Exercise Remodels Akkermansia-Associated Eicosanoid Metabolism to Alleviate Intestinal Senescence: Multi-Omics Insights
Abstract
1. Introduction
2. Materials and Methods
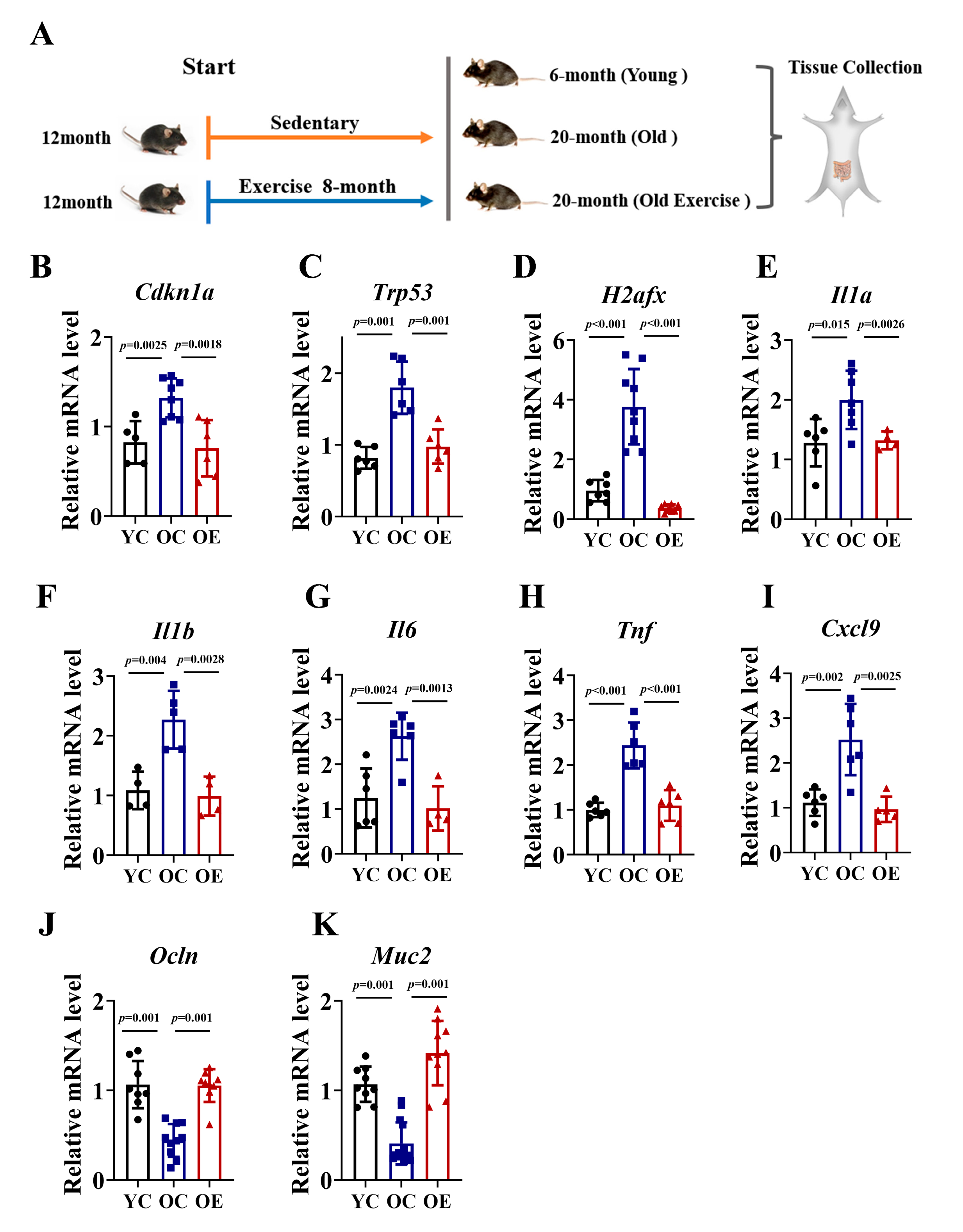
2.1. Animals
2.2. Intestinal Inflammatory and Cellular Senescence
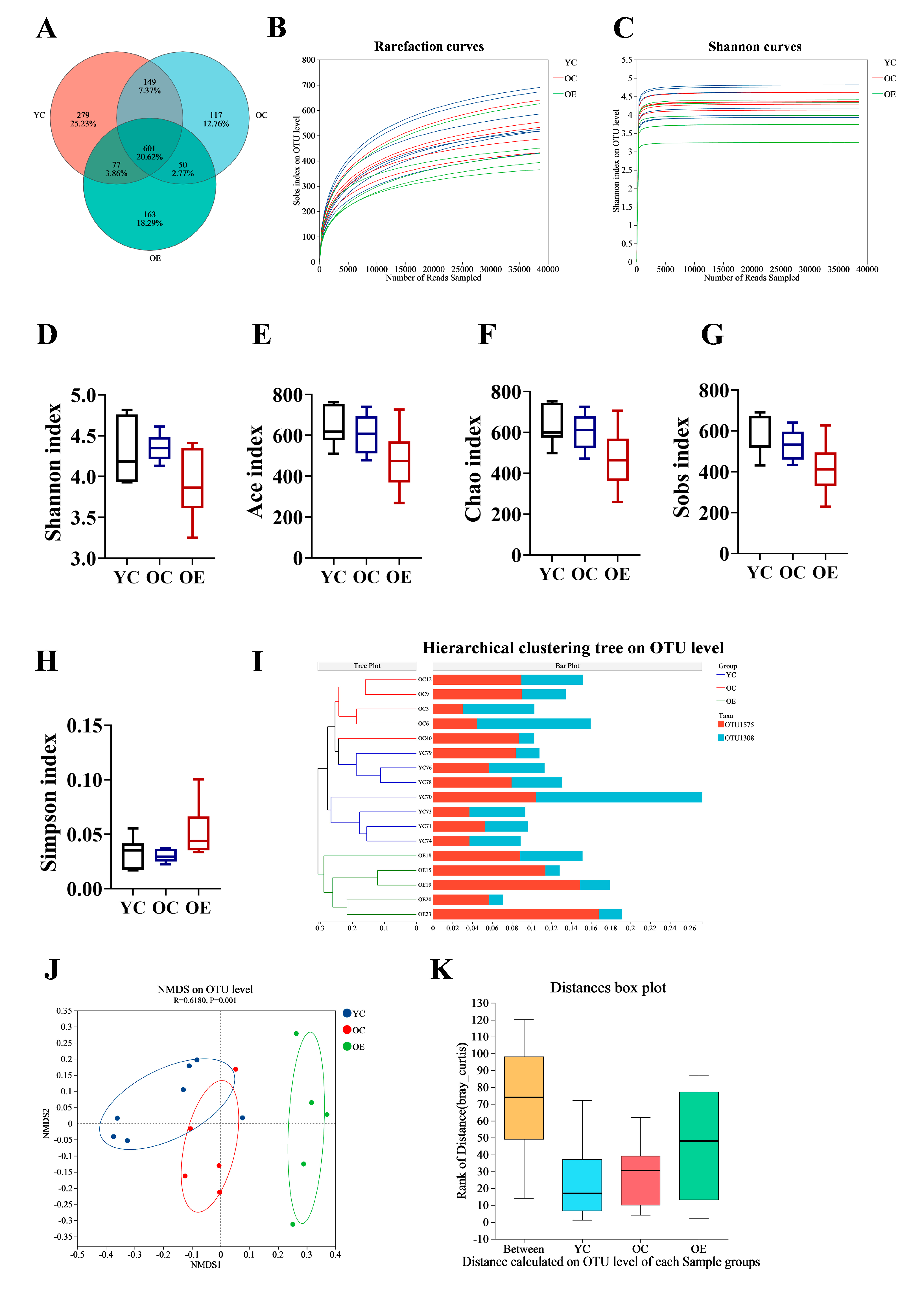
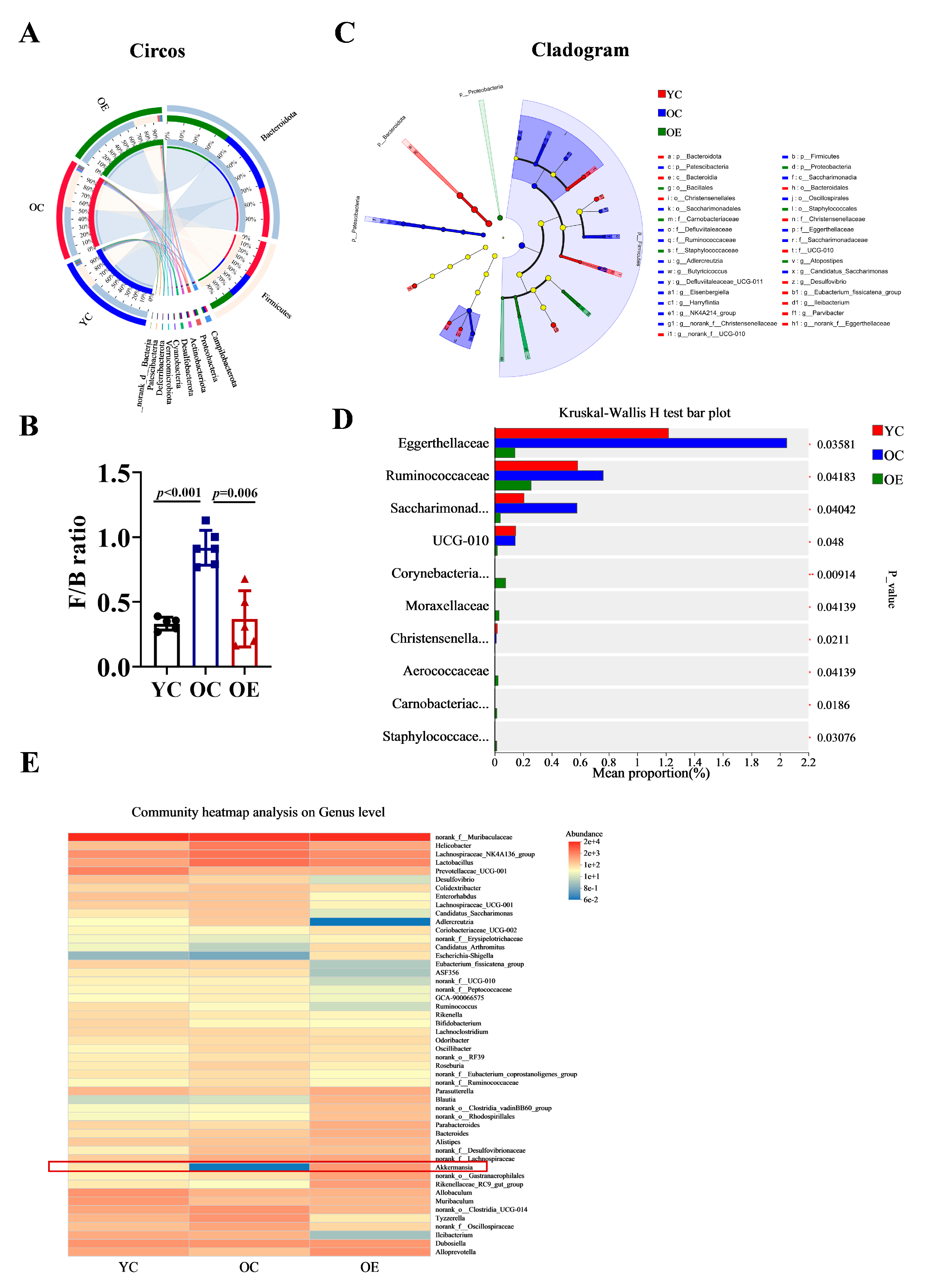
2.3. Microbiota Analysis
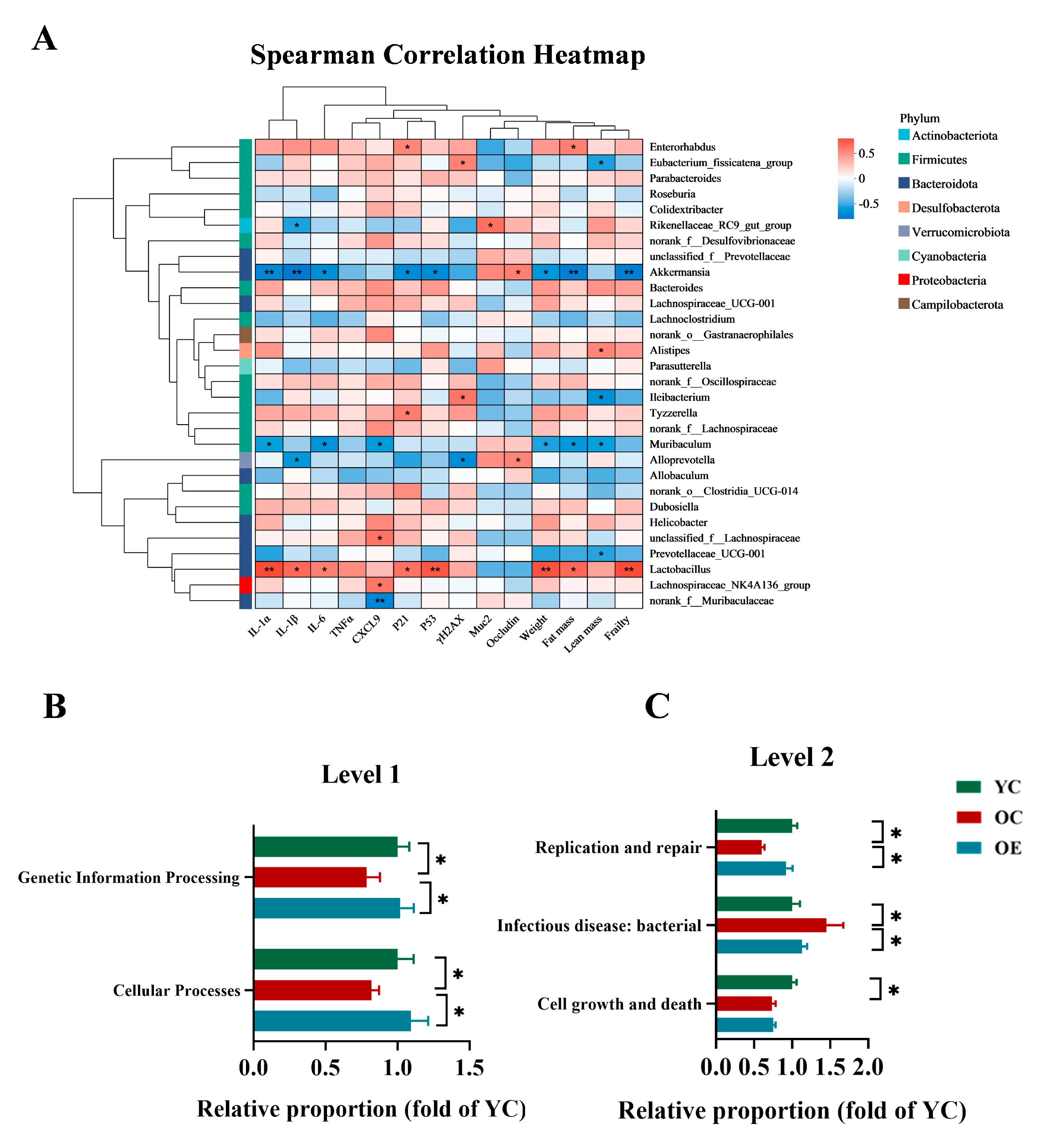
2.4. Untargeted Metabolomic Analysis
2.5. Statistical Analysis
3. Results
3.1. Exercise Attenuated Age-Associated Intestinal Senescence and Inflammatory Burden in Aged Mice
3.2. Exercise Remodeled Microbiota Composition in Aged Mice
3.3. Exercise Attenuated Senescence and Inflammation Through Microbiome-Associated Modulation in Aged Mice
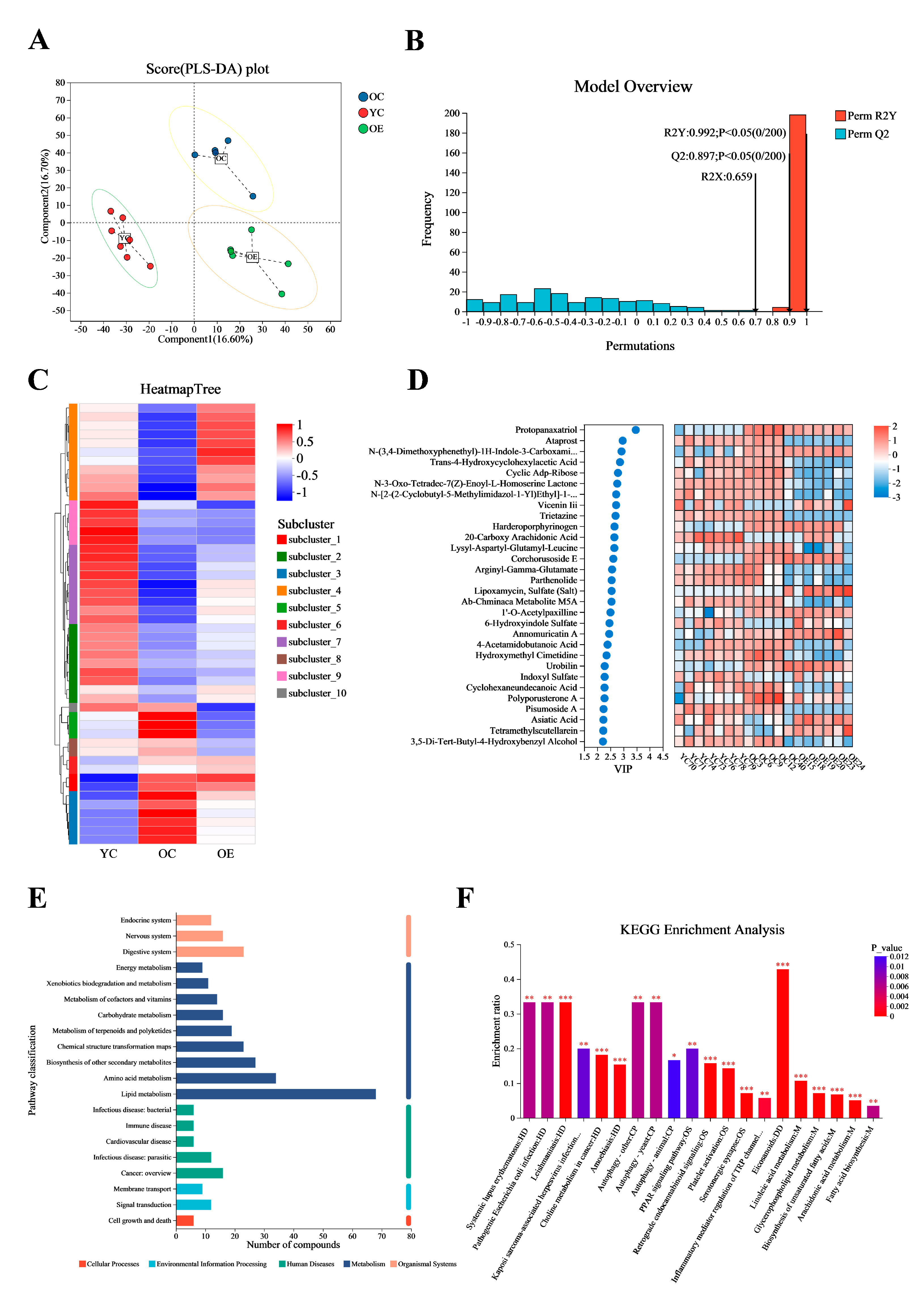
3.4. Exercise Changed the Microbial Metabolites in Aged Mice
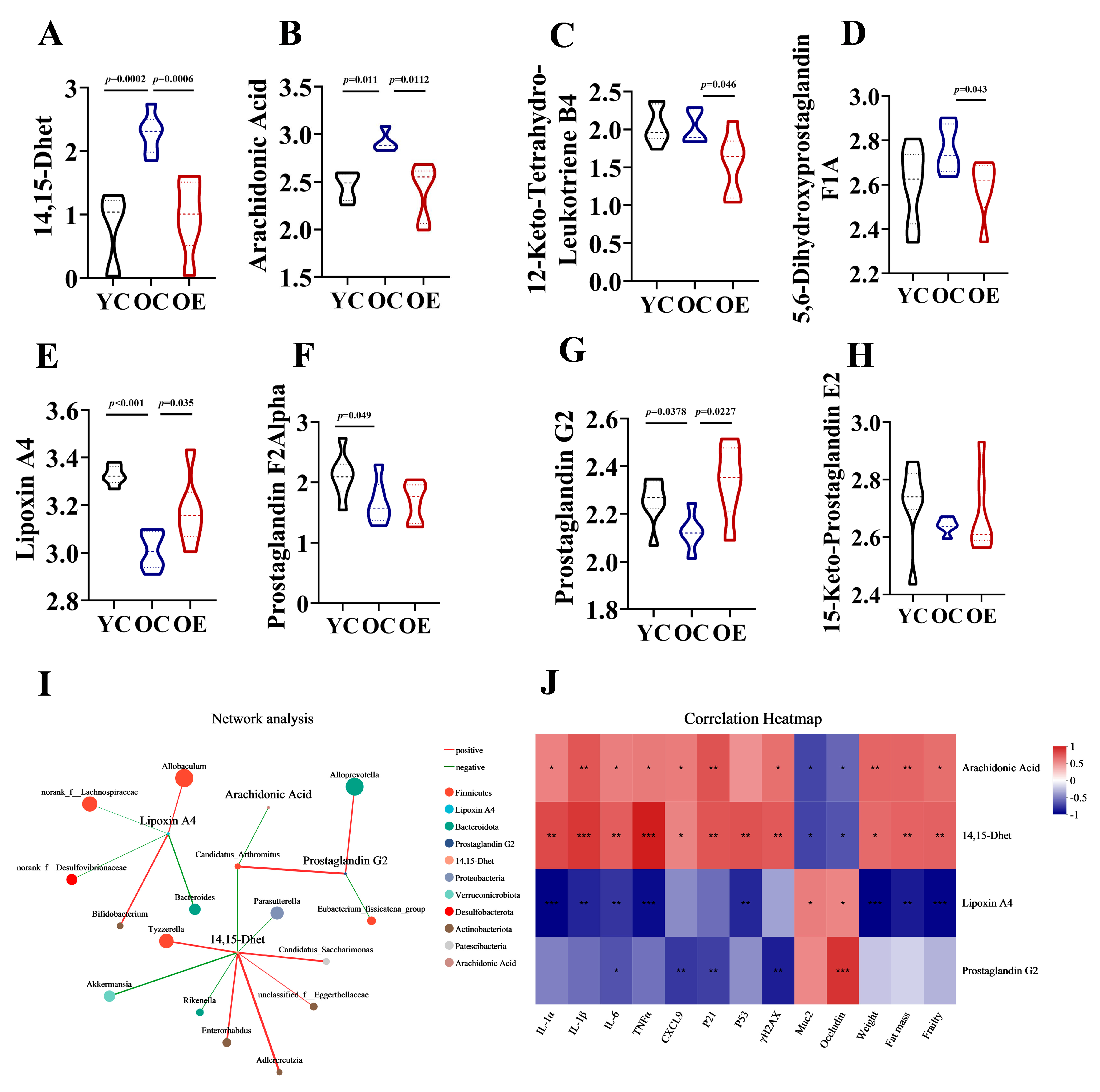
3.5. Exercise Mitigated Intestinal Aging Through Eicosanoids Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, L.; Yang, J.; Fu, X.; Zhang, Y.; Syed, J.N.; Tian, Y. Alpha-Glucosidase Inhibitors in Aging and Aging-Related Diseases: Clinical Applications and Relevant Mechanisms. Aging Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.M.; Pedrosa, S.S.; Kirkland, J.L.; Reis, F.; Madureira, A.R. The senotherapeutic potential of phytochemicals for age-related intestinal disease. Ageing Res. Rev. 2024, 104, 102619. [Google Scholar] [CrossRef]
- Du, Y.; Gao, Y.; Zeng, B.; Fan, X.; Yang, D.; Yang, M. Effects of anti-aging interventions on intestinal microbiota. Gut Microbes 2021, 13, 1994835. [Google Scholar] [CrossRef]
- Strasser, B.; Wolters, M.; Weyh, C.; Krüger, K.; Ticinesi, A. The Effects of Lifestyle and Diet on Gut Microbiota Composition, Inflammation and Muscle Performance in Our Aging Society. Nutrients 2021, 13, 2045. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, K.; Williams, G.; Hoedt, E.C.; Collins, C.E.; Keely, S.; Tally, N.J. Diet-microbiota associations in gastrointestinal research: A systematic review. Gut Microbes 2024, 16, 2350785. [Google Scholar] [CrossRef]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, J.; Huang, J.; Wang, M.; Wu, L.; Wu, T.; Chen, N. Exerkine irisin mitigates cognitive impairment by suppressing gut-brain axis-mediated inflammation. J. Adv. Res. 2024, 9, S2090-1232(24)00485-5. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef]
- Brooks, C.N.; Wight, M.E.; Azeez, O.E.; Bleich, R.M.; Zwetsloot, K.A. Growing old together: What we know about the influence of diet and exercise on the aging host’s gut microbiome. Front. Sports Act. Living 2023, 5, 1168731. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, C.; Zou, X.; Li, H.; Yang, G.; Su, X.; Mo, Z. Multi-omics insights implicate the remodeling of the intestinal structure and microbiome in aging. Front. Genet. 2024, 15, 1450064. [Google Scholar] [CrossRef]
- Schnabl, B.; Damman, C.J.; Carr, R.M. Metabolic dysfunction-associated steatotic liver disease and the gut microbiome: Pathogenic insights and therapeutic innovations. J. Clin. Investig. 2025, 135, e186423. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, S.; Niu, Y.; Fu, L. Exercise protects intestinal epithelial barrier from high fat diet- induced permeabilization through SESN2/AMPKα1/HIF-1α signaling. J. Nutr. Biochem. 2022, 107, 109059. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.D.; Sokol, H.; Doré, J.; Furet, J. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Zhu, S.; Han, M.; Liu, S.; Fan, L.; Shi, H.; Li, P. Composition and diverse differences of intestinal microbiota in ulcerative colitis patients. Front. Cell. Infect. Microbiol. 2022, 12, 953962. [Google Scholar] [CrossRef]
- Yang, H.; Wang, T.; Qian, C.; Wang, H.; Yu, D.; Shi, M.; Zhao, C. Gut microbial-derived phenylacetylglutamine accelerates host cellular senescence. Nat. Aging 2025, 5, 401–418. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Lorenzetti, F. Role of eicosanoids in liver repair, regeneration and cancer. Biochem. Pharmacol. 2021, 192, 114732. [Google Scholar] [CrossRef]
- Tunctan, B. CYP-derived eicosanoids in inflammatory diseases. Prostaglandins Other Lipid Mediat. 2020, 148, 106424. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef] [PubMed]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Bradley, E.; Haran, J. The human gut microbiome and aging. Gut Microbes. 2024, 16, 2359677. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, J.; Yang, Y.; Zhang, L.; Wang, X. Effects of exercise interventions on cognitive functions in healthy populations: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 92, 102116. [Google Scholar] [CrossRef]
- Efremova, I.; Maslennikov, R.; Medvedev, O.; Kudryavtseva, A.; Avdeeva, A.; Krasnov, G.; Ivashkin, V. Gut Microbiota and Biomarkers of Intestinal Barrier Damage in Cirrhosis. Microorganisms 2024, 12, 463. [Google Scholar] [CrossRef]
- Kang, E.J.; Kim, J.H.; Kim, Y.E.; Lee, H.; Jung, K.B.; Chang, D.H.; Lee, C.H. The secreted protein Amuc_1409 from Akkermansia muciniphila improves gut health through intestinal stem cell regulation. Nat. Commun. 2024, 15, 2983. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Gagnon, E.; Mitchell, P.L.; Manikpurage, H.D.; Abner, E.; Taba, N.; Esko, T.; Arsenault, B.J. Impact of the gut microbiota and associated metabolites on cardiometabolic traits, chronic diseases and human longevity: A Mendelian randomization study. J. Transl. Med. 2023, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Honarpisheh, P.; Bryan, R.M.; McCullough, L.D. Aging Microbiota-Gut-Brain Axis in Stroke Risk and Outcome. Circ. Res. 2022, 130, 1112–1144. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Pan, J.; Guo, M.; Duan, H.; Zhang, H.; Narbad, A.; Chen, W. Gut microbiota and anti-aging: Focusing on spermidine. Crit. Rev. Food Sci. Nutr. 2024, 64, 10419–10437. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Ravipati, S.; Pousinis, P.; Menni, C.; Mangino, M.; Abhishek, A.; Doherty, M. Omega-6 oxylipins generated by soluble epoxide hydrolase are associated with knee osteoarthritis. J. Lipid Res. 2018, 59, 1763–1770. [Google Scholar] [CrossRef]
- Bergmann, C.B.; Hammock, B.D.; Wan, D.; Gogolla, F.; Goetzman, H.; Caldwell, C.C.; Supp, D.M. TPPU treatment of burned mice dampens inflammation and generation of bioactive DHET which impairs neutrophil function. Sci. Rep. 2021, 11, 16555. [Google Scholar] [CrossRef]
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Cdkn1a | GTCAGGCTGGTCTGCCTCCG | CGGTCCCGTGGACAGTGAGCAG |
| Trp53 | CCGACCTATCCTTACCATCATCA | AGGCACAAACACGAACCTCAA |
| H2afx | GGCCTGTGGACAAGAGTTCTAT | GCCCATTAAATCTCCCCACT |
| Muc2 | GCTGACGAGTGGTTGGTGAATG | GATGAGGTGGCAGACAGGAGAC |
| Ocln | CATCAGCCATGTCCGTGAGG | GGGGCGACGTCCATTTGTAG |
| Il1a | GCACCTTACACCTACCAGAGT | AAACTTCTGCCTGACGAGCTT |
| Il1b | TTGAAGTTGACGGACCCCA | ATGAGTGATACTGCCTGCCTGA |
| Tnf | AGGGTCTGGGCCATAGAACT | CAGCCTCTTCTCATTCCTGC |
| Cxcl9 | AGTGTGGAGTTCGAGGAACCCT | TGCAGGAGCATCGTGCATT |
| Il6 | CCAGAAACCGCTATGAAGTTCC | TTGTCACCAGCATCAGTCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Liu, X.; Li, Y.; Li, S.; Huang, Y.; Liu, S.; Shao, H.; Shen, Y.; Fu, L. Exercise Remodels Akkermansia-Associated Eicosanoid Metabolism to Alleviate Intestinal Senescence: Multi-Omics Insights. Microorganisms 2025, 13, 1379. https://doi.org/10.3390/microorganisms13061379
Yu C, Liu X, Li Y, Li S, Huang Y, Liu S, Shao H, Shen Y, Fu L. Exercise Remodels Akkermansia-Associated Eicosanoid Metabolism to Alleviate Intestinal Senescence: Multi-Omics Insights. Microorganisms. 2025; 13(6):1379. https://doi.org/10.3390/microorganisms13061379
Chicago/Turabian StyleYu, Chunxia, Xuanyu Liu, Yitong Li, Silin Li, Yating Huang, Sujuan Liu, Heng Shao, Yanna Shen, and Li Fu. 2025. "Exercise Remodels Akkermansia-Associated Eicosanoid Metabolism to Alleviate Intestinal Senescence: Multi-Omics Insights" Microorganisms 13, no. 6: 1379. https://doi.org/10.3390/microorganisms13061379
APA StyleYu, C., Liu, X., Li, Y., Li, S., Huang, Y., Liu, S., Shao, H., Shen, Y., & Fu, L. (2025). Exercise Remodels Akkermansia-Associated Eicosanoid Metabolism to Alleviate Intestinal Senescence: Multi-Omics Insights. Microorganisms, 13(6), 1379. https://doi.org/10.3390/microorganisms13061379







