Abstract
Campylobacter jejuni and Campylobacter coli are the two main campylobacter species that cause foodborne campylobacteriosis. Recent studies have reported that Campylobacter spp. are prone to developing resistance to antibiotics commonly used for their treatment, with many C. coli strains identified as multidrug-resistant. This study presents the results of the whole-genome sequencing analysis of the multidrug-resistant C. coli strain BCT3 isolated in Greece from a stool specimen of a pediatric patient presenting with diarrhea. The strain was isolated using selective culture media and, based on antimicrobial susceptibility tests, was found to be resistant to ciprofloxacin, tetracycline, erythromycin, azithromycin, clarithromycin, and doxycycline. To further characterize it, we performed whole-genome sequencing, which identified strain BCT3 as C. coli. Moreover, multilocus sequence typing assigned the BCT3 to the sequence type (ST) 872, belonging to clonal complex ST-828. The presence of multiple virulence genes revealed its pathogenic potential. The detection of antimicrobial resistance genes and mutated alleles was indicative of its resistance to fluoroquinolones, macrolides, and tetracyclines, supporting the observed phenotype. To our knowledge, this is the first reported clinical case of such a multidrug-resistant C. coli strain in Greece.
1. Introduction
Campylobacter is a major cause of bacterial gastroenteritis worldwide [1] and is associated with multiple gastrointestinal and neurological disorders, including inflammatory bowel disease (IBD), colorectal cancer, and Guillain–Barré syndrome [2,3,4]. Although Campylobacter infection can have a fatal outcome in children, the elderly, and immunocompromised individuals [5], the majority of cases do not require any treatment, as the disease is self-limited and the symptoms are mild [6]. Campylobacter jejuni and Campylobacter coli are the two most commonly detected species in humans, as compared to Campylobacter lari and Campylobacter upsaliensis [5].
The main route of Campylobacter transmission is through the consumption of raw or undercooked meat, especially poultry, while other transmissive sources can include the soil, water, and contact with domestic or wild animals [7,8]. It is widely acknowledged that chicken meat is a significant source of Campylobacter infections in humans on a global scale. Campylobacter spp. have been observed to colonize the intestinal tract of avian hosts in substantial numbers. Also, the transmission of these bacteria among flocks can result in elevated infection rates within the poultry population. At the level of the slaughterhouse, improper handling of chickens can result in carcasses becoming contaminated with Campylobacter from the intestinal content. The occurrence of cross-contamination is a potential hazard, with the transmission of bacteria from contaminated poultry products to water sources or food being a possibility [9]. It has also been demonstrated that due to the failure to comply with strict safety requirements during the slaughtering process, pig and cattle carcasses can become contaminated with Campylobacter [10]. Additionally, the bacterium was found at the highest rates in the neck region of the subjects in comparison with other anatomical regions. This could possibly be due to elevated exposure of these regions to the contents of the intestinal tract during the processing stage [11]. A study conducted in Greece in 2020 revealed that the prevalence of Campylobacter spp. in carcasses was found to be 70.42% positive for the presence of the bacterium. Furthermore, 73.94% of the cecum-based samples were found to be positive for Campylobacter spp. [12].
Distinctive genetic virulence factors of human Campylobacter isolates are the cadF and flaA genes. The cadF gene encodes the CadF adhesive fibronectin and facilitates adhesion to host cells. FlaA, the flagellin protein that is encoded by the flaA gene, is a part of the bacterial filament and is important for Campylobacter mobility [13,14,15]. The flaA locus is a short variable region (SVR) that is used in Campylobacter genotyping and phylogenetic analyses [16,17]. Among other significant virulence indicators that are associated with better host invasion are the ciaB and virB11 genes [13].
The invasion of the intestinal tract by Campylobacter, particularly the crypts, is established by specific adhesion to the host’s epithelium proteins, accompanied by the colonization of intestinal cells. This is followed by Campylobacter proliferation and secretion of toxins, necrotizing the intestinal villi, resulting in damage to the epithelium of the intestines through the opening of the tight junctions and the shielding barrier, the release of electrolytes, and the initiation of inflammatory responses, resulting in severe and bloody diarrhea [18]. Campylobacter has been reported to induce the secretion of cytokines and Interleukin-8. Another bacterial protein used for adhesion and host invasion is JlpA, a lipoprotein of the surface that also binds to Hsp90α. This interaction activates both NF-κΒ and mitogen-activated protein (MAP) kinase, leading to proinflammatory responses [19].
The first-line treatment against C. jejuni and C. coli includes the administration of antimicrobials from the macrolide class (erythromycin, clarithromycin, and azithromycin) and tetracycline and its derivative doxycycline, and antimicrobials of the quinolone class (ciprofloxacin) [17,20]. Other treatment options for persistent campylobacteriosis cases are aminoglycosides (gentamicin, kanamycin, etc.) and carbapenems (meropenem and imipenem) [21,22].
Over the past few years, the extensive use of drugs in livestock farming has contributed to the development of high resistance towards different groups of antimicrobials used against C. jejuni and C. coli [23]. According to the 2022 surveillance report of the European Centre for Disease Prevention and Control (ECDC), the highest rate of resistance to macrolides for C. coli (38.5%) was detected in Greece, followed by Portugal (26.9%) and Spain (19.3%) [24]. Additionally, the “European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022” revealed that in contrast with C. jejuni, C. coli had elevated multidrug-resistance (MDR) in 9.0% of human, 8.3% of broiler, 16.9% of fattening turkey, 39.3% of young calf, and 9.5% of fattening pig isolates [25].
The outcomes of the antimicrobial resistance (AMR) assessment of foodborne pathogens like Campylobacter spp. are deeply concerning, underscoring an immediate imperative for the establishment of an integrated surveillance system to restrain the overuse of antibiotics. A “One Health” approach is imperative to curtail the excessive utilization of antibiotics in the context of animal farming and to forestall the spread of AMR through the food supply chain. The implementation of the “One Health” concept in the context of poultry farming necessitates a multifaceted approach and effective collaboration between food production, human health, and regulatory bodies. This could include enhanced biosecurity measures, more rational antibiotic usage, and more robust food safety regulations [11]. Relevant to these concepts, in the current study, we present the genome sequence of a multidrug-resistant C. coli strain, isolated from the stool of a 3-year-old male patient hospitalized with diarrheal symptoms.
2. Materials and Methods
2.1. Strain Isolation and Antimicrobial Susceptibility Testing
A strain of Campylobacter coli was isolated from a child’s diarrheic stool sample using Campylobacter Agar (Oxoid, Basingstoke, UK) that contains the antibiotics vancomycin, polymyxin B, amphotericin B, and trimethoprim to inhibit the growth of competing intestinal flora and allow for the selective isolation of Campylobacter spp. For culture, a Campylobacter agar plate was inoculated and incubated under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) at 42 °C for 40–48 h. After the incubation period, the plates were examined for the presence of Campylobacter spp. Colonies with characteristic morphology were presumptively identified and further confirmed using the VITEK® MS PRIME system (bioMérieux, Craponne, France), a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) system, following the manufacturer’s instructions and established protocols [26]. Antimicrobial susceptibility testing was performed according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standardized disk diffusion method [27]. Mueller–Hinton agar supplemented with 5% defibrinated horse blood and 20 mg/L β-NAD (Bioprepare, Keratea, Greece) was used as the testing medium. The bacterial inoculum was adjusted to a 0.5 McFarland turbidity standard using a densitometer (DensiCHEK Plus, bioMérieux, Craponne, France), according to EUCAST recommendations. The plates were incubated under microaerophilic conditions at 42 °C for 24 h. Inhibition zones were measured and interpreted in accordance with EUCAST guidelines [28].
2.2. Genome Sequencing and Bioinformatics Analysis
Genomic DNA of C. coli strain BCT3 was extracted using the MagCore Nucleic Acid Extraction Kit for bacteria and the respective equipment DNAex-MagCore-02 (RBC Bioscience Corp., New Taipei City, Taiwan). Whole-genome sequencing (WGS) of the BCT3 strain was performed by Novogene (Novogene, Cambridge, UK) on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) following standard procedures, including library preparation and 2 × 250 bp paired-end sequencing. After sequencing, the raw reads of the BCT3 strain were assessed for their quality with FastQC v0.11.9 [29] and trimmed using Cutadapt v4.5 [30]. Subsequently, the trimmed reads were assembled de novo using the Unicycler v0.5.1 assembly pipeline [31], and the quality of the obtained assembly was checked with Quast v5.0.2 [32]. Species identification of the BCT3 strain was conducted with the Type (Strain) Genome Server (TYGS) [33]. In order to obtain the reference-guided assembly, the initial assembly was further processed using the assembly tool Ragout v2.3 [34] with the Campylobacter coli reference genome for strain FDAARGOS_735 (GenBank accession number: CP046317.1). The completeness of the final reference-guided genome assembly was checked with Busco v5.0.0 [35], and its alignment against the chromosomal sequence of the reference strain was obtained using the Circoletto suite [36]. The genome map of C. coli BCT3, visualizing the genomic features and labeling of genes and proteins, was acquired with DNAPlotter [37]. A search to identify plasmid sequences was conducted using PlasmidFinder [38], and variant calling was carried out with Snippy v4.3.6 [39]. Multilocus sequence typing (MLST) and core genome MLST analysis were conducted with CLC genomics workbench v24.0.2 (Qiagen, Hilden, Germany) with the C. jejuni/C. coli MLST [40,41] and core genome C. jejuni/C. coli MLST (cgMLST) v1.0 schemes [42] using the PubMLST database [43]. The functional annotation of the final genome was made using the Rapid Annotation Subsystem Technology (RAST) server [44]. The presence of virulence genes in the C. coli BCT3 genome was explored using CLC genomics workbench v24.0.2 as well as the ABRicate v1.0.1 pipeline [45] through CamPype v1.0 [46] against the virulence factor database (VFDB) [47]. The antimicrobial resistance (AMR) of the C. coli BCT3 strain was explored using CLC genomics workbench v24.0.2 and CamPype v1.0, ABRicate v1.0.1 and AMRFinderPlus v3.11.2 [48] against ResFinder [49], the Comprehensive Antibiotic Resistance Database (CARD) [50], the ARG-ANNOT peptide marker database [51], MEGARes 2.0 [52], and the NCBI [48]. Only genes with alignment coverage exceeding 90% and sequence identity above 80% were considered as present in the genome [53,54].
3. Results and Discussion
3.1. General Characteristics of the Genome of Campylobacter coli Strain BCT3
A high-quality de novo assembly of the genome under investigation was obtained, comprising 44 contigs with a total length of 1,690,752 bp and a GC% content of 31%. A total of 11 contigs exceeded 50,000 bp, and the assembly showed strong continuity with an N50 value of 162,311 bp and an L50 of 4. Genome analysis using the TYGS server identified strain BCT3 as Campylobacter coli, which agreed with the results of the Vitek MS Prime system (Figure 1A).
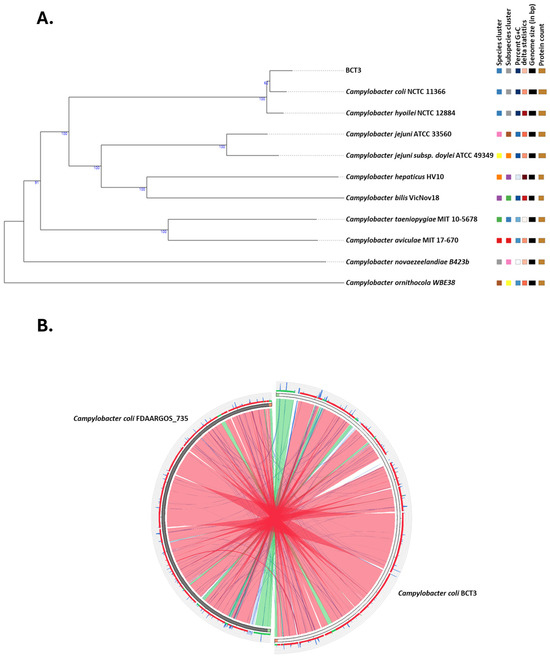
Figure 1.
Phylogenetic and comparative genomic analyses of Campylobacter coli BCT3. Phylogenetic tree showing the relationship among the C. coli BCT3 and type strains in the TYGS database (A). Circular genome alignment between the C. coli BCT3 strain and the C. coli reference strain FDAARGOS_735 (B). Colored ribbons correspond to different levels of % identity according to BLASTn local alignment (red > 98%, green ≤ 98%, blue ≤ 95%, and orange ≤ 90%).
A reference-guided assembly produced a single scaffold of 1,731,905 bp in length with a number of unplaced contigs of a total length of 14,884 bp (0.87% of the total) and a completeness of 99.8%. Furthermore, alignment of C. coli BCT3 against the reference genome of strain FDAARGOS_735 revealed significant similarity, with most regions being syntenic (Figure 1B). Variant calling revealed a total of 6094 variants in the C. coli BCT3 genome compared to the reference sequence, out of which 4744 were accounted as Single Nucleotide Polymorphisms (SNPs). In addition, a small number of insertions (70) and deletions (52) were observed, suggesting minimal structural disruption in terms of added or missing nucleotides. Finally, no plasmids were identified according to the in silico analysis.
The circular genome map of the C. coli BCT3 strain is shown in Figure 2A. The analysis of the protein-encoding genes of strain BCT3 with RAST showed that 29% of the annotated features were categorized into subsystems, while 71% of proteins could not be assigned to subsystem categories. The results indicated that the genome of the isolated strain harbors genes associated with “Amino acid and protein metabolism” (148 counts), “Protein metabolism” (118 counts), and “Cofactors, vitamins, prosthetic groups, pigments” (78 counts), among others (Figure 2B).
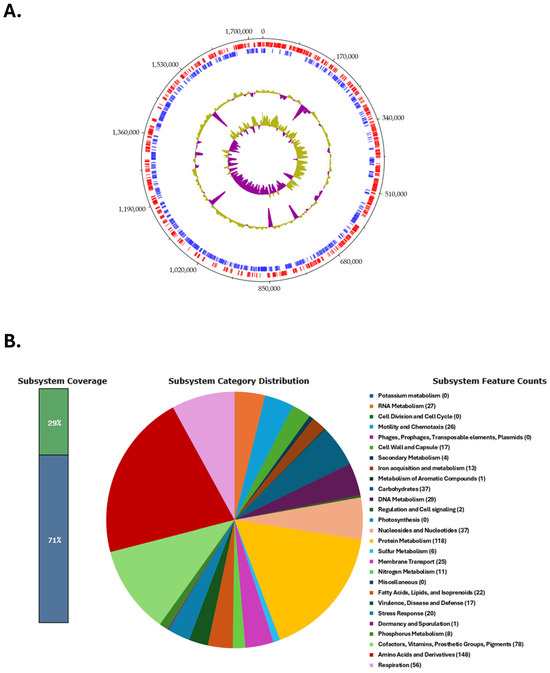
Figure 2.
Genome characteristics of the Campylobacter coli BCT3 strain. Circular genome map of the C. coli BCT3 strain generated using DNAPlotter (A). Tracks from inside to outside represent the GC skew, GC content, and reverse (blue) and forward (red) coding sequences (CDSs). Functional categorization of the protein-encoding genes of the C. coli BCT3 genome assigned to subsystem categories (B).
3.2. Multilocus Sequence Typing
The BCT3 genome was analyzed with the C. jejuni/C. coli MLST scheme targeting seven housekeeping genes (aspA, glnA, gltA, glyA, pgm, tkt, and uncA) [40,41,55]. The results obtained indicated that the BCT3 strain possessed aspA (33), glnA (39), gltA (30), pgm (113), glyA (82), tkt (44), and uncA (17) and was assigned to sequence type (ST) 872 belonging to the ST-828 clonal complex (CC) (Figure 3). Isolates of C. coli assigned to ST-872 have been identified in different hosts, predominantly in chickens [56,57,58,59,60,61,62,63] and humans [58,64,65,66,67,68,69,70], but also in geese [61] and swine [71]. It has also been suggested that C. coli of ST-872 is a potential foodborne risk for human infection through chicken consumption [56].
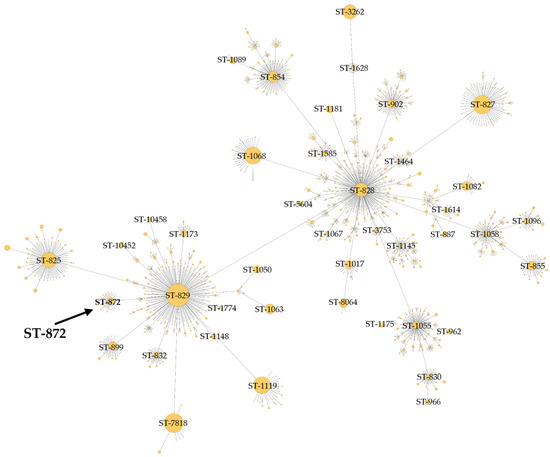
Figure 3.
Minimum spanning tree of clonal complex ST-828 constructed for C. coli BCT3 according to the C. jejuni/C. coli MLST scheme.
The genome of C. coli BCT3 was also subjected to analysis using the Campylobacter jejuni/C. coli core-genome MLST (cgMLST) scheme [42] and it was assigned to cgST 19335, but inconclusively.
3.3. Virulence Factors
The pathogenic potential of the C. coli BCT3 strain was further investigated. Our analysis revealed the presence of multiple genes that contribute to the virulence of Campylobacter spp. by facilitating adhesion, invasion, motility, chemotaxis, and evasion of the host innate immune system [72,73,74,75]. Adhesion and invasion genes included cadF and pebA (Table 1). The cadF gene encodes an outer membrane protein that binds fibronectin, promoting, in this way, adhesion to host epithelial cells and enabling colonization [74,76]. The pebA gene encodes a bifunctional protein that serves both as an adhesin and the substrate-binding component of an ABC transporter for aspartate and glutamate, contributing to host cell adhesion [77]. The invasion-associated genes ciaB and ciaC, which mediate the interaction of the pathogen with host cells, were also detected [72]. Genes encoding core structural components of the flagellum included flgB, flgC, flgD, flgE, flgF, flgG, flgH, flgI, fliD, fliE, fliF, fliG, fliL, flaC, flaD, and flaG. Of note, a partial flaA was detected after manual investigation, most probably due to a local assembly incompleteness. Genes responsible for motor function, secretion, and export comprised pflA, motA, flhA, flhB, flhF, flhG, fliI, fliP, fliQ, fliR, and fliS, while additional genes contributing to flagellar assembly and structural components included flgJ, flgK, flgM, flgP, flgQ, fliW, and fliY [72,74,75,78,79,80,81]. Chemotaxis-related genes involved in host colonization, cheA, cheV, cheW, and cheY, were also found, further increasing the virulence potential of the C. coli BCT3 strain [72,82,83]. Furthermore, we identified the presence of the two-component regulatory system genes flgS and flgR, along with rpoN, which encodes σ54, indicating the potential for σ54-dependent regulation of flagellar gene expression [72,84]. Additional regulatory genes included fliA, which encodes the flagellar-specific sigma factor σ28, and the motor switch proteins fliM and fliN, involved in controlling flagellar rotation [72,79]. Genes involved in flagellin glycosylation and post-translational modification, including pseA-pseI, ptmA, ptmB, and eptC, were also detected, supporting the completeness of the pseudaminic acid biosynthesis pathway and indicating the potential of alternative glycosylation mechanisms essential for flagellar function [59,79,85,86,87].
Other virulence genes detected were those involved in lipooligosaccharide (LOS) biosynthesis that play a significant role in the pathogenicity of Campylobacter spp. [72]. Genes gmhA and gmhB, involved in the biosynthesis of heptose sugars, an important component of LOS, were found to be present. Genes waaC and waaF encoding heptosyltransferases that add heptose sugar molecules to the LOS core structure were also detected [72,88,89,90,91,92]. Furthermore, gene waaV contributing to LOS core assembly was found along with hldD and hldE, which are associated with LOS biosynthesis [59,93]. The presence of all of these genes indicates a fully functional LOS biosynthetic pathway. Finally, genes protecting the C. coli BCT3 strain from host immune responses related to capsule biosynthesis and export included kpsD, kpsF, kpsM, kpsS, and kpsT [72,92]. Several of the aforementioned virulence genes have also been reported for C. coli strains of ST-872 [57,59,61,63].

Table 1.
Virulence genes found in the C. coli BCT3 strain.
Table 1.
Virulence genes found in the C. coli BCT3 strain.
| Gene | Function | Ref. |
|---|---|---|
| cadF | Outer membrane fibronectin-binding protein | [57,74] |
| pebA | Binding protein of an ABC transporter system | [77,94] |
| ciaB | Invasion antigen | [72,74] |
| ciaC | Invasion antigen | [72,74] |
| flgB | Flagellar basal body rod protein | [79,94] |
| flgC | Flagellar basal body rod protein | [79] |
| flgD | Flagellar basal body rod modification protein | [79] |
| flgE | Flagellar hook protein | [72,79,95] |
| flgF | Flagellar basal body rod protein | [80] |
| flgG | Flagellar basal body rod protein | [74] |
| flgH | Flagellar L-ring protein precursor | [80] |
| flgI | Flagellar P-ring protein precursor | [96] |
| flgJ | Flagellar protein involved in peptidoglycan hydrolysis | [96] |
| flgK | Flagellar hook-associated protein | [79] |
| flgM | Negative regulator of flagellin synthesis | [97] |
| flgP | Flagellar motility protein | [72] |
| flgQ | Flagellar motility protein | [75] |
| flgR | σ54—associated transcriptional activator | [74] |
| flgS | Signal transduction histidine kinase | [74] |
| flhA | Flagellar biosynthesis protein | [72,74] |
| flhB | Flagellar biosynthesis protein | [74] |
| flhF | Flagellar biosynthesis regulator | [79] |
| flhG | ATP-binding protein | [79] |
| flaC | Secreted flagellin | [72] |
| flaD | Flagellar protein | [81] |
| flaG | Flagellar filament length control | [72,97] |
| fliA | Flagellar biosynthesis sigma factor | [72] |
| fliD | Flagellar hook-associated protein | [79,98] |
| fliE | Flagellar hook–basal body complex protein | [79] |
| fliF | Flagellar M-ring protein | [74,79] |
| fliG | Flagellar motor switch protein | [74,79] |
| fliI | Flagellum-specific ATPse | [79] |
| fliL | Flagellar basal body protein | [79] |
| fliM | Flagellar motor switch protein | [79] |
| fliN | Flagellar motor switch protein | [79] |
| fliP | Flagellar biosynthesis protein | [74] |
| fliQ | Flagellar biosynthesis protein | [74] |
| fliR | Flagellar biosynthetic protein | [74] |
| fliS | Flagellar secretion chaperone | [72,78,79] |
| fliW | Flagellar assembly protein | [72,78] |
| fliY | Flagellar motor switch protein | [74] |
| rpoN | RNA polymerase factor σ54 | [84] |
| pflA | Paralysed flagellum protein | [74] |
| motA | Flagellar motor protein | [72,74] |
| cheA | Histidine kinase sensor | [72,74,82,83] |
| cheV | Phosphotransferase | [72,74] |
| cheW | Phosphotransferase | [72,74] |
| cheY | Cytoplasmic response regulator | [72,74] |
| pseA | Pseudaminic acid biosynthesis protein | [87] |
| pseB | UDP-N-acetylglucosamine 4,6-dehydratase | [87,99] |
| pseC | UDP-4-amino-4,6-dideoxy-N-acetyl-beta-L-altrosamine transaminase | [87] |
| pseD | Motility accessory factor | [87] |
| pseE | Motility accessory factor | [79,87] |
| pseF | Acylneuraminate cytidylyltransferase | [87] |
| pseG | UDP-2,4-diacetamido-2,4,6-trideoxy-beta-L-altropyranose hydrolase | [87] |
| pseH | UDP-4-amino-4, 6-dideoxy-N-acetyl-beta-L-altrosamine N-acetyltransferase | [87] |
| pseI | N-acetylneuraminic acid synthetase | [100] |
| eptC | Phosphoethanolamine transferase | [59] |
| ptmA | Flagellin modification protein | [79,86] |
| ptmB | Acylneuraminate cytidylyltransferase | [79,86] |
| gmhA | Phosphoheptose isomerase | [91,92] |
| gmhB | DD-heptose 17-bisphosphate phosphatase | [91] |
| waaC | Heptosyltransferase I | [90,92] |
| waaF | Heptosyltransferase II | [92] |
| waaV | Glucosyltransferase | [92,93] |
| hldD | ADP-glyceromanno-heptose 6-epimerase | [59] |
| hldE | Bifunctional D-beta-D-heptose 7-phosphate kinase/D-beta-D-heptose 1-phosphate adenylyltransferase | [59] |
| kpsD | Capsule polysaccharide export system periplasmic protein | [72,92] |
| kpsF | D-arabinose 5-phosphate isomerase | [92] |
| kpsM | Capsule polysaccharide export system inner membrane protein | [72,92] |
| kpsS | Capsule polysaccharide modification protein | [92] |
| kpsT | Capsule polysaccharide export ATP-binding protein | [92] |
3.4. Antimicrobial Resistance
Laboratory tests of the C. coli BCT3 strain showed its high resistance to multiple antibiotics, including ciprofloxacin, tetracycline, erythromycin, azithromycin, clarithromycin, and doxycycline (Table 2).

Table 2.
Resistance of the C. coli BCT3 strain to different antibiotics.
The observed MDR exerted by strain BCT3 could be attributed to the presence of AMR genes and mutated alleles (Table 3), which were detected in the genome sequence. These genes included tet(O), one of the most prevalent resistance genes in Campylobacter spp. [101], which confers resistance to tetracycline, doxycycline, and minocycline [102,103] and blaOXA-61, blaOXA-489, and blaOXA-605, contributing to resistance against beta-lactam [104,105].

Table 3.
Antimicrobial resistance genes found in the C. coli BCT3 strain.
The analysis also showed the existence of aminoglycoside resistance genes, including ant(6)-Ia, aad9, and aadE [110,114]. Additionally, mutations gyrA (T86I) and 23S (A2075G) were found, providing resistance to quinolones and macrolides, respectively [79,107,110,113]. Furthermore, a major multidrug efflux pump, CmeABC, which expels toxic compounds and plays a key role in Campylobacter resistance to a wide range of structurally diverse antimicrobials, was identified [107,111,115,116,117]. The full functionality of the CmeABC efflux pump requires the presence of all three components [107]. In addition, the cmeR gene, which encodes the transcriptional repressor CmeR regulating cmeABC expression, was also detected, albeit with 76.7% identity to the reference sequence [107,111]. This reduced identity may suggest a potential alteration in the regulatory function of CmeR, which could lead to the overexpression of CmeABC and contribute to increased antimicrobial resistance in the C. coli BCT3 strain [118,119]. Finally, another multidrug efflux pump, CmeDEF, was also present [74,107]. Although it may differ in function and in capacity to expel antibiotics and other toxic compounds from cmeABC, the presence of both of these systems in the C. coli BCT3 strain may enhance its antimicrobial resistance and adaptability to stresses [74,107,111,112].
It should be emphasized that other strains belonging to ST-872 within the ST-828 clonal complex isolated from human and chicken hosts have been found to be multidrug resistant (resistant to more than three different antimicrobial classes) [25,59,61,66,67]. These findings highlight the need for continuous monitoring and further assessment of the antibiotic resistance, virulence, and epidemiological behavior of strains belonging to this sequence type.
4. Conclusions
This investigation of the genome of the C. coli BCT3 strain isolated in Greece from a pediatric diarrhea case has provided important insights into its genetic composition, virulence potential, and antimicrobial resistance. The analysis revealed that the C. coli BCT3 strain belonged to ST-872 of clonal complex ST-828. Isolates of this type have been previously associated with human and chicken infections [57,59,61,63,67]. The identification of virulence-associated genes contributing to C. coli BCT3 strain immune evasion and intestinal colonization of the host revealed the pathogenic potential of this strain. Laboratory tests and analysis of AMR genes of the BCT3 strain validated its resistance to multiple antibiotics, including fluoroquinolones, macrolides, and tetracyclines. The MDR of the BCT3 strain, particularly its resistance to macrolides and fluoroquinolones, which are commonly used for the treatment of human campylobacteriosis, highlights potential challenges for clinical treatment [120,121]. The genome analysis reveals several characteristics of the strain. Nevertheless, further functional studies are needed to uncover novel aspects of its pathogenicity and antimicrobial resistance, particularly under simulated food processing or clinical conditions. Our findings emphasize the need to study multidrug-resistant strains of C. coli to understand the underlying resistance mechanisms and develop effective treatments for Campylobacter infections. The properties of the isolated multidrug-resistant strain BCT3 highlight the necessity for implementing suitable measures, ranging from livestock farming to health systems. Overall, this necessity arises from the concept of “One Health”, which emphasizes the interconnection between the health of humans, animals, plants, and the environment.
Author Contributions
Conceptualization, K.P., A.I. and S.C.; methodology, K.P. and A.S.; formal analysis, K.P., A.I., A.S. and E.O.; investigation, G.C., N.M., A.P., D.T., I.K. and S.C.; writing—original draft preparation, K.P., A.I., A.S., I.K. and E.I.K.; writing—review and editing, K.P., A.I., A.S. and N.V.; supervision, K.P., A.I., N.V. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Athens Medical Center (protocol code: 183/21 February 2025).
Informed Consent Statement
Informed consent was given by the parents of the child.
Data Availability Statement
The whole-genome shotgun project has been deposited in GenBank under the bioproject ID PRJNA1237805.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaakoush, N.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Esan, O.B.; Pearce, M.; van Hecke, O.; Roberts, N.; Collins, D.R.J.; Violato, M.; McCarthy, N.; Perera, R.; Fanshawe, T.R. Factors associated with sequelae of Campylobacter and non-typhoidal Salmonella infections: A systematic review. EBioMedicine 2017, 15, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Leonhard, S.E.; van der Eijk, A.A.; Andersen, H.; Antonini, G.; Arends, S.; Attarian, S.; Barroso, F.A.; Bateman, K.J.; Batstra, M.R.; Benedetti, L.; et al. An international perspective on preceding infections in Guillain-Barré syndrome: The IGOS-1000 cohort. Neurology 2022, 99, e1299–e1313. [Google Scholar] [CrossRef]
- WHO. Campylobacter. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 21 November 2024).
- Veronese, P.; Dodi, I. Campylobacter jejuni/coli infection: Is it still a concern? Microorganisms 2024, 12, 2669. [Google Scholar] [CrossRef]
- Quino, W.; Caro-Castro, J.; Hurtado, V.; Flores-León, D.; Gonzalez-Escalona, N.; Gavilan, R.G. Genomic analysis and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli in Peru. Front. Microbiol. 2022, 12, 802404. [Google Scholar] [CrossRef]
- Rukambile, E.; Sintchenko, V.; Muscatello, G.; Kock, R.; Alders, R. Infection, colonization and shedding of Campylobacter and Salmonella in animals and their contribution to human disease: A review. Zoonoses Public Health 2019, 66, 562–578. [Google Scholar] [CrossRef]
- Popa, S.A.; Morar, A.; Ban-Cucerzan, A.; Imre, K. Last decade mini-review of the scientific progresses in the monitoring of the occurrence and antimicrobial susceptibility profile of poultry origin Campylobacter spp. within the European Union countries. Rev. Rom. Med. Vet. 2022, 32, 75–82. [Google Scholar]
- Popa, S.A.; Morar, A.; Ban-Cucerzan, A.; Tîrziu, E.; Herman, V.; Imre, M.; Florea, T.; Morar, D.; Pătrînjan, R.-T.; Imre, K. First study in the frequency of isolation and phenotypic antimicrobial resistance profiles of pig and cattle origin Campylobacter strains in Romania. Vet. Res. Commun. 2024, 48, 2621–2627. [Google Scholar] [CrossRef]
- Popa, S.A.; Herman, V.; Tîrziu, E.; Morar, A.; Ban-Cucerzan, A.; Imre, M.; Pătrînjan, R.-T.; Imre, K. Public health risk of Campylobacter spp. isolated from slaughterhouse and retail poultry meat: Prevalence and antimicrobial resistance profiles. Pathogens 2025, 14, 316. [Google Scholar] [CrossRef]
- Natsos, G.; Mouttotou, N.K.; Magiorkinis, E.; Ioannidis, A.; Rodi-Burriel, A.; Chatzipanagiotou, S.; Koutoulis, K.C. Prevalence of and risk factors for Campylobacter spp. colonization of broiler chicken flocks in Greece. Foodborne Pathog. Dis. 2020, 17, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Bunduruș, I.A.; Balta, I.; Ștef, L.; Ahmadi, M.; Peț, I.; McCleery, D.; Corcionivoschi, N. Overview of virulence and antibiotic resistance in Campylobacter spp. livestock isolates. Antibiotics 2023, 12, 402. [Google Scholar] [CrossRef]
- Elmi, A.; Nasher, F.; Dorrell, N.; Wren, B.; Gundogdu, O. Revisiting Campylobacter jejuni virulence and fitness factors: Role in sensing, adapting, and competing. Front. Cell. Infect. Microbiol. 2021, 10, 607704. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Wołkowicz, T.; Osek, J. Antimicrobial resistance and virulence-associated traits of Campylobacter jejuni isolated from poultry food chain and humans with diarrhea. Front. Microbiol. 2018, 9, 1508. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, V.; Ioannidis, A.; Magiorkinis, E.; Bagos, P.; Nicolaou, C.; Legakis, N.; Chatzipanagiotou, S. Multilocus sequence typing (and phylogenetic analysis) of Campylobacter jejuni and Campylobacter coli strains isolated from clinical cases in Greece. BMC Res. Notes 2013, 6, 359. [Google Scholar] [CrossRef]
- Magana, M.; Chatzipanagiotou, S.; Burriel, A.R.; Ioannidis, A. Inquiring into the gaps of Campylobacter surveillance methods. Vet. Sci. 2017, 4, 36. [Google Scholar] [CrossRef]
- Aguilar, C.; Jiménez-Marín, Á.; Martins, R.P.; Garrido, J.J. Interaction between Campylobacter and intestinal epithelial cells leads to a different proinflammatory response in human and porcine host. Vet. Immunol. Immunopathol. 2014, 162, 14–23. [Google Scholar] [CrossRef]
- Ikejiofor, O.K.; Tion, M.T.; Shima, F.K. Campylobacter: Virulence factors and pathogenesis. In Recent Advances in Bacterial Biofilm Studies—Formation, Regulation, and Eradication in Human Infections; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Kovač, J.; Čadež, N.; Stessl, B.; Stingl, K.; Gruntar, I.; Ocepek, M.; Trkov, M.; Wagner, M.; Smole Možina, S. High genetic similarity of ciprofloxacin-resistant Campylobacter jejuni in central Europe. Front. Microbiol. 2015, 6, 1169. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Tang, M.; Pu, J.; Zhang, J.; Lu, J.; Zhang, Y.; Gao, Y. Aminoglycoside resistance and possible mechanisms in Campylobacter spp. isolated from chicken and swine in Jiangsu, China. Front. Microbiol. 2021, 12, 716185. [Google Scholar] [CrossRef]
- Nunes, A.; Oleastro, M.; Alves, F.; Liassine, N.; Lowe, D.M.; Benejat, L.; Ducounau, A.; Jehanne, Q.; Borges, V.; Gomes, J.P.; et al. Recurrent Campylobacter jejuni infections with in vivo selection of resistance to macrolides and carbapenems: Molecular characterization of resistance determinants. Microbiol. Spectr. 2023, 11, e0107023. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic use in livestock and residues in food—A public health threat: A review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Campylobacteriosis Annual Epidemiological Report for 2022. 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/campylobacteriosis-annual-epidemiological-report-2022 (accessed on 24 October 2024).
- EFSA; European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022. EFSA J. 2024, 22, e8583. [Google Scholar] [CrossRef]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.-E.; Rolain, J.M.; Raoult, D. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009, 49, 543–551. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing Disk Diffusion Test Methodology. 2024. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology (accessed on 15 March 2024).
- EUCAST. European Committee on Antimicrobial Susceptibility Testing, Clinical Breakpoints—Breakpoints and Guidance. Version 14.0. 2024. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 15 March 2024).
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 20 July 2024).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Raney, B.; Paten, B.; Pham, S. Ragout—A reference-assisted assembly tool for bacterial genomes. Bioinformatics 2014, 30, i302–i309. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Zdobnov, E.M. BUSCO: Assessing genomic data quality and beyond. Curr. Protoc. 2021, 1, e323. [Google Scholar] [CrossRef]
- Darzentas, N. Circoletto: Visualizing sequence similarity with Circos. Bioinformatics 2010, 26, 2620–2621. [Google Scholar] [CrossRef]
- Carver, T.; Thomson, N.; Bleasby, A.; Berriman, M.; Parkhill, J. DNAPlotter: Circular and linear interactive genome visualization. Bioinformatics 2009, 25, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Snippy: Fast Bacterial Variant Calling from NGS Reads. 2015. Available online: https://github.com/tseemann/snippy (accessed on 20 July 2024).
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; On, S.L.W.; Wang, G.; Fontanoz, S.; Lastovica, A.J.; Mandrell, R.E. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. J. Clin. Microbiol. 2005, 43, 2315–2329. [Google Scholar] [CrossRef]
- Cody, A.J.; Bray, J.E.; Jolley, K.A.; McCarthy, N.D.; Maiden, M.C.J. Core genome multilocus sequence typing scheme for stable, comparative analyses of Campylobacter jejuni and C. coli human disease isolates. J. Clin. Microbiol. 2017, 55, 2086–2097. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Seemann, T. Abricate. Github. 2014. Available online: https://github.com/tseemann/abricate (accessed on 20 July 2024).
- Ortega-Sanz, I.; Barbero-Aparicio, J.A.; Canepa-Oneto, A.; Rovira, J.; Melero, B. CamPype: An open-source workflow for automated bacterial whole-genome sequencing analysis focused on Campylobacter. BMC Bioinform. 2023, 24, 291. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the reference gene catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 2021, 11, 12728. [Google Scholar] [CrossRef]
- Florensa, A.F.; Kaas, R.S.; Clausen, P.; Aytan-Aktug, D.; Aarestrup, F.M. ResFinder—An open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from genotypes. Microb. Genom. 2022, 8, 000748. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, J.M. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 2014, 58, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Doster, E.; Lakin, S.M.; Dean, C.J.; Wolfe, C.; Young, J.G.; Boucher, C.; Belk, K.E.; Noyes, N.R.; Morley, P.S. MEGARes 2.0: A database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data. Nucleic Acids Res. 2019, 48, D561–D569. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Zarske, M.; Luu, H.Q.; Deneke, C.; Knüver, M.-T.; Thieck, M.; Hoang, H.T.T.; Bretschneider, N.; Pham, N.T.; Huber, I.; Stingl, K. Identification of knowledge gaps in whole-genome sequence analysis of multi-resistant thermotolerant Campylobacter spp. BMC Genom. 2024, 25, 156. [Google Scholar] [CrossRef]
- Maiden, M.C.J.; Bygraves, J.A.; Feil, E.; Morelli, G.; Russell, J.E.; Urwin, R.; Zhang, Q.; Zhou, J.; Zurth, K.; Caugant, D.A.; et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 1998, 95, 3140–3145. [Google Scholar] [CrossRef]
- Tedersoo, T.; Roasto, M.; Mäesaar, M.; Kisand, V.; Ivanova, M.; Meremäe, K. The prevalence, counts, and MLST genotypes of Campylobacter in poultry meat and genomic comparison with clinical isolates. Poult. Sci. 2022, 101, 101703. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, Q.; Tang, H.; Wang, Z.; Yin, Y.; Ren, F.; Kong, L.; Jiao, X.; Huang, J. Characterization and prevalence of Campylobacter spp. from broiler chicken rearing period to the slaughtering process in Eastern China. Front. Vet. Sci. 2020, 7, 227. [Google Scholar] [CrossRef]
- Elhadidy, M.; Miller, W.G.; Arguello, H.; Álvarez-Ordóñez, A.; Dierick, K.; Botteldoorn, N. Molecular epidemiology and antimicrobial resistance mechanisms of Campylobacter coli from diarrhoeal patients and broiler carcasses in Belgium. Transbound. Emerg. Dis. 2019, 66, 463–475. [Google Scholar] [CrossRef]
- Phu, D.H.; Wongtawan, T.; Wintachai, P.; Nhung, N.T.; Yen, N.T.P.; Carrique-Mas, J.; Turni, C.; Omaleki, L.; Blackall, P.J.; Thomrongsuwannakij, T. Molecular characterization of Campylobacter spp. isolates obtained from commercial broilers and native chickens in Southern Thailand using whole genome sequencing. Poult. Sci. 2024, 103, 103485. [Google Scholar] [CrossRef] [PubMed]
- Audu, B.J.; Norval, S.; Bruno, L.; Meenakshi, R.; Marion, M.; Forbes, K.J. Genomic diversity and antimicrobial resistance of Campylobacter spp. from humans and livestock in Nigeria. J. Biomed. Sci. 2022, 29, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Chen, X.; Li, Y.; Guo, J.; Gao, J.; Jiao, X.; Tang, Y.; Huang, J. Prevalence and genetic characterization of Campylobacter from clinical poultry cases in China. Microbiol. Spectr. 2023, 11, e00797-23. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, F.; Liu, T.; Ouyang, C.; Wang, X.; Li, M.; Huang, Z.; Huang, J.; Wang, L.; Wang, X. Identification of a multidrug resistance genomic island harboring a nonfunctional optrA gene in Campylobacter coli of chicken origin. Vet. Microbiol. 2024, 293, 110083. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, J.; Li, F.; Yang, B.; Ren, X.; Wang, Y.; Hu, Y.; Dong, Y.; Wang, W.; Zhang, J.; et al. Antimicrobial resistance and genomic characterization of Campylobacter jejuni and Campylobacter coli isolated from retail chickens in Beijing, China. Microorganisms 2024, 12, 1601. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Liu, Y.; Jiang, J.; Shen, Z.; Chen, Q.; Ma, X. Multilocus sequence types and antimicrobial resistance of Campylobacter jejuni and C. coli isolates of human patients from Beijing, China, 2017–2018. Front. Microbiol. 2020, 11, 554784. [Google Scholar] [CrossRef]
- Mossong, J.; Mughini-Gras, L.; Penny, C.; Devaux, A.; Olinger, C.; Losch, S.; Cauchie, H.M.; van Pelt, W.; Ragimbeau, C. Human Campylobacteriosis in Luxembourg, 2010–2013: A case-control study combined with multilocus sequence typing for source attribution and risk factor analysis. Sci. Rep. 2016, 6, 20939. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Liu, Y.; Cui, Q.; Qin, X.; Niu, Y.; Wang, C.; Wang, T.; Chen, Q.; Ding, S.; et al. Genomic insights into the increased occurrence of Campylobacteriosis caused by antimicrobial-resistant Campylobacter coli. mBio 2022, 13, e02835-22. [Google Scholar] [CrossRef]
- Gao, F.; Tu, L.; Chen, M.; Chen, H.; Zhang, X.; Zhuang, Y.; Luo, J.; Chen, M. Erythromycin resistance of clinical Campylobacter jejuni and Campylobacter coli in Shanghai, China. Front. Microbiol. 2023, 14, 1145581. [Google Scholar] [CrossRef]
- Kim, S.Y.; An, D.; Jeong, H.; Kim, J. Antimicrobial susceptibility patterns and genetic diversity of Campylobacter spp. isolates from patients with diarrhea in South Korea. Microorganisms 2024, 12, 94. [Google Scholar] [CrossRef]
- Jehanne, Q.; Bénéjat, L.; Ducournau, A.; Aptel, J.; Pivard, M.; Gillet, L.; Jauvain, M.; Lehours, P. Increasing rates of erm(B) and erm(N) in human Campylobacter coli and Campylobacter jejuni erythromycin-resistant isolates between 2018 and 2023 in France. Antimicrob. Agents Chemother. 2025, 69, e01668-24. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.K.; Dallas, J.F.; Wilson, D.J.; Strachan, N.J.C.; McCarthy, N.D.; Jolley, K.A.; Colles, F.M.; Rotariu, O.; Ogden, I.D.; Forbes, K.J.; et al. Evolution of an agriculture-associated disease causing Campylobacter coli clade: Evidence from national surveillance data in Scotland. PLoS ONE 2010, 5, e15708. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Sakata, J.; Nakamura, H.; Yamamoto, S.; Murakami, S. Phylogenetic diversity and antimicrobial resistance of Campylobacter coli from humans and animals in Japan. Microbes Environ. 2019, 34, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Sharafutdinov, I.; Harrer, A.; Soltan Esmaeili, D.; Linz, B.; Backert, S. Campylobacter virulence factors and molecular host–pathogen interactions. Curr. Top. Microbiol. Immunol. 2021, 431, 169–202. [Google Scholar] [CrossRef]
- Guerry, P. Campylobacter flagella: Not just for motility. Trends Microbiol. 2007, 15, 456–461. [Google Scholar] [CrossRef]
- Kreling, V.; Falcone, F.H.; Kehrenberg, C.; Hensel, A. Campylobacter sp.: Pathogenicity factors and prevention methods—New molecular targets for innovative antivirulence drugs? Appl. Microbiol. Biotechnol. 2020, 104, 10409–10436. [Google Scholar] [CrossRef]
- Bolton, D.J. Campylobacter virulence and survival factors. Food Microbiol. 2015, 48, 99–108. [Google Scholar] [CrossRef]
- Konkel, M.E.; Talukdar, P.K.; Negretti, N.M.; Klappenbach, C.M. Taking control: Campylobacter jejuni binding to fibronectin sets the stage for cellular adherence and invasion. Front. Microbiol. 2020, 11, 564. [Google Scholar] [CrossRef]
- Pina-Mimbela, R.; Madrid, J.A.; Kumar, A.; Torrelles, J.B.; Rajashekara, G. Polyphosphate kinases modulate Campylobacter jejuni outer membrane constituents and alter its capacity to invade and survive in intestinal epithelial cells in vitro. Emerg. Microbes Infect. 2015, 4, e77. [Google Scholar] [CrossRef]
- Radomska, K.A.; Wösten, M.M.S.M.; Ordoñez, S.R.; Wagenaar, J.A.; van Putten, J.P.M. Importance of Campylobacter jejuni FliS and FliW in flagella biogenesis and flagellin secretion. Front. Microbiol. 2017, 8, 1060. [Google Scholar] [CrossRef]
- Dias, T.S.; Panzenhagen, P.; Figueira, A.A.; Costa, G.A.; Rossi, D.A.; de Melo, R.T.; Pereira, V.L.A.; de Aquino, M.H.C. Genomic characterisation of Campylobacter jejuni Cj26: A high-level ciprofloxacin/erythromycin-resistant strain isolated from a poultry carcass in southern Brazil. J. Glob. Antimicrob. Resist. 2023, 34, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Karki, A.B.; Khatri, B.; Fakhr, M.K. Transcriptome analysis of Campylobacter jejuni and Campylobacter coli during cold stress. Pathogens 2023, 12, 960. [Google Scholar] [CrossRef] [PubMed]
- Admasie, A.; Wei, X.; Johnson, B.; Burns, L.; Pawar, P.; Aurand-Cravens, A.; Voloshchuk, O.; Dudley, E.G.; Sisay Tessema, T.; Zewdu, A.; et al. Genomic diversity of Campylobacter jejuni and Campylobacter coli isolated from the Ethiopian dairy supply chain. PLoS ONE 2024, 19, e0305581. [Google Scholar] [CrossRef] [PubMed]
- Marchant, J.; Wren, B.; Ketley, J. Exploiting genome sequence: Predictions for mechanisms of Campylobacter chemotaxis. Trends Microbiol. 2002, 10, 155–159. [Google Scholar] [CrossRef]
- Zautner, A.E.; Tareen, A.M.; Groß, U.; Lugert, R. Chemotaxis in Campylobacter jejuni. Eur. J. Microbiol. Immunol. 2012, 2, 24–31. [Google Scholar] [CrossRef]
- Wösten, M.M.S.M.; Wagenaar, J.A.; van Putten, J.P.M. The FlgS/FlgR Two-component signal transduction system regulates the fla Regulon in Campylobacter jejuni. J. Biol. Chem. 2004, 279, 16214–16222. [Google Scholar] [CrossRef]
- Salah Ud-Din, A.I.M.; Roujeinikova, A. Flagellin glycosylation with pseudaminic acid in Campylobacter and Helicobacter: Prospects for development of novel therapeutics. Cell Mol. Life Sci. 2018, 75, 1163–1178. [Google Scholar] [CrossRef]
- Guerry, P.; Doig, P.; Alm, R.A.; Burr, D.H.; Kinsella, N.; Trust, T.J. Identification and characterization of genes required for post-translational modification of Campylobacter coli VC167 flagellin. Mol. Microbiol. 1996, 19, 369–378. [Google Scholar] [CrossRef]
- Guerry, P.; Ewing, C.P.; Schirm, M.; Lorenzo, M.; Kelly, J.; Pattarini, D.; Majam, G.; Thibault, P.; Logan, S. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 2006, 60, 299–311. [Google Scholar] [CrossRef]
- Oldfield, N.J.; Moran, A.P.; Millar, L.A.; Prendergast, M.M.; Ketley, J.M. Characterization of the Campylobacter jejuni heptosyltransferase ii gene, waaf, provides genetic evidence that extracellular polysaccharide is lipid a core independent. J. Bacteriol. 2002, 184, 2100–2107. [Google Scholar] [CrossRef]
- Taylor, P.L.; Blakely, K.M.; de Leon, G.P.; Walker, J.R.; McArthur, F.; Evdokimova, E.; Zhang, K.; Valvano, M.A.; Wright, G.D.; Junop, M.S. Structure and function of sedoheptulose-7-phosphate isomerase, a critical enzyme for lipopolysaccharide biosynthesis and a target for antibiotic adjuvants. J. Biol. Chem. 2008, 283, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Klena, J.D.; Gray, S.A.; Konkel, M.E. Cloning, sequencing, and characterization of the lipopolysaccharide biosynthetic enzyme heptosyltransferase I gene (waaC) from Campylobacter jejuni and Campylobacter coli. Gene 1998, 222, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Karlyshev, A.V.; Champion, O.L.; Churcher, C.; Brisson, J.-R.; Jarrell, H.C.; Gilbert, M.; Brochu, D.; St Michael, F.; Li, J.; Wakarchuk, W.W.; et al. Analysis of Campylobacter jejuni capsular loci reveals multiple mechanisms for the generation of structural diversity and the ability to form complex heptoses. Mol. Microbiol. 2005, 55, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, N.; Mangan, J.A.; Laing, K.G.; Hinds, J.; Linton, D.; Al-Ghusein, H.; Barrell, B.G.; Parkhill, J.; Stoker, N.G.; Karlyshev, A.V.; et al. Whole genome comparison of Campylobacter jejuni human isolates using a low-cost microarray reveals extensive genetic diversity. Genome Res. 2001, 11, 1706–1715. [Google Scholar] [CrossRef]
- Parker, C.T.; Horn, S.T.; Gilbert, M.; Miller, W.G.; Woodward, D.L.; Mandrell, R.E. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J. Clin. Microbiol. 2005, 43, 2771–2781. [Google Scholar] [CrossRef]
- Poudel, S.; Li, T.; Chen, S.; Zhang, X.; Cheng, W.H.; Sukumaran, A.T.; Kiess, A.S.; Zhang, L. Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter isolated from broilers and broiler meat raised without antibiotics. Microbiol. Spectr. 2022, 10, e0025122. [Google Scholar] [CrossRef]
- Matsunami, H.; Barker, C.S.; Yoon, Y.H.; Wolf, M.; Samatey, F.A. Complete structure of the bacterial flagellar hook reveals extensive set of stabilizing interactions. Nat. Commun. 2016, 7, 13425. [Google Scholar] [CrossRef]
- Reid, A.N.; Pandey, R.; Palyada, K.; Naikare, H.; Stintzi, A. Identification of Campylobacter jejuni genes involved in the response to acidic pH and stomach transit. Appl. Environ. Microbiol. 2008, 74, 1583–1597. [Google Scholar] [CrossRef]
- Inoue, T.; Barker, C.S.; Matsunami, H.; Aizawa, S.I.; Samatey, F.A. The FlaG regulator is involved in length control of the polar flagella of Campylobacter jejuni. Microbiology 2018, 164, 740–750. [Google Scholar] [CrossRef]
- Freitag, C.M.; Strijbis, K.; van Putten, J.P.M. Host cell binding of the flagellar tip protein of Campylobacter jejuni. Cell Microbiol. 2017, 19, e12714. [Google Scholar] [CrossRef]
- Goon, S.; Kelly, J.F.; Logan, S.M.; Ewing, C.P.; Guerry, P. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol. Microbiol. 2003, 50, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Song, W.S.; Park, M.; Ki, D.U.; Yoon, S. Structural analysis of the pseudaminic acid synthase PseI from Campylobacter jejuni. Biochem. Biophys. Res. Commun. 2022, 635, 252–258. [Google Scholar] [CrossRef] [PubMed]
- The National Antimicrobial Resistance Monitoring System. Global Resistome Data. 2025. Available online: https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/global-resistome-data (accessed on 20 April 2025).
- Li, W.; Atkinson, G.C.; Thakor, N.S.; Allas, U.; Lu, C.C.; Chan, K.Y.; Tenson, T.; Schulten, K.; Wilson, K.S.; Hauryliuk, V.; et al. Mechanism of tetracycline resistance by ribosomal protection protein Tet(O). Nat. Commun. 2013, 4, 1477. [Google Scholar] [CrossRef] [PubMed]
- Benites, C.; Anampa, D.; Torres, D.; Avalos, I.; Rojas, M.; Conte, C.; Lázaro, C. Prevalence, tetracycline resistance and tet(O) gene identification in pathogenic Campylobacter strains isolated from chickens in retail markets of Lima, Peru. Antibiotics 2022, 11, 1580. [Google Scholar] [CrossRef]
- Casagrande Proietti, P.; Guelfi, G.; Bellucci, S.; De Luca, S.; Di Gregorio, S.; Pieramati, C.; Franciosini, M.P. Beta-lactam resistance in Campylobacter coli and Campylobacter jejuni chicken isolates and the association between blaOXA-61 gene expression and the action of β-lactamase inhibitors. Vet. Microbiol. 2020, 241, 108553. [Google Scholar] [CrossRef]
- Gomes, C.N.; Campioni, F.; Barker, D.O.R.; Che, E.V.; Duque, S.S.; Taboada, E.N.; Falcão, J.P. Antimicrobial resistance genotypes and phenotypes of Campylobacter coli isolated from different sources over a 16-year period in Brazil. J. Glob. Antimicrob. Resist. 2023, 33, 109–113. [Google Scholar] [CrossRef]
- Marotta, F.; Janowicz, A.; Romantini, R.; Di Marcantonio, L.; Di Timoteo, F.; Romualdi, T.; Zilli, K.; Barco, L.; D’Incau, M.; Mangone, I.; et al. Genomic and antimicrobial surveillance of Campylobacter population in Italian poultry. Foods 2023, 12, 2919. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Zhang, Q.; Shen, J. Antimicrobial resistance in Campylobacter spp. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Cobo-Díaz, J.F.; González del Río, P.; Álvarez-Ordóñez, A. Whole Resistome Analysis in Campylobacter jejuni and C. coli Genomes Available in Public Repositories. Front. Microbiol. 2021, 12, 662144. [Google Scholar] [CrossRef]
- Ghatak, S.; Milton, A.A.P.; Das, S.; Momin, K.M.; Srinivas, K.; Pyngrope, D.A.; Priya, G.B. Campylobacter coli of porcine origin exhibits an open pan-genome within a single clonal complex: Insights from comparative genomic analysis. Front. Cell. Infect. Microbiol. 2024, 14, 1449856. [Google Scholar] [CrossRef]
- Hormeño, L.; Palomo, G.; Borge, C.; Vadillo, S.; Píriz, S.; Domínguez, L.; Campos, M.J.; Quesada, A. ant(6)-I genes encoding aminoglycoside o-nucleotidyltransferases are widely spread among streptomycin resistant strains of Campylobacter jejuni and Campylobacter coli. Front. Microbiol. 2018, 9, 2515. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Shen, Z.; Wang, Y.; Deng FLiu, D.; Naren, G.; Dai, L.; Su, C.; Wang, B.; Wang, S.; Wu, C.; et al. Emergence of a potent multidrug efflux pump variant that enhances Campylobacter resistance to multiple antibiotics. mBio 2016, 7, e01543-16. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, D.A.; Korolik, V. Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol. Lett. 2007, 277, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Portes, A.B.; Panzenhagen, P.; dos Santos, A.M.P.; de Jesus, A.C.S.; Ochioni, A.C.; Duque, S.S.; Aburjaile, F.F.; Brenig, B.; Azevedo, V.; Conte Junior, C.A. Draft genomes of multidrug-resistant Campylobacter jejuni and Campylobacter coli strains from Brazil representing novel sequence types. Microbiol. Resour. Announc. 2024, 13, e00524. [Google Scholar] [CrossRef]
- Pinto-Alphandary, H.; Mabilat, C.; Courvalin, P. Emergence of aminoglycoside resistance genes aadA and aadE in the genus Campylobacter. Antimicrob. Agents Chemother. 1990, 34, 1294–1296. [Google Scholar] [CrossRef]
- Lin, J.; Michel, L.O.; Zhang, Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 2002, 46, 2124–2131. [Google Scholar] [CrossRef]
- Klenotic, P.A.; Moseng, M.A.; Morgan, C.E.; Yu, E.W. Structural and functional diversity of resistance-nodulation-cell division transporters. Chem. Rev. 2021, 121, 5378–5416. [Google Scholar] [CrossRef]
- Bravo, V.; Katz, A.; Porte, L.; Weitzel, T.; Varela, C.; Gonzalez-Escalona, N.; Blondel, C.J. Genomic analysis of the diversity, antimicrobial resistance and virulence potential of clinical Campylobacter jejuni and Campylobacter coli strains from Chile. PLoS Neglected Trop. Dis. 2021, 15, e0009207. [Google Scholar] [CrossRef]
- Grinnage-Pulley, T.; Zhang, Q. Genetic basis and functional consequences of differential expression of the CmeABC efflux pump in Campylobacter jejuni Isolates. PLoS ONE 2015, 10, e0131534. [Google Scholar] [CrossRef]
- Lin, J.; Akiba, M.; Sahin, O.; Zhang, Q. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob. Agents Chemother. 2005, 49, 1067–1075. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, C.; Ye, Y.; Liu, Y.; Wang, A.; Li, Y.; Zhou, X.; Pan, H.; Zhang, J.; Xu, X. Molecular identification of multidrug-resistant Campylobacter species from diarrheal patients and poultry meat in Shanghai, China. Front. Microbiol. 2018, 9, 1642. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).