Zika Virus Infection Modulates Extracellular Vesicle Biogenesis and Morphology in Human Umbilical Cord Endothelial Cells: A Proteomic and Microscopic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Human Umbilical Cord Vein Endothelial Cells (HUVECs)
2.2. ZIKV Supply and Titer
2.3. Determination of Factor VIII and ZIKV Envelope Protein by Immunofluorescence
2.4. Isolation of Exosomes of HUVECs
2.5. Characterization of HUVECs and Exosomes by Flow Cytometry
2.6. Proteome Analysis of ZIKV-Infected HUVECs
2.6.1. Protein Extraction
2.6.2. Isoelectrofocusing
2.6.3. 2D Electrophoresis
2.6.4. In Silico Analysis
2.7. Analysis of Exosomal Proteins by Western Blot
2.8. Detection of ZIKV Viral Elements in EVs from Infected HUVECs
Evaluation of the Infectivity of EVs from HUVECs Infected with Zika Virus
2.9. EV Analysis by Cryo-TEM
2.10. EV Analysis by NTA
Statistical Analysis
3. Results
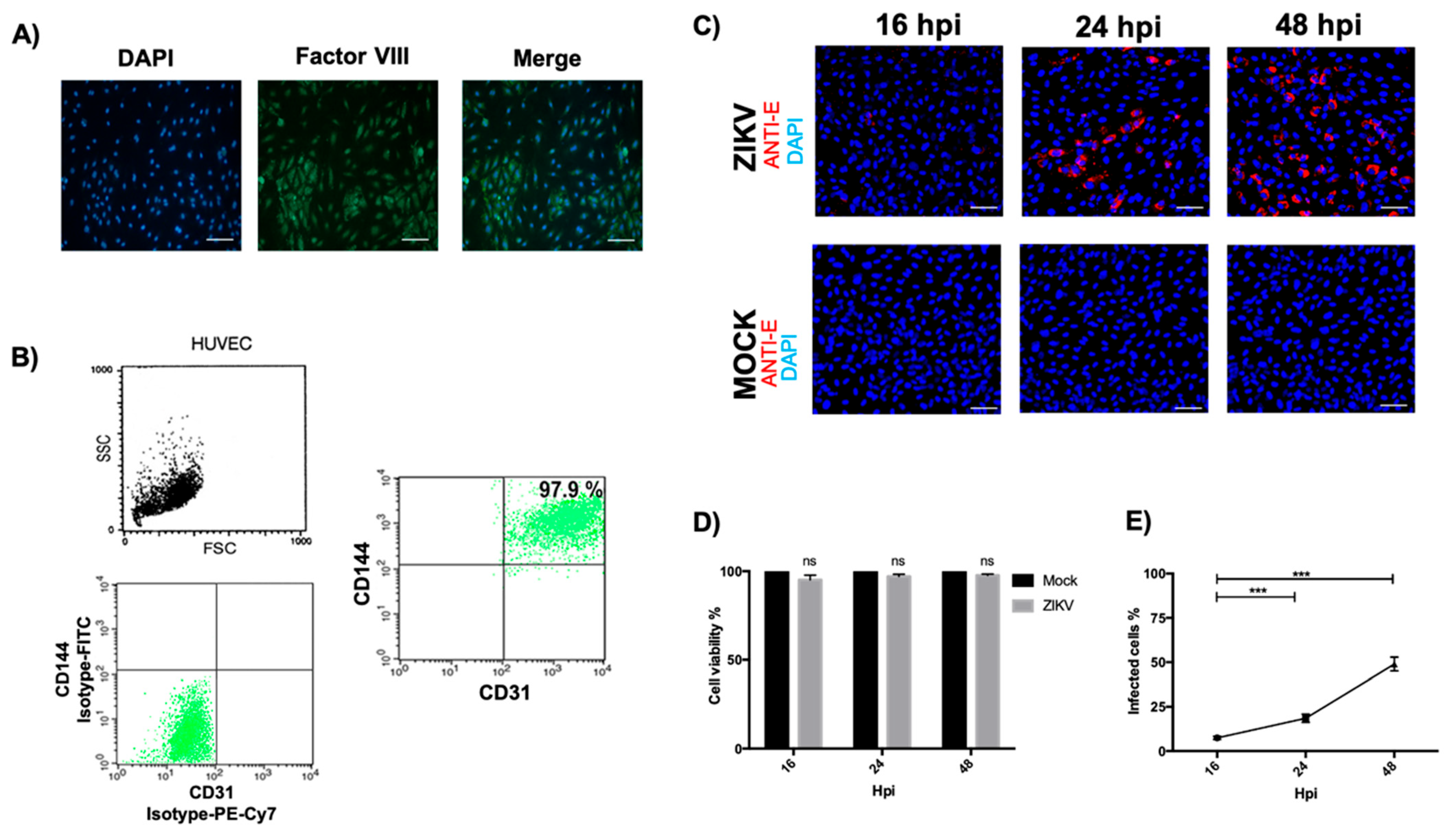
3.1. Optimal HUVEC Isolation and Evaluation of Permissibility to ZIKV Infection
3.2. ZIKV Infection Changes the Proteome of HUVECs
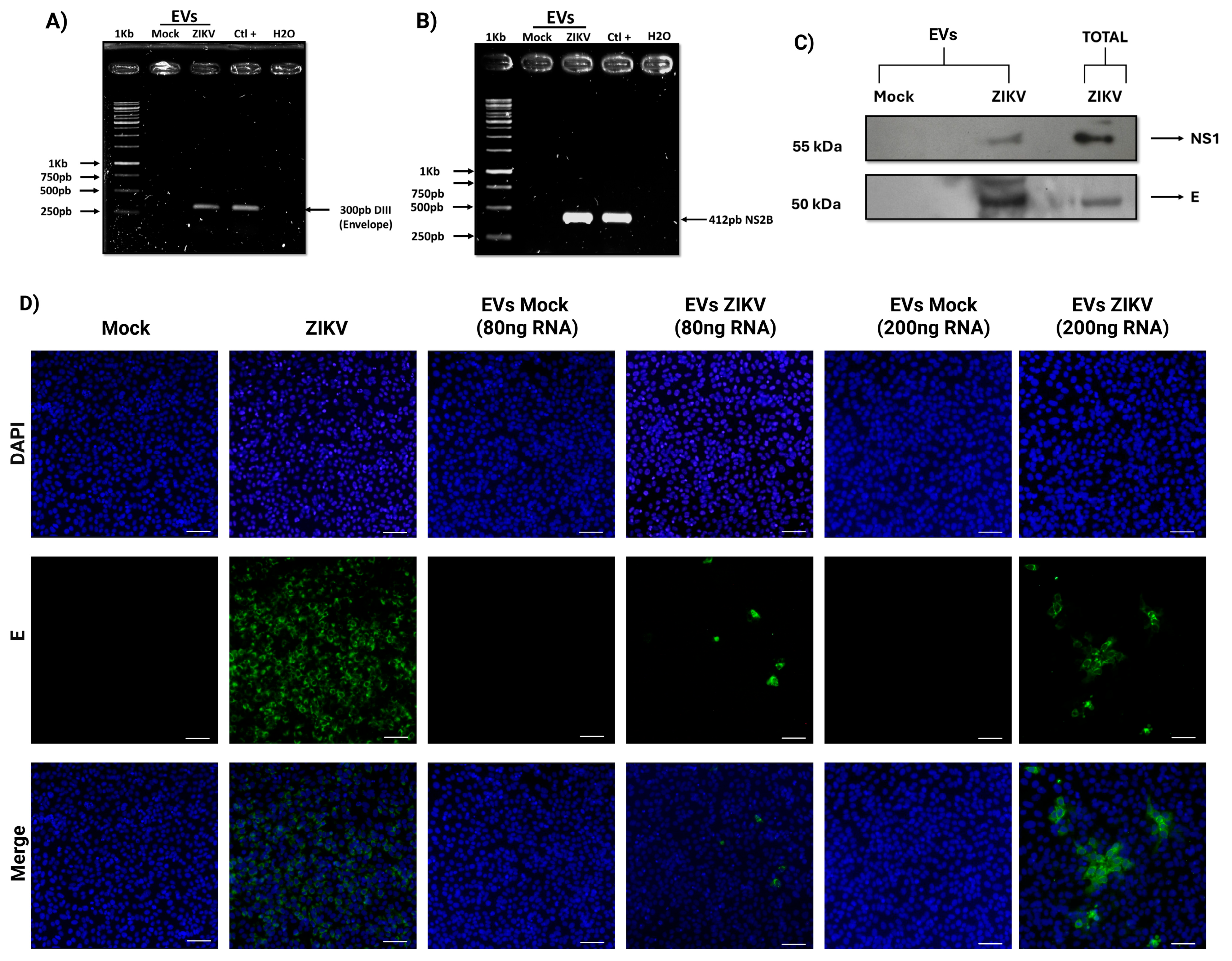
3.3. EVs Isolated from ZIKV-Infected HUVECs Act as Vehicles for Transporting Infectious Viral Elements
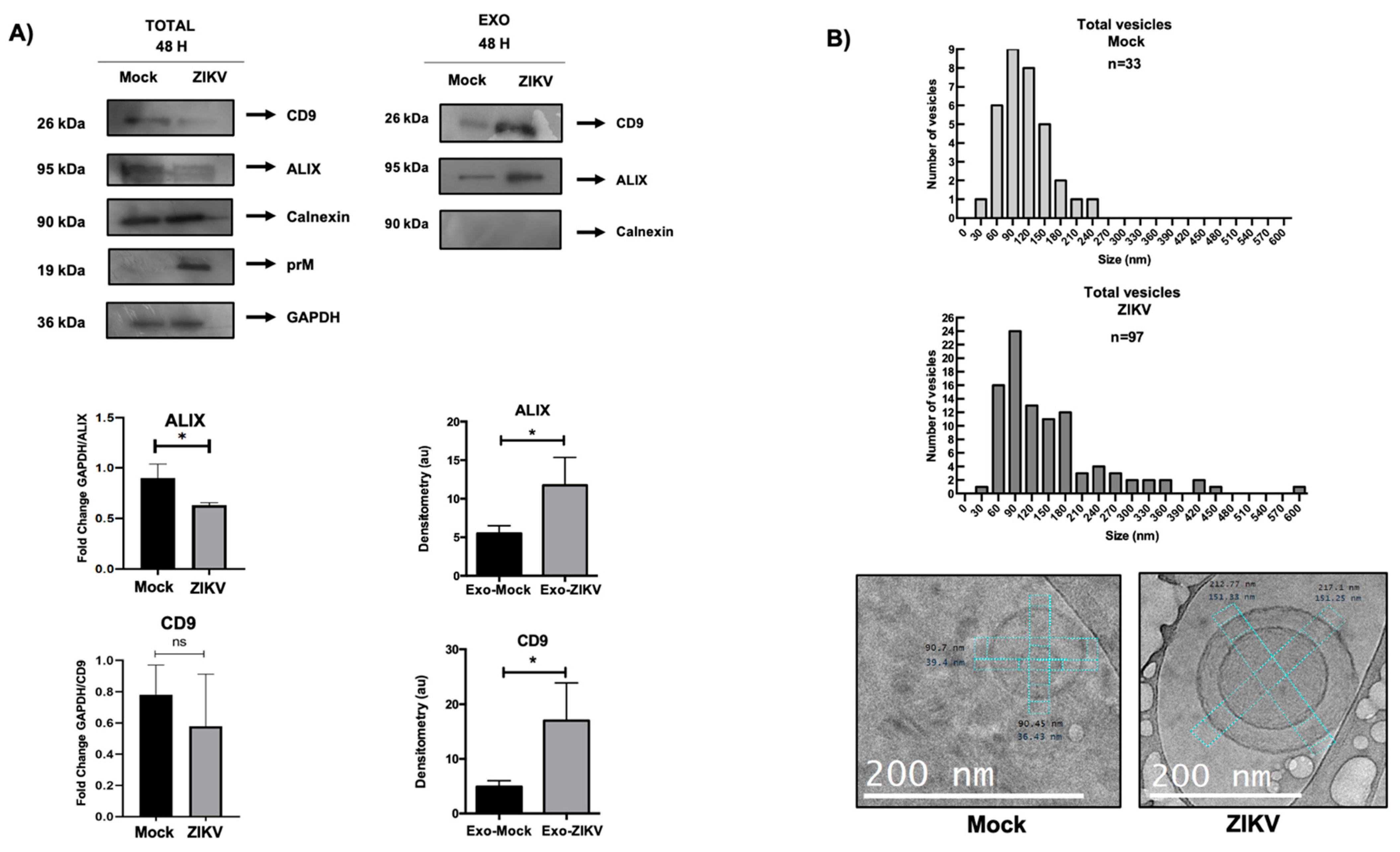
3.4. Proteins Involved in Exosome Biogenesis Are Altered During Infection of ZIKV-Infected HUVECs
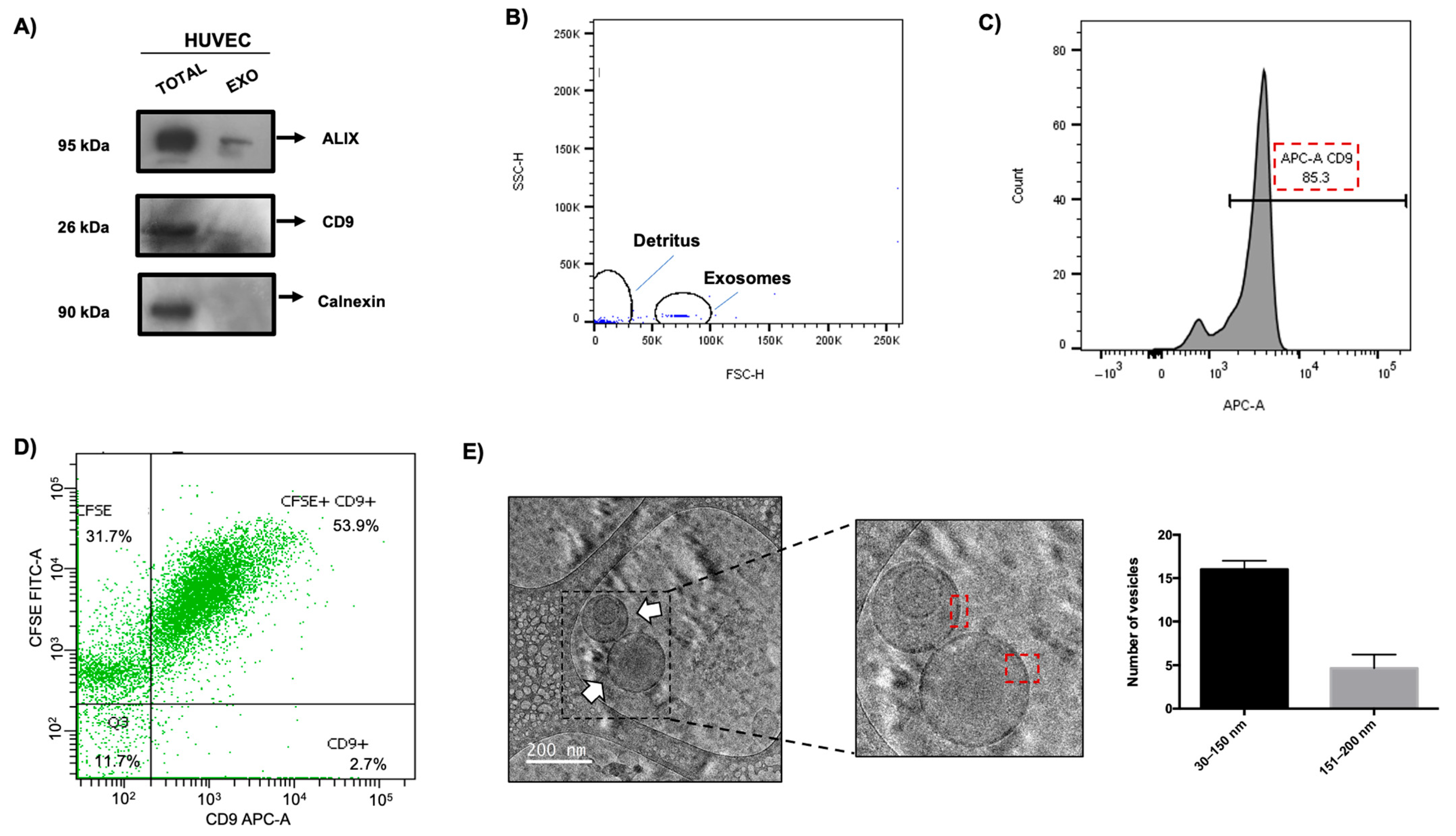
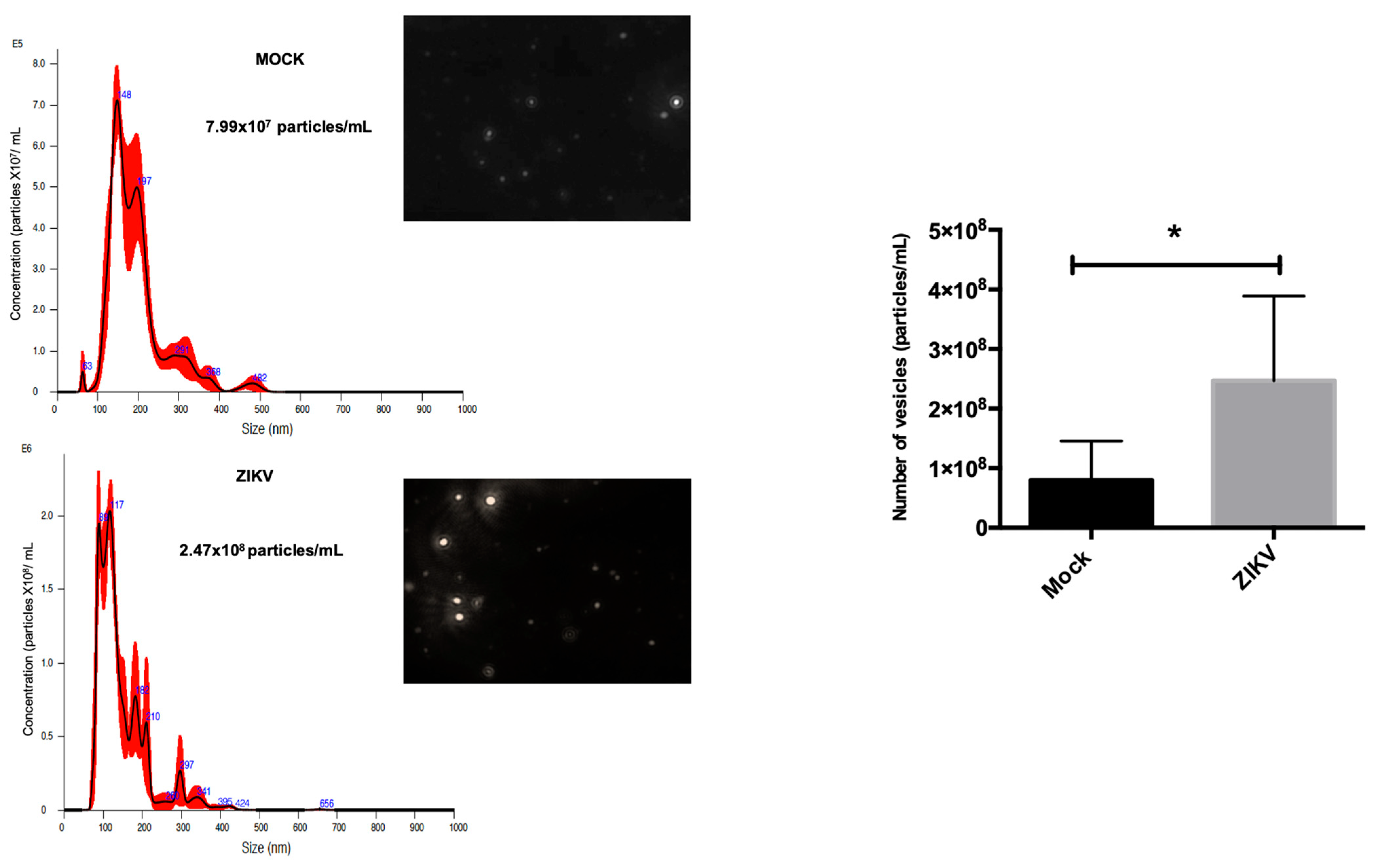
3.5. Isolation and Characterization of EVs from HUVECs
3.6. HUVEC EVs During ZIKV Infection Show Differential Regulation in Exosomal Proteins and Quantity and Size of These Vesicles
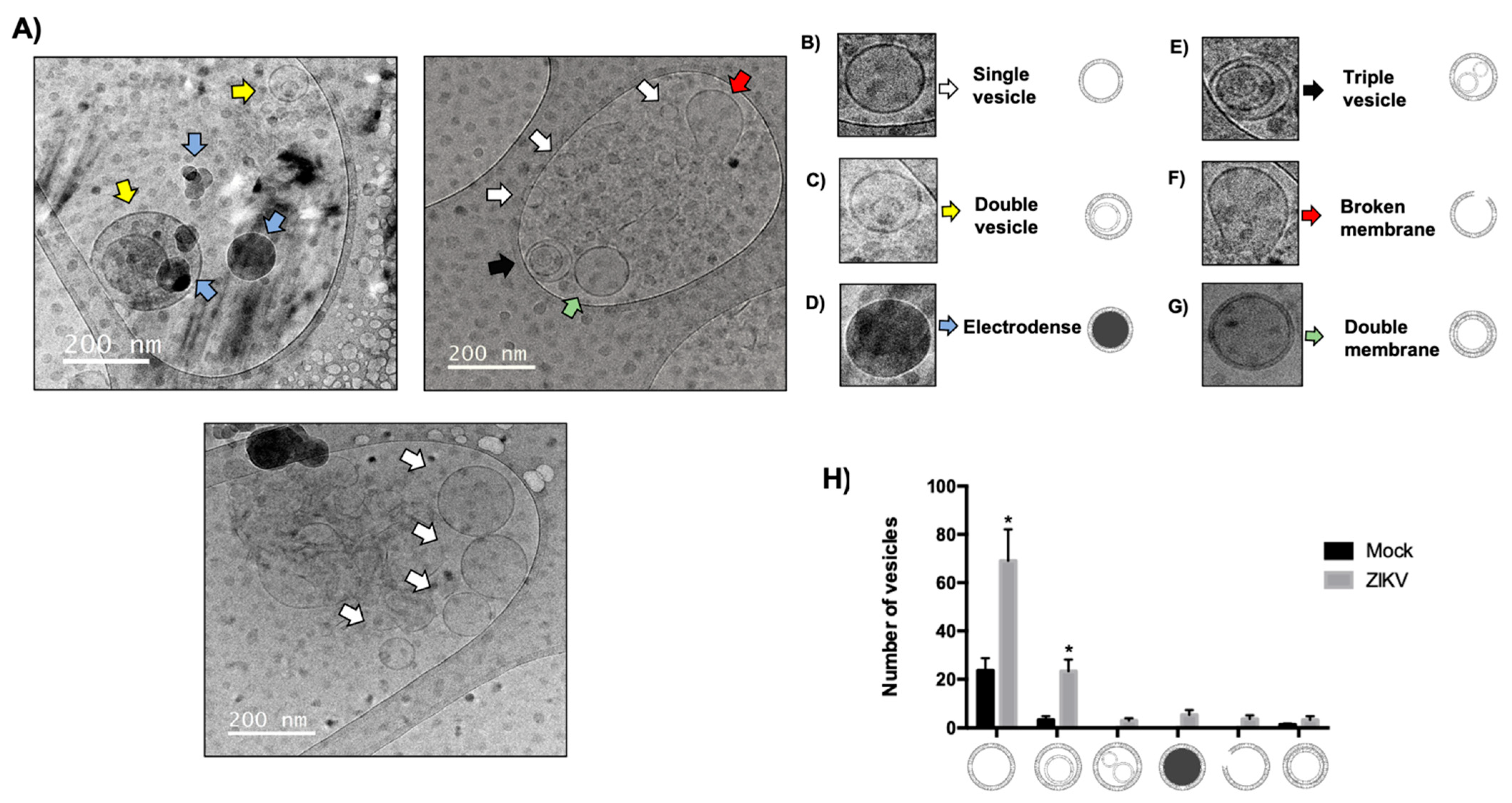
3.7. Determination and Alteration of Morphological Populations of EVs During ZIKV Infection in HUVECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierson, T.C.; Diamond, M.S. The emergence of Zika virus and its new clinical syndromes. Nature 2018, 560, 573–581. [Google Scholar] [CrossRef]
- Besnard, M.; Lastere, S.; Teissier, A.; Cao–Lormeau, V.; Musso, D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014, 19, 20751. [Google Scholar] [CrossRef]
- Chang, C.; Ortiz, K.; Ansari, A.; Gershwin, M.E. The Zika outbreak of the 21st century. J. Autoimmun. 2016, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Lennemann, N.; Ouyang, Y.; Bramley, J.; Morosky, S.; Marques, E.; Coyne, C. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef]
- Mlakar, M.; Korva, M.; Tul, N.; Popovic, M.; Poljsak, M.; Mraz, J.; Kolenc, M.; Resman, K.; Vesnaver, T.; Fabjan, V.; et al. Zika virus associated with microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Quicke, K.; Bowen, J.; Johnson, E.; McDonald, C.; Ma, H.; O’neal, J.; Rajakumar, A.; Wrammert, J.; Rimawi, B.; Pulendran, B.; et al. Zika virus infects human plancental macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Petitt, M.; Puerta–Guardado, H.; Micchlmayr, D.; Wang, C.; Fang–Hoover, J.; Harris, E.; Pereira, L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef]
- Peng, H.; Liu, B.; Doueu, T.; He, Y.; Wang, S.; Tang, H.; Ren, H.; Zhao, P.; Qi, Z.; Qin, Z. Zika virus induces autophagy in human umbilical vein endothelial cells. Virus 2018, 10, 259. [Google Scholar] [CrossRef]
- Puerta–Guardado, H.; Glasner, D.; Espinosa, D.; Biering, S.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, R.; Harris, E. Flavivirus NS1 triggers tissue–specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep. 2019, 26, 1598–1613. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Gu, L.; Sims, B.; Matthews, Q.L. Exosome Biogenesis and Biological Function in Response to Viral Infections. Open Virol. J. 2018, 12, 134–148. [Google Scholar] [CrossRef]
- Chahar, H.S.; Corsello, T.; Kudlicki, A.S.; Komaravelli, N.; Casola, A. Respiratory Syncytial Virus Infection Changes Cargo Composition of Exosome Released from Airway Epithelial Cells. Sci. Rep. 2018, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The role of extracellular vesicles in COVID–19 virus infection. Infect. Genet. Evol. 2020, 85, 104422. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Huang, M.B.; Campbell, P.E.; Roth, W.W.; Campbell, T.; Khan, M.; Newman, G.; Villinger, F.; Powell, M.D.; Bond, V.C. Genetic characterization of HIV type 1 Nef–induced vesicle secretion. AIDS Res. Hum. Retroviruses 2010, 26, 173–192. [Google Scholar] [CrossRef]
- Arenaccio, C.; Chiozzini, C.; Columba–Cabezas, S.; Manfredi, F.; Affabris, E.; Baur, A.; Federico, M. Exosomes from human immunodeficiency virus type 1 (HIV–1)–infected cells license quiescent CD4+ T lymphocytes to replicate HIV–1 through a Nef– and ADAM17–dependent mechanism. J. Virol. 2014, 88, 11529–11539. [Google Scholar] [CrossRef]
- Vora, A.; Zhou, W.; Londono–Renteria, B.; Woodson, M.; Sherman, M.B.; Colpitts, T.M.; Neelakanta, G.; Sultana, H. Arthropod EVs mediate dengue virus transmission through interaction with a tetraspanin domain containing glycoprotein Tsp29Fb. Proc. Natl. Acad. Sci. USA 2018, 115, E6604–E6613. [Google Scholar] [CrossRef]
- Martínez–Rojas, P.P.; Quiroz–García, E.; Monroy–Martínez, V.; Agredano–Moreno, L.T.; Jiménez–García, L.F.; Ruiz–Ordaz, B.H. Participation of Extracellular Vesicles from Zika–Virus–Infected Mosquito Cells in the Modification of Naïve Cells’ Behavior by Mediating Cell–to–Cell Transmission of Viral Elements. Cells 2020, 9, 123. [Google Scholar] [CrossRef]
- Wang, J.; Wu, F.; Liu, C.; Dai, W.; Teng, Y.; Su, W.; Kong, W.; Gao, F.; Cai, L.; Hou, A.; et al. Exosomes Released from Rabies Virus–Infected Cells May be Involved in the Infection Process. Virol. Sin. 2019, 34, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Reyes–Ruiz, J.M.; Osuna–Ramos, J.F.; De Jesús–González, L.A.; Hurtado–Monzón, A.M.; Farfan–Morales, C.N.; Cervantes–Salazar, M.; Bolaños, J.; Cigarroa–Mayorga, O.E.; Martín–Martínez, E.S.; Medina, F.; et al. Isolation and characterization of exosomes released from mosquito cells infected with dengue virus. Virus Res. 2019, 266, 1–14. [Google Scholar] [CrossRef]
- Zhou, W.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerg. Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J. Diversity of extracellular vesicles in human ejaculates revealed by cryo–electron microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef]
- Zonneveld, M.I.; Brisson, A.R.; van Herwijnen, M.J.; Tan, S.; van de Lest, C.H.; Redegeld, F.A.; Garssen, J.; Wauben, M.H.; Nolte–’t Hoen, E.N. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell. Vesicles 2014, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Zabeo, D.; Cvjetkovic, A.; Lässer, C.; Schorb, M.; Lötvall, J.; Höög, J.L. Exosomes purified from a single cell type have diverse morphology. J. Extracell Vesicles 2017, 6, 1329476. [Google Scholar] [CrossRef] [PubMed]
- Issman, L.; Brenner, B.; Talmon, Y.; Aharon, A. Cryogenic transmission electron microscopy nanostructural study of shed microparticles. PLoS ONE 2013, 8, e83680. [Google Scholar] [CrossRef]
- Coleman, B.M.; Hanssen, E.; Lawson, V.A.; Hill, A.F. Prion–infected cells regulate the release of exosomes with distinct ultrastructural features. FASEB J. 2012, 26, 4160–4173. [Google Scholar] [CrossRef]
- Chumchanchira, C.; Ramphan, S.; Paemanee, A.; Roytrakul, S.; Lithanatudom, P.; Smith, D.R. A 2D–proteomic analysis identifies proteins differentially regulated by two different dengue virus serotypes. Sci. Rep. 2024, 14, 8287. [Google Scholar] [CrossRef] [PubMed]
- Abere, B.; Wikan, N.; Ubol, S.; Auewarakul, P.; Paemanee, A.; Kittisenachai, S.; Roytrakul, S.; Smith, D.R. Proteomic analysis of chikungunya virus infected microgial cells. PLoS ONE 2012, 7, e34800. [Google Scholar] [CrossRef]
- Cervantes, M.A.V.; Martinez, J.A.V.; Garcia, L.D.G.; Ortega, O.L.; Romero, H.A.; Estrada, A.M.; Castillo, M.M.; Pliego, A.F.; Reyes, G.L.; Repetto, A.C.H.; et al. Zika virus infection induces expression of NRF2 and antioxidant systems in trophoblast cells. Virus Genes 2023, 59, 781–785. [Google Scholar] [CrossRef]
- Zhang, Z.; Rong, L.; Li, Y.P. Flaviviridae Viruses and Oxidative Stress: Implications for Viral Pathogenesis. Oxid. Med. Cell Longev. 2019, 2019, 1409582. [Google Scholar] [CrossRef]
- Almeida, L.T.; Ferraz, A.C.; da Silva Caetano, C.C.; da Silva Menegatto, M.B.; Dos Santos Pereira Andrade, A.C.; Lima, R.L.S.; Camini, F.C.; Pereira, S.H.; da Silva Pereira, K.Y.; de Mello Silva, B.; et al. Zika virus induces oxidative stress and decreases antioxidant enzyme activities in vitro and in vivo. Virus Res. 2020, 286, 198084. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin–Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular cesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 3, e12404. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Hong, C.S.; Donnenberg, V.S.; Donnenberg, A.D.; Whiteside, T.L. Evaluation of Exosome Proteins by on-Bead Flow Cytometry. Cytom. A 2021, 99, 372–381. [Google Scholar] [CrossRef]
- Fortunato, D.; Mladenović, D.; Criscuoli, M.; Loria, F.; Veiman, K.L.; Zocco, D.; Koort, K.; Zarovni, N. Opportunities and Pitfalls of Fluorescent Labeling Methodologies for Extracellular Vesicle Profiling on High-Resolution Single-Particle Platforms. Int. J. Mol. Sci. 2021, 22, 10510. [Google Scholar] [CrossRef]
- Neyroud, A.S.; Chiechio, R.M.; Moulin, G.; Ducarre, S.; Heichette, C.; Dupont, A.; Budzynski, M.; Even-Hernandez, P.; Faro, M.J.L.; Yefimova, M.; et al. Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification. Int. J. Mol. Sci. 2022, 23, 11676. [Google Scholar] [CrossRef]
- Liu, F.; Vermesh, O.; Mani, V.; Ge, T.J.; Madsen, S.J.; Sabour, A.; Hsu, E.C.; Gowrishankar, G.; Kanada, M.; Jokerst, J.V.; et al. The Exosome Total Isolation Chip. ACS Nano 2017, 11, 10712–10723. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Kugeratski, F.G.; Kalluri, R. A novel machine learning algorithm picks proteome signature to specifically identify cancer exosomes. eLife 2024, 12, RP90390. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, Y.; Liu, N.; Lv, D.; Chen, Y.; Liu, Z.; Jin, X.; Xiao, M.; Lavillette, D.; Zhong, J.; et al. Extracellular vesicles from Zika virus-infected cells display viral E protein that binds ZIKV-neutralizing antibodies to prevent infection enhancement. EMBO J. 2023, 42, e112096. [Google Scholar] [CrossRef]
- Safadi, D.E.; Lebeau, G.; Lagrave, A.; Mélade, J.; Grondin, L.; Rosanaly, S.; Begue, F.; Hoareau, M.; Veeren, B.; Roche, M.; et al. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses 2023, 15, 364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef]
- Fikatas, A.; Dehairs, J.; Noppen, S.; Doijen, J.; Vanderhoydonc, F.; Meyen, E.; Swinnen, J.V.; Pannecouque, C.; Schols, D. Deciphering the Role of Extracellular Vesicles Derived from ZIKV-Infected hcMEC/D3 Cells on the Blood-Brain Barrier System. Viruses 2021, 13, 2363. [Google Scholar] [CrossRef]
- York, S.B.; Sun, L.; Cone, A.S.; Duke, L.C.; Cheerathodi, M.R.; Meckes, D.G., Jr. Zika Virus Hijacks Extracellular Vesicle Tetraspanin Pathways for Cell-to-Cell Transmission. mSphere 2021, 6, e0019221. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.; Sagaradze, G.; Orlova, E.; Shtam, T.; Proskura, K.; Kamyshinsky, R.; Yunusova, N.; Alexandrova, A.; Efimenko, A.; Tamkovich, S. Total Blood Exosomes in Breast Cancer: Potential Role in Crucial Steps of Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 7341. [Google Scholar] [CrossRef]
- Emelyanov, A.; Shtam, T.; Kamyshinsky, R.; Garaeva, L.; Verlov, N.; Miliukhina, I.; Kudrevatykh, A.; Gavrilov, G.; Zabrodskaya, Y.; Pchelina, S.; et al. Cryo-electron microscopy of extracellular vesicles from cerebrospinal fluid. PLoS ONE 2020, 15, e0227949. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, X.; Zhao, X.; Yang, X.; Wang, H. F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases. J. Hematol. Oncol. 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Saarikangas, J.; Zhao, H.; Pykäläinen, A.; Laurinmäki, P.; Mattila, P.K.; Kinnunen, P.K.; Butcher, S.J.; Lappalainen, P. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr. Biol. 2009, 19, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Erdmann, K.S.; Roux, A.; Habermann, B.; Werner, H.; De Camilli, P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell 2005, 9, 791–804. [Google Scholar] [CrossRef]
- Kapoor, K.S.; Kong, S.; Sugimoto, H.; Guo, W.; Boominathan, V.; Chen, Y.L.; Biswal, S.L.; Terlier, T.; McAndrews, K.M.; Kalluri, R. Single Extracellular Vesicle Imaging and Computational Analysis Identifies Inherent Architectural Heterogeneity. ACS Nano 2024, 18, 11717–11731. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
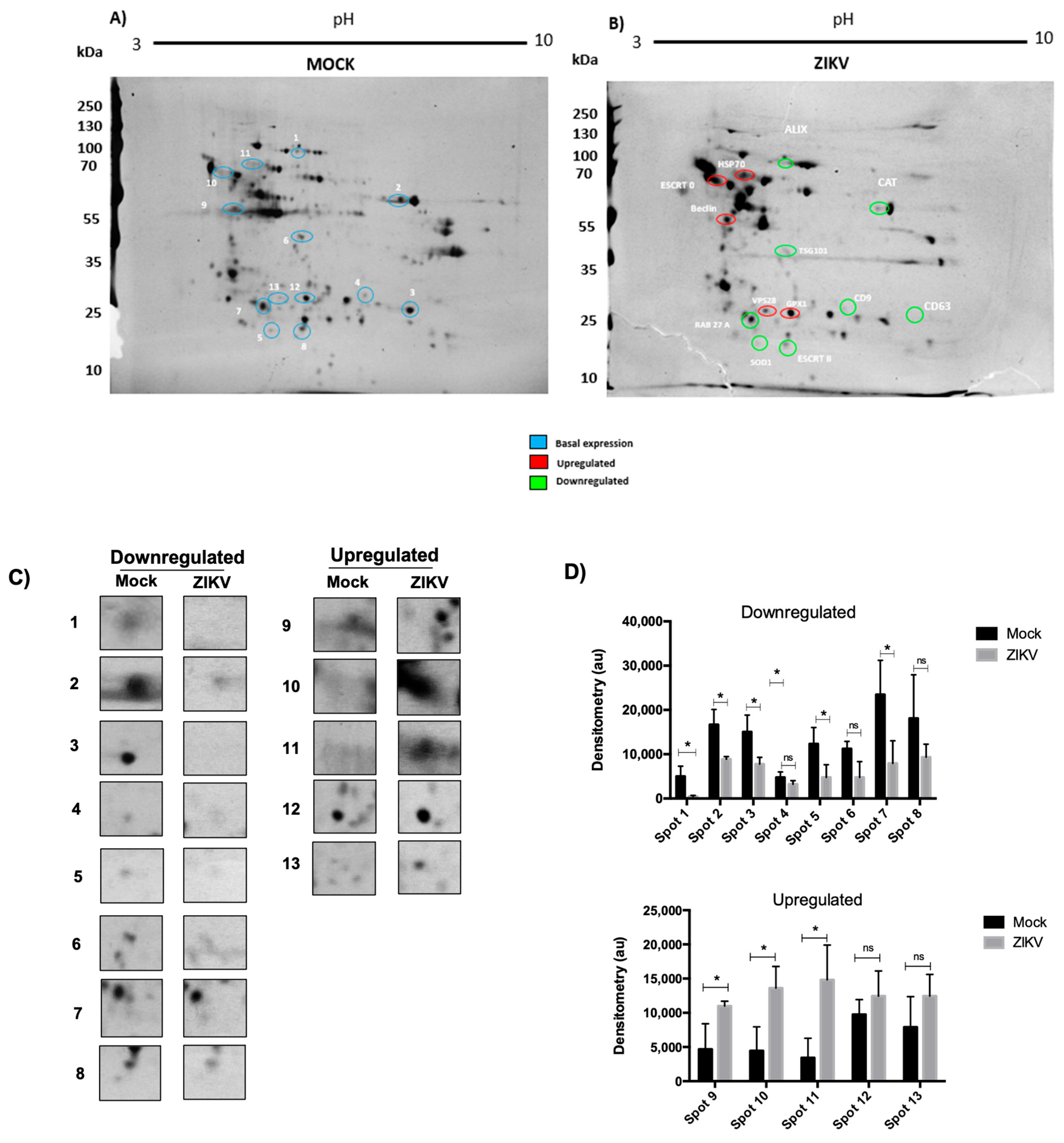

| Spots | Protein | MW | pI | Funtion | Regulation During Infection |
|---|---|---|---|---|---|
| 1 | ALIX | 96.02 | 6.13 | Protein cargo, biogenesis exosomes | Down |
| 2 | Catalase | 59.75 | 6.9 | Oxidative stress regulator | Down |
| 3 | CD63 | 25.637 | 8.14 | Tetraspanin exosomes | Down |
| 4 | CD9 | 25.41 | 6.8 | Tetraspanin exosomes | Down |
| 5 | SOD-1 | 15.93 | 5.7 | Oxidative stress regulator | Down |
| 6 | TSG101 | 43.9 | 6.06 | ESCRT-I interacting protein, for sorting | Down |
| 7 | RAB27A | 24.86 | 5.09 | GTPase leads to MVBs | Down |
| 8 | ESCRT II (VPS 25) | 20.74 | 5.97 | Exosome biogenesis | Down |
| 9 | BECLIN-1 | 51.896 | 4.83 | Precursor of autophagy | Up |
| 10 | STAM (ESCRT 0) | 59.18 | 4.7 | Exosome biogenesis | Up |
| 11 | HSP70 | 70.03 | 5.5 | Heat shock protein and protein exosome | Up |
| 12 | GPX1 | 22.08 | 6.15 | Oxidative stress regulator | Up |
| 13 | VPS28 | 25.42 | 5.37 | ESCRT-I regulatory protein | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez-Cervantes, M.A.; Flores-Pliego, A.; Benitez-Zeferino, Y.R.; Cruz-Holguín, V.J.; Herrera Moro-Huitron, L.; Helguera-Repetto, A.C.; Meza-Sánchez, D.E.; Maravillas-Montero, J.L.; Cayetano-Castro, N.; Mancilla-Ramírez, J.; et al. Zika Virus Infection Modulates Extracellular Vesicle Biogenesis and Morphology in Human Umbilical Cord Endothelial Cells: A Proteomic and Microscopic Analysis. Microorganisms 2025, 13, 1402. https://doi.org/10.3390/microorganisms13061402
Velázquez-Cervantes MA, Flores-Pliego A, Benitez-Zeferino YR, Cruz-Holguín VJ, Herrera Moro-Huitron L, Helguera-Repetto AC, Meza-Sánchez DE, Maravillas-Montero JL, Cayetano-Castro N, Mancilla-Ramírez J, et al. Zika Virus Infection Modulates Extracellular Vesicle Biogenesis and Morphology in Human Umbilical Cord Endothelial Cells: A Proteomic and Microscopic Analysis. Microorganisms. 2025; 13(6):1402. https://doi.org/10.3390/microorganisms13061402
Chicago/Turabian StyleVelázquez-Cervantes, Manuel Adrián, Arturo Flores-Pliego, Yazmín Rocío Benitez-Zeferino, Victor Javier Cruz-Holguín, Luis Herrera Moro-Huitron, Addy Cecilia Helguera-Repetto, David Eduardo Meza-Sánchez, José Luis Maravillas-Montero, Nicolás Cayetano-Castro, Javier Mancilla-Ramírez, and et al. 2025. "Zika Virus Infection Modulates Extracellular Vesicle Biogenesis and Morphology in Human Umbilical Cord Endothelial Cells: A Proteomic and Microscopic Analysis" Microorganisms 13, no. 6: 1402. https://doi.org/10.3390/microorganisms13061402
APA StyleVelázquez-Cervantes, M. A., Flores-Pliego, A., Benitez-Zeferino, Y. R., Cruz-Holguín, V. J., Herrera Moro-Huitron, L., Helguera-Repetto, A. C., Meza-Sánchez, D. E., Maravillas-Montero, J. L., Cayetano-Castro, N., Mancilla-Ramírez, J., Casarrubias-Betancourt, A., León-Reyes, G., Martínez-Castillo, M., Wong-Baeza, I., De Jesús-González, L. A., Baeza-Ramírez, M. I., & León-Juárez, M. (2025). Zika Virus Infection Modulates Extracellular Vesicle Biogenesis and Morphology in Human Umbilical Cord Endothelial Cells: A Proteomic and Microscopic Analysis. Microorganisms, 13(6), 1402. https://doi.org/10.3390/microorganisms13061402









