar] [CrossRef]
- Lu, L.; Xiao, M.; Xu, X. Enterobacter agglomerans B1 producing β-galactosidase with transglycosylation activity: Screening, identification, fermentation conditions, and galacto- oligosaccharides synthesis. Acta Microbiol. Sin. 2008, 48, 38–44. [Google Scholar] [PubMed]
- Dong, Z.; Shao, T.; Li, J.; Yang, L.; Yuan, X. Effect of alfalfa microbiota on fermentation quality and bacterial community succession in fresh or sterile Napier grass silages. J. Dairy Sci. 2020, 103, 4288–4301. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Li, S.; Wang, S.; Li, S.; Ke, Y.; Gao, R.; Wang, L.; Zhou, Z.; Wu, Z.; et al. Pretreatment of sweet sorghum silages with Lactobacillus plantarum and cellulase with two different raw material characteristics: Fermentation profile, carbohydrate composition, in vitro rumen fermentation and microbiota communities. Chem. Biol. Technol. Agric. 2025, 12, 33. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Guo, Q.; Sudu, B.; Han, H. Modulation of the microbial community and the fermentation characteristics of wrapped natural grass silage inoculated with composite bacteria. Chem. Biol. Technol. Agric. 2025, 12, 50. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Zhang, B.; Bao, J. pH shifting adaptive evolution stimulates the low pH tolerance of Pediococcus acidilactici and high L-lactic acid fermentation efficiency. Bioresour. Technol. 2025, 416, 131813. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.M.A.; Gomes, O.P.; Evangelista, F.P.; Azevedo, C.F.; da Silva, V.P.; e Silva, F.F. Microbiome of rehydrated corn and sorghum grain silages treated with microbial inoculants in different fermentation periods. Sci. Rep. 2022, 12, 16864. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Biochem. Microbiol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, R.; Wang, C.; Dong, W.; Zhang, Z.; Zhao, L.; Zhang, X. Effects of Cellulase and Lactobacillus plantarum on Fermentation Quality, Chemical Composition, and Microbial Community of Mixed Silage of Whole-Plant Corn and Peanut Vines. Appl. Biochem. Biotechnol. 2022, 194, 2465–2480. [Google Scholar] [CrossRef]
- Xiong, H.; Zhu, Y.; Wen, Z.; Liu, G.; Guo, Y.; Sun, B. Effects of Cellulase, Lactobacillus plantarum, and Sucrose on Fermentation Parameters, Chemical Composition, and Bacterial Community of Hybrid Pennisetum Silage. Fermentation 2022, 8, 356. [Google Scholar] [CrossRef]
- Chen, G.; Chen, D.; Zhou, W.; Peng, Y.; Chen, C.; Shen, W.; Zeng, X.; Yuan, Q. Improvement of metabolic syndrome in high-fat diet-induced mice by yeast β-glucan is linked to inhibited proliferation of Lactobacillus and Lactococcus in gut microbiota. J. Agric. Food Chem. 2021, 69, 7581–7592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).