Healthcare-Associated Clostridioides difficile Infection: A Hospital-Based Retrospective Study in North Eastern Romania
Abstract
1. Introduction
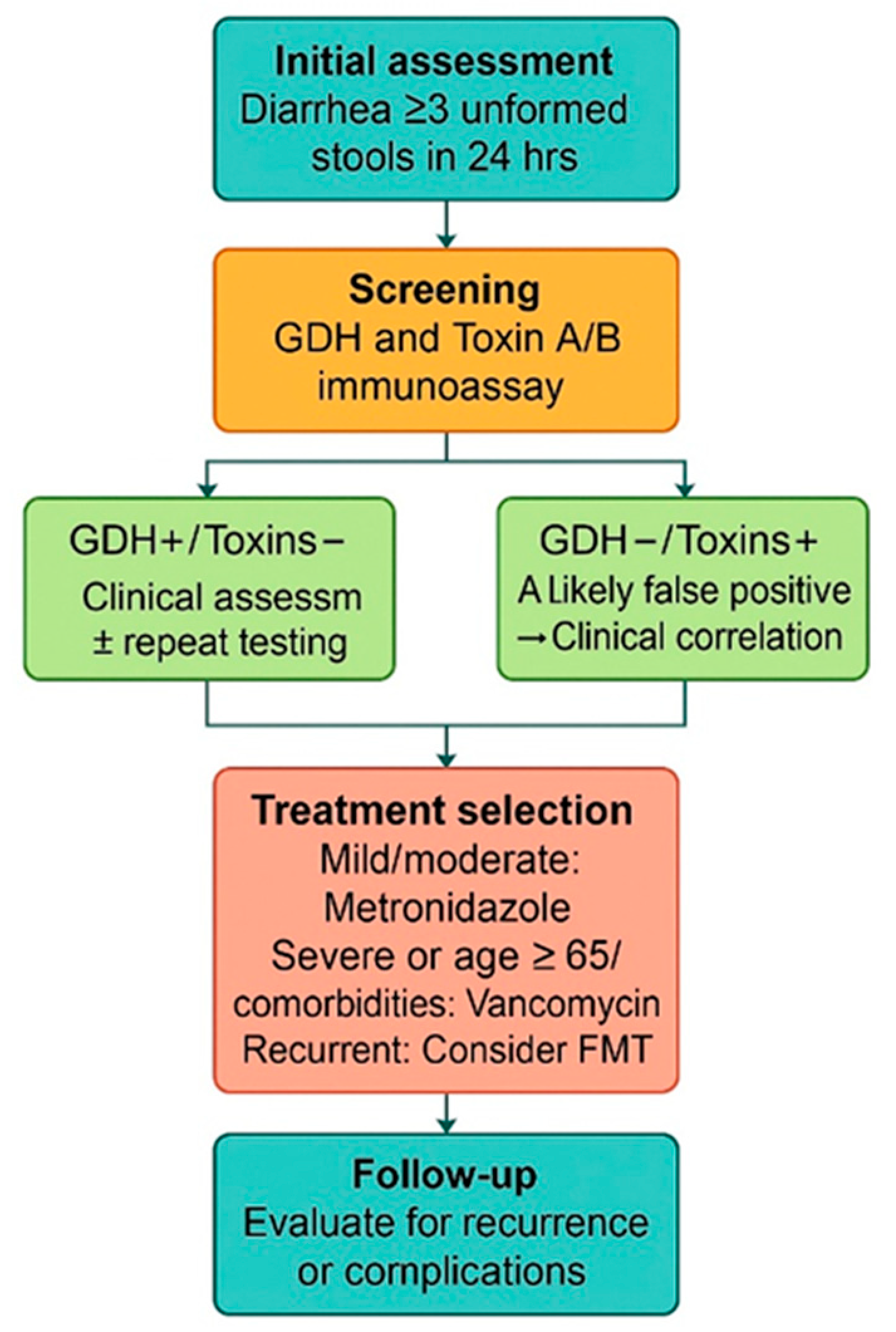
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CD | Clostridioides difficile |
| CDI | Clostridioides difficile infection |
| CI | Confidence interval |
| ELISA | Enzyme-linked immunoassay |
| FMT | Fecal microbiota transplantation |
| GDH | Glutamate dehydrogenase |
| ICU | Intensive care unit |
| LOS | Length of stay |
| ROC | Receiver-operating characteristic curve |
References
- CDC. Clostridium difficile Infection (CDI) Surveillance. Available online: https://www.cdc.gov/healthcare-associated-infections/php/haic-eip/cdiff.html (accessed on 19 September 2024).
- Fu, Y.; Luo, Y.; Grinspan, A.M. Epidemiology of Community-Acquired and Recurrent Clostridioides difficile Infection. Ther. Adv. Gastroenterol. 2021, 14, 175628482110162. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.K.; Hernandez, A.V.; Donskey, C.J.; Fraser, T.G. Risk Factors for Recurrent Clostridium difficile Infection: A Systematic Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2015, 36, 452–460. [Google Scholar] [CrossRef]
- Brown, K.A.; Khanafer, N.; Daneman, N.; Fisman, D.N. Meta-Analysis of Antibiotics and the Risk of Community-Associated Clostridium difficile Infection. Antimicrob. Agents Chemother. 2013, 57, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Asgary, R.; Snead, J.A.; Wahid, N.A.; Ro, V.; Halim, M.; Stribling, J.C. Risks and Preventive Strategies for Clostridioides difficile Transmission to Household or Community Contacts during Transition in Healthcare Settings. Emerg. Infect. Dis. 2021, 27, 1776–1782. [Google Scholar] [CrossRef]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile Infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Weese, J.S. Clostridium difficile in Food–-Innocent Bystander or Serious Threat? Clin. Microbiol. Infect. 2010, 16, 3–10. [Google Scholar] [CrossRef]
- Weese, J.S.; Avery, B.P.; Rousseau, J.; Reid-Smith, R.J. Detection and Enumeration of Clostridium difficile Spores in Retail Beef and Pork. Appl. Environ. Microbiol. 2009, 75, 5009–5011. [Google Scholar] [CrossRef]
- Antonelli, M.; Martin-Loeches, I.; Dimopoulos, G.; Gasbarrini, A.; Vallecoccia, M.S. Clostridium difficile (Formerly Clostridium difficile) Infection in the Critically Ill: An Expert Statement. Intensive Care Med. 2020, 46, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Slimings, C.; Riley, T.V. Antibiotics and Healthcare Facility-Associated Clostridium difficile Infection: Systematic Review and Meta-Analysis 2020 Update. J. Antimicrob. Chemother. 2021, 76, 1676–1688. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Willey, S.H.; Chang, T.W.; Lowe, B. Cephalosporin-Associated Pseudomembranous Colitis Due to Clostridium difficile. JAMA 1979, 242, 2683–2685. [Google Scholar] [CrossRef]
- Smart, R.F.; Ramsden, D.A.; Gear, M.W.L.; Nicol, A.; Lennox, W.M. Severe Pseudomembranous Colitis after Lincomycin and Clindamycin. Br. J. Surg. 2005, 63, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Asempa, T.; Nicolau, D. Clostridium difficile Infection in the Elderly: An Update on Management. Clin. Interv. Aging 2017, 12, 1799–1809. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Gerding, D.N. Clinical Recognition and Diagnosis of Clostridium difficile Infection. Clin. Infect. Dis. 2008, 46, S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.J.; LaMont, J.T. Pathogenesis and Clinical Manifestations of Clostridium difficile Diarrhea and Colitis. Curr. Top. Microbiol. Immunol. 2000, 250, 109–125. [Google Scholar] [CrossRef]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile Infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Papatheodorou, P.; Schwan, C. Binary Clostridium difficile Toxin (CDT)—A Virulence Factor Disturbing the Cytoskeleton. Anaerobe 2018, 53, 21–29. [Google Scholar] [CrossRef]
- Crobach, M.J.T.; Planche, T.; Eckert, C.; Barbut, F.; Terveer, E.M.; Dekkers, O.M.; Wilcox, M.H.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Diagnostic Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2016, 22, S63–S81. [Google Scholar] [CrossRef]
- Crobach, M.J.T.; Baktash, A.; Duszenko, N.; Kuijper, E.J. Diagnostic Guidance for C. Difficile Infections. In Updates on Clostridium difficile in Europe: Advances in Microbiology, Infectious Diseases and Public Health; Mastrantonio, P., Rupnik, M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 8, pp. 27–44. ISBN 978-3-319-72799-8. [Google Scholar]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Johnson, S.; Louie, T.J.; Gerding, D.N.; Cornely, O.A.; Chasan-Taber, S.; Fitts, D.; Gelone, S.P.; Broom, C.; Davidson, D.M.; for the Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, Metronidazole, or Tolevamer for Clostridium difficile Infection: Results from Two Multinational, Randomized, Controlled Trials. Clin. Infect. Dis. 2014, 59, 345–354. [Google Scholar] [CrossRef]
- Di, X.; Bai, N.; Zhang, X.; Liu, B.; Ni, W.; Wang, J.; Wang, K.; Liang, B.; Liu, Y.; Wang, R. A Meta-Analysis of Metronidazole and Vancomycin for the Treatment of Clostridium difficile Infection, Stratified by Disease Severity. Braz. J. Infect. Dis. 2015, 19, 339–349. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Gasbarrini, A. Fecal Microbiota Transplantation for the Treatment of Clostridium difficile Infection: A Systematic Review. J. Clin. Gastroenterol. 2014, 48, 693. [Google Scholar] [CrossRef] [PubMed]
- Kassam, Z.; Lee, C.H.; Yuan, Y.; Hunt, R.H. Fecal Microbiota Transplantation forClostridium difficileInfection: Systematic Review and Meta-Analysis. Off. J. Am. Coll. Gastroenterol. ACG 2013, 108, 500. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.N.; Widlak, M.; Bhala, N.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T.H. Systematic Review with Meta-analysis: The Efficacy of Faecal Microbiota Transplantation for the Treatment of Recurrent and Refractory Clostridium difficile Infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef]
- Ianiro, G.; Maida, M.; Burisch, J.; Simonelli, C.; Hold, G.; Ventimiglia, M.; Gasbarrini, A.; Cammarota, G. Efficacy of Different Faecal Microbiota Transplantation Protocols for Clostridium difficile Infection: A Systematic Review and Meta-Analysis. United Eur. Gastroenterol. J. 2018, 6, 1232–1244. [Google Scholar] [CrossRef]
- Guery, B.; Galperine, T.; Barbut, F. Clostridium difficile: Diagnosis and Treatments. BMJ 2019, 366, l4609. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Jesdale, W.M.; Tjia, J.; Lapane, K. Recurrent Clostridium difficile Infection among Medicare Patients in Nursing Homes: A Population-Based Cohort Study. Medicine (Baltimore) 2017, 96, e6231. [Google Scholar] [CrossRef]
- Di Bella, S.; Sanson, G.; Monticelli, J.; Zerbato, V.; Principe, L.; Giuffrè, M.; Pipitone, G.; Luzzati, R. Clostridium difficile Infection: History, Epidemiology, Risk Factors, Prevention, Clinical Manifestations, Treatment, and Future Options. Clin. Microbiol. Rev. 2024, 37, e00135-23. [Google Scholar] [CrossRef]
- Fonseca, F.; Forrester, M.; Advinha, A.M.; Coutinho, A.; Landeira, N.; Pereira, M. Clostridium difficile Infection in Hospitalized Patients-A Retrospective Epidemiological Study. Healthcare 2023, 12, 76. [Google Scholar] [CrossRef]
- Karas, J.A.; Enoch, D.A.; Aliyu, S.H. A Review of Mortality Due to Clostridium difficile Infection. J. Infect. 2010, 61, 1–8. [Google Scholar] [CrossRef]
- Mada, P.K.; Alam, M.U. Clostridium difficile Infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bagdasarian, N.; Rao, K.; Malani, P.N. Diagnosis and Treatment of Clostridium difficile in Adults: A Systematic Review. JAMA 2015, 313, 398. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.; Emirian, A.; Le Monnier, A.; Cathala, L.; De Montclos, H.; Goret, J.; Berger, P.; Petit, A.; De Chevigny, A.; Jean-Pierre, H.; et al. Prevalence and Pathogenicity of Binary Toxin–Positive Clostridium difficile Strains That Do Not Produce Toxins A and B. New Microbes New Infect. 2015, 3, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lyras, D.; O’Connor, J.R.; Howarth, P.M.; Sambol, S.P.; Carter, G.P.; Phumoonna, T.; Poon, R.; Adams, V.; Vedantam, G.; Johnson, S.; et al. Toxin B Is Essential for Virulence of Clostridium difficile. Nature 2009, 458, 1176–1179. [Google Scholar] [CrossRef]
- Carter, G.P.; Chakravorty, A.; Pham Nguyen, T.A.; Mileto, S.; Schreiber, F.; Li, L.; Howarth, P.; Clare, S.; Cunningham, B.; Sambol, S.P.; et al. Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections. mBio 2015, 6, e00551-15. [Google Scholar] [CrossRef]
- Li, Z.; Lee, K.; Rajyaguru, U.; Jones, C.H.; Janezic, S.; Rupnik, M.; Anderson, A.S.; Liberator, P. Ribotype Classification of Clostridium difficile Isolates Is Not Predictive of the Amino Acid Sequence Diversity of the Toxin Virulence Factors TcdA and TcdB. Front. Microbiol. 2020, 11, 1310. [Google Scholar] [CrossRef]
- Di Bella, S.; Ascenzi, P.; Siarakas, S.; Petrosillo, N.; Di Masi, A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins 2016, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; LaMont, J.T. Clostridium difficile—More Difficult than Ever. N. Engl. J. Med. 2008, 359, 1932–1940. [Google Scholar] [CrossRef]
- Stămăteanu, L.O.; Pleşca, C.E.; Miftode, I.L.; Bădescu, A.C.; Manciuc, D.C.; Hurmuzache, M.E.; Roșu, M.F.; Miftode, R.Ș.; Obreja, M.; Miftode, E.G. “Primum, Non Nocere”: The Epidemiology of Toxigenic Clostridium difficile Strains in the Antibiotic Era—Insights from a Prospective Study at a Regional Infectious Diseases Hospital in Eastern Europe. Antibiotics 2024, 13, 461. [Google Scholar] [CrossRef]
- Conrad, J.; Giesbrecht, K.; Aguilar, R.C.; Gräfe, S.K.; Ullah, A.; Hunfeld, K.-P.; Lübbert, C.; Pützfeld, S.; Reuken, P.A.; Schmitz-Rode, M.; et al. Comparative Effectiveness of Vancomycin and Metronidazole on Event-Free Survival after Initial Infection in Patients with Clostridium difficile—A German Multicentre Cohort Study. Clin. Microbiol. Infect. 2024, 30, 1433–1438. [Google Scholar] [CrossRef]
- Zar, F.A.; Bakkanagari, S.R.; Moorthi, K.M.L.S.T.; Davis, M.B. A Comparison of Vancomycin and Metronidazole for the Treatment of Clostridium difficile—Associated Diarrhea, Stratified by Disease Severity. Clin. Infect. Dis. 2007, 45, 302–307. [Google Scholar] [CrossRef]
- Stevens, V.W.; Nelson, R.E.; Schwab-Daugherty, E.M.; Khader, K.; Jones, M.M.; Brown, K.A.; Greene, T.; Croft, L.D.; Neuhauser, M.; Glassman, P.; et al. Comparative Effectiveness of Vancomycin and Metronidazole for the Prevention of Recurrence and Death in Patients with Clostridium difficile Infection. JAMA Intern. Med. 2017, 177, 546. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Polyzos, K.A.; Patouni, K.; Rafailidis, P.I.; Samonis, G.; Falagas, M.E. Treatment Failure and Recurrence of Clostridium difficile Infection Following Treatment with Vancomycin or Metronidazole: A Systematic Review of the Evidence. Int. J. Antimicrob. Agents 2012, 40, 1–8. [Google Scholar] [CrossRef]
- Carter, G.P.; Rood, J.I.; Lyras, D. The Role of Toxin A and Toxin B in the Virulence of Clostridium difficile. Trends Microbiol. 2012, 20, 21–29. [Google Scholar] [CrossRef]
- Loo, V.G.; Bourgault, A.-M.; Poirier, L.; Lamothe, F.; Michaud, S.; Turgeon, N.; Toye, B.; Beaudoin, A.; Frost, E.H.; Gilca, R.; et al. Host and Pathogen Factors for Clostridium difficile Infection and Colonization. N. Engl. J. Med. 2011, 365, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- To, K.B.; Napolitano, L.M. Clostridium difficile Infection: Update on Diagnosis, Epidemiology, and Treatment Strategies. Surg. Infect. 2014, 15, 490–502. [Google Scholar] [CrossRef]
- Mulki, R.; Baumann, A.J.; Alnabelsi, T.; Sandhu, N.; Alhamshari, Y.; Wheeler, D.S.; Perloff, S.; Katz, P.O. Body Mass Index Greater than 35 Is Associated with Severe Clostridium difficile Infection. Aliment. Pharmacol. Ther. 2017, 45, 75–81. [Google Scholar] [CrossRef]
- Nathanson, B.H.; Higgins, T.L.; McGee, W.T. The Dangers of Extreme Body Mass Index Values in Patients with Clostridium difficile. Infection 2017, 45, 787–793. [Google Scholar] [CrossRef]
- Dolan, R.D.; Abougergi, M.S.; Schulman, A.R. Morbid Obesity Increases 30-Day Readmission and Morbidity in Clostridiodes difficile Infection. Obes. Surg. 2021, 31, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Negrut, N.; Bungau, S.; Behl, T.; Khan, S.A.; Vesa, C.M.; Bustea, C.; Nistor-Cseppento, D.C.; Rus, M.; Pavel, F.-M.; Tit, D.M. Risk Factors Associated with Recurrent Clostridium difficile Infection. Healthcare 2020, 8, 352. [Google Scholar] [CrossRef]
- Chakra, C.N.A.; Pepin, J.; Sirard, S.; Valiquette, L. Risk Factors for Recurrence, Complications and Mortality in Clostridium difficile Infection: A Systematic Review. PLoS ONE 2014, 9, e98400. [Google Scholar] [CrossRef]
- Jump, R.L. Clostridium difficile Infection in Older Adults. Aging Health 2013, 9, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Karas, J.A.; Bradshaw, S.; Mahmud, W.; Enoch, D.A. Mortality in Hospitalized Older Adults Associated with Clostridium difficile Infection at a District Hospital. Infect. Dis. Rep. 2010, 2, e8. [Google Scholar] [CrossRef][Green Version]
- Abou Chakra, C.N.; McGeer, A.; Labbé, A.-C.; Simor, A.E.; Gold, W.L.; Muller, M.P.; Powis, J.; Katz, K.; Garneau, J.R.; Fortier, L.-C.; et al. Factors Associated with Complications of Clostridium difficile Infection in a Multicenter Prospective Cohort. Clin. Infect. Dis. 2015, 61, 1781–1788. [Google Scholar] [CrossRef]
- Qu, H.-Q.; Jiang, Z.-D. Clostridium difficile Infection in Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 285–294. [Google Scholar] [CrossRef]
- Stămăteanu, L.O.; Miftode, I.L.; Pleșca, C.E.; Dorneanu, O.S.; Roșu, M.F.; Miftode, I.D.; Obreja, M.; Miftode, E.G. Symptoms, Treatment, and Outcomes of COVID-19 Patients Coinfected with Clostridium difficile: Single-Center Study from NE Romania during the COVID-19 Pandemic. Antibiotics 2023, 12, 1091. [Google Scholar] [CrossRef]
- Shakov, R.; Salazar, R.S.; Kagunye, S.K.; Baddoura, W.J.; DeBari, V.A. Diabetes Mellitus as a Risk Factor for Recurrence of Clostridium difficile Infection in the Acute Care Hospital Setting. Am. J. Infect. Control 2011, 39, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Atramont, A.; Lindecker-Cournil, V.; Rudant, J.; Tajahmady, A.; Drewniak, N.; Fouard, A.; Singer, M.; Leone, M.; Legrand, M. Association of Age with Short-Term and Long-Term Mortality Among Patients Discharged from Intensive Care Units in France. JAMA Netw. Open 2019, 2, e193215. [Google Scholar] [CrossRef] [PubMed]
- Sammons, J.S.; Localio, R.; Xiao, R.; Coffin, S.E.; Zaoutis, T. Clostridium difficile Infection Is Associated with Increased Risk of Death and Prolonged Hospitalization in Children. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 1–8. [Google Scholar] [CrossRef]
- CDC. About C. diff. Available online: https://www.cdc.gov/c-diff/about/index.html (accessed on 17 March 2025).
- Agnoletti, F.; Arcangeli, G.; Barbanti, F.; Barco, L.; Brunetta, R.; Cocchi, M.; Conedera, G.; D’Este, L.; Drigo, I.; Spigaglia, P.; et al. Survey, Characterization and Antimicrobial Susceptibility of Clostridium difficile from Marine Bivalve Shellfish of North Adriatic Sea. Int. J. Food Microbiol. 2019, 298, 74–80. [Google Scholar] [CrossRef]
- McFarland, L.V.; Mulligan, M.E.; Kwok, R.Y.Y.; Stamm, W.E. Nosocomial Acquisition of Clostridium difficile Infection. N. Engl. J. Med. 1989, 320, 204–210. [Google Scholar] [CrossRef]
- Samore, M.H.; Venkataraman, L.; DeGirolami, P.C.; Arbeit, R.D.; Karchmer, A.W. Clinical and Molecular Epidemiology of Sporadic and Clustered Cases of Nosocomial Clostridium difficile Diarrhea. Am. J. Med. 1996, 100, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Boven, A.; Vlieghe, E.; Engstrand, L.; Andersson, F.L.; Callens, S.; Simin, J.; Brusselaers, N. Clostridium difficile Infection-Associated Cause-Specific and All-Cause Mortality: A Population-Based Cohort Study. Clin. Microbiol. Infect. 2023, 29, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Pechal, A.; Lin, K.; Allen, S.; Reveles, K. National Age Group Trends in Clostridium difficile Infection Incidence and Health Outcomes in United States Community Hospitals. BMC Infect. Dis. 2016, 16, 682. [Google Scholar] [CrossRef] [PubMed]

| Epidemiological Characteristics | N (%) | Mean LOS ± SD | Median/Limits | FANOVA Test |
|---|---|---|---|---|
| Symptoms | ||||
| Watery stools | 533 (99.8%) | 10.92 ± 7.38 | 11/1-83 | p = 0.349 |
| Fever | 130 (24.3%) | 12.42 ± 8.15 | 12/1-63 | p = 0.007 |
| Vomiting | 115 (21.6%) | 11.81 ± 10.25 | 12/1-83 | p = 0.136 |
| Abdominal pain | 394 (73.8%) | 10.59 ± 7.55 | 10/1-83 | p = 0.098 |
| Demographic data | ||||
| Male Female | 252 (47.2%) 282 (52.8%) | 11.03 ± 6.55 10.79 ± 8.06 | 11/1-43 11/1-83 | p = 0.711 |
| Rural Urban | 249 (46.6%) 285 (53.4%) | 11.13 ± 7.14 10.71 ± 7.58 | 11/1-63 11/1-83 | p = 0.508 |
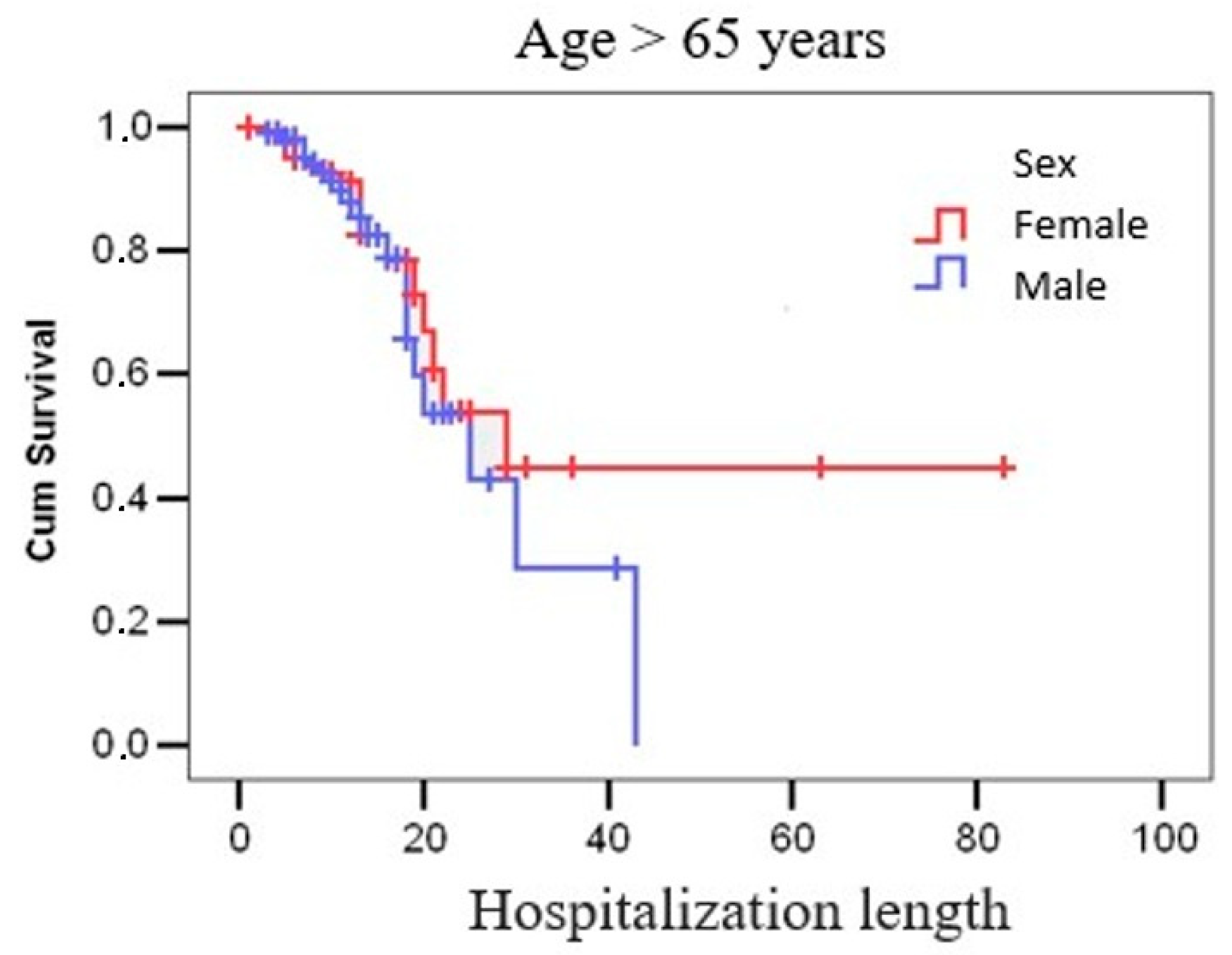
| ≤65 years >65 years | 301 (56.4%) 233 (43.6%) | 9.98 ± 6.27 12.10 ± 8.46 | 10/1-46 12/1-83 | p = 0.001 |
| Comorbidities | Toxin A + GDH n = 89 | Toxin B + GDH n = 23 | Toxin A + B + GDH n = 422 | Chi2 Test |
|---|---|---|---|---|
| Gastrointestinal, n (%) | 7 (7.9%) | 6 (26.1%) | 98 (23.2%) | p = 0.001 |
| Cardiovascular, n (%) | 12 (13.5%) | 5 (21.7%) | 108 (25.6%) | p = 0.036 |
| Pulmonary, n (%) | 5 (5.6%) | 2 (8.7%) | 42 (10.0%) | p = 0.396 |
| Renal, n (%) | 4 (4.5%) | 6 (26.1%) | 42 (10.0%) | p = 0.015 |
| Hemodialysis, n (%) | 2 (2.2%) | 1 (4.3%) | 9 (2.1%) | p = 0.823 |
| Neurological, n (%) | 9 (10.1%) | 7 (30.4%) | 54 (12.8%) | p = 0.065 |
| Psychiatric, n (%) | 5 (5.6%) | 2 (8.7%) | 21 (5.0%) | p = 0.759 |
| Diabetes, n (%) | 11 (12.4%) | 6 (26.1%) | 65 (15.4%) | p = 0.301 |
| Oncological, n (%) | 18 (20.2%) | 5 (21.7%) | 50 (11.8%) | p = 0.073 |
| Obesities, n (%) | 10 (11.2%) | 1 (4.3%) | 29 (6.9%) | p = 0.333 |
| Laboratory parameters | ||||
| Inflammatory syndrome, n (%) | 82 (92.1%) | 22 (95.7%) | 374 (88.6%) | p = 0.331 |
| Elevated transaminases, n (%) | 17 (19.1%) | 11 (47.8%) | 100 (23.7%) | p = 0.025 |
| Kidney failure, n (%) | 27 (30.3%) | 10 (43.5%) | 114 (27.0%) | p = 0.231 |
| Evolution | Toxin A + GDH n = 89 | Toxin B + GDH n = 23 | Toxin A + B + GDH n = 422 | Chi2 Square Test |
|---|---|---|---|---|
| Favorable outcome, n (%) | 85 (95.5%) | 22 (95.7%) | 377 (89.3%) | p = 0.097 |
| FMT, n (%) | 1 (1.1%) | 0 (0.0%) | 8 (1.9%) | p = 0.582 |
| Recurrence, n (%) | 17 (19.1%) | 3 (13.0%) | 128 (30.3%) | p = 0.020 |
| Mortality, n (%) | 4 (4.5%) | 1 (4.3%) | 45 (10.7%) | p = 0.097 |
| Evolution | Metronidazole | Chi2 Test | |||
|---|---|---|---|---|---|
| Yes (n = 245) | No (n = 289) | ||||
| n | % | n | % | ||
| Favorable outcome | 228 | 93.1 | 256 | 88.6 | p = 0.050 |
| FMT | 3 | 1.2 | 6 | 2.1 | p = 0.340 |
| Recurrence | 42 | 17.1 | 106 | 36.7 | p = 0.001 |
| Mortality | 17 | 6.9 | 33 | 11.4 | p = 0.050 |
| Evolution | Vancomycin | Chi2 Test | |||
| Yes (n = 369) | No (n = 165) | ||||
| n | % | n | % | ||
| Favorable outcome | 326 | 88.3 | 158 | 95.8 | p = 0.004 |
| FMT | 8 | 2.2 | 1 | 0.6 | p = 0.178 |
| Recurrence | 133 | 36.0 | 15 | 9.1 | p = 0.001 |
| Mortality | 43 | 11.7 | 7 | 4.2 | p = 0.004 |
| Evolution | Vancomycin + Metronidazole | Chi2 Test | |||
| Yes (n = 80) | No (n = 454) | ||||
| n | % | n | % | ||
| Favorable outcome | 70 | 87.5 | 414 | 91.2 | p = 0.198 |
| FMT | 2 | 2.5 | 7 | 1.5 | p = 0.401 |
| Recurrence | 27 | 33.8 | 121 | 26.7 | p = 0.121 |
| Mortality | 10 | 12.5 | 40 | 8.8 | p = 0.198 |
| Toxin Type | Recurrence Rate (%) | Treatment Used | Mortality Rate (%) | Favorable Outcome (%) | ESCMID 2014 Recommendations |
|---|---|---|---|---|---|
| A + GDH n = 89 | 19.1 | Vancomycin 65.1% Metronidazole 51.6% Metronidazole + Vancomycin 17.9% | 4.5 | 95.5 | NON-SEVERE DISEASE - Metronidazole orally 500 mg × 3 daily for 10 days - Vancomycin orally 125 mg × 4 daily for 10 days - Fidaxomicin orally 200 mg × 2 daily for 10 days SEVERE DISEASE - Vancomycin orally 125 mg × 4 daily for 10 days - Fidaxomicin orally 200 mg × 2 daily for 10 days |
| B + GDH n = 23 | 13.0 | Vancomycin 69.5% Metronidazole 39.1% Metronidazole + Vancomycin 8.6% | 4.3 | 95.7 | |
| A + B + GDH n = 422 | 30.3 | Vancomycin 70% Metronidazole 45% Metronidazole + Vancomycin 14.6% | 10.7 | 89.3 |
| Characteristics | Metronidazole (n = 165) | Vancomycin (n = 289) | Vancomycin + Metronidazole (n = 80) | CI 95% | p-Value |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 55 ± 16.2 | 61 ± 15.8 | 61 ± 14.9 | −8.9–3.1 | <0.001 |
| Hospitalization, days (mean ± SD) | 7.9 ± 4.3 | 12.3 ± 6.8 | 12.0 ± 6.5 | −9.8–2.2 | <0.001 |
| Characteristics | Metronidazole (N = 165) | Vancomycin (N = 289) | Vancomycin + Metronidazole (N = 80) | Chi2 Test p-Value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 82 (49.7%) | 157 (54.3%) | 43 (53.8%) | 0.624 |
| Male | 83 (50.3%) | 132 (45.7%) | 37 (46.2%) | |
| Environment, n (%) | ||||
| Urban | 98 (59.4%) | 147 (50.9%) | 40 (50.0%) | 0.181 |
| Rural | 67 (40.6%) | 142 (49.1%) | 40 (50.0%) | |
| Clinical symptoms | ||||
| Fever | 35 (21.2%) | 80 (27.7%) | 15 (18.8%) | 0.099 |
| Vomiting | 32 (19.4%) | 69 (23.9%) | 16 (20.0%) | 0.470 |
| Abdominal pain | 133 (80.6%) | 198 (68.5%) | 62 (77.5%) | 0.013 |
| Comorbidities | ||||
| Gastroenterological | 35 (21.2%) | 59 (20.4%) | 17 (21.3%) | 0.976 |
| Cardiovascular | 24 (14.5%) | 91 (31.5%) | 11 (13.8%) | <0.001 |
| Pulmonary | 11 (6.7%) | 32 (11.1%) | 6 (7.5%) | 0.238 |
| Renal | 11 (6.7%) | 32 (11.1%) | 10 (12.5%) | 0.170 |
| Hemodialysis | 4 (2.4%) | 9 (3.1%) | 0 (0%) | 0.311 |
| Neurological | 20 (12.1%) | 42 (14.5%) | 7 (8.8%) | 0.371 |
| Psychiatric | 5 (3.0%) | 16 (5.5%) | 6 (7.5%) | 0.197 |
| Diabetes | 17 (10.3%) | 50 (17.3%) | 15 (18.8%) | 0.077 |
| Oncological | 16 (9.7%) | 48 (16.6%) | 10 (12.5%) | 0.102 |
| Obesity | 5 (3.0%) | 29 (10.0%) | 6 (7.5%) | 0.019 |
| Logistic Regression Models Deceased, Age > 65 Years Assessed Variables | Odds Ratio (OR) | 95% CI | p Value |
|---|---|---|---|
| Pulmonary | 5.344 | 3.664–7.795 | 0.001 |
| Pulmonary | 2.858 | 1.573–5.195 | 0.001 |
| Renal | 2.200 | 1.194–4.053 | 0.011 |
| Pulmonary | 2.667 | 1.432–5.006 | 0.002 |
| Renal | 1.798 | 0.807–4.007 | 0.151 |
| Diabetes | 1.349 | 0.636–2.860 | 0.436 |
| Asymptotic 95% Confidence Interval | |||||
|---|---|---|---|---|---|
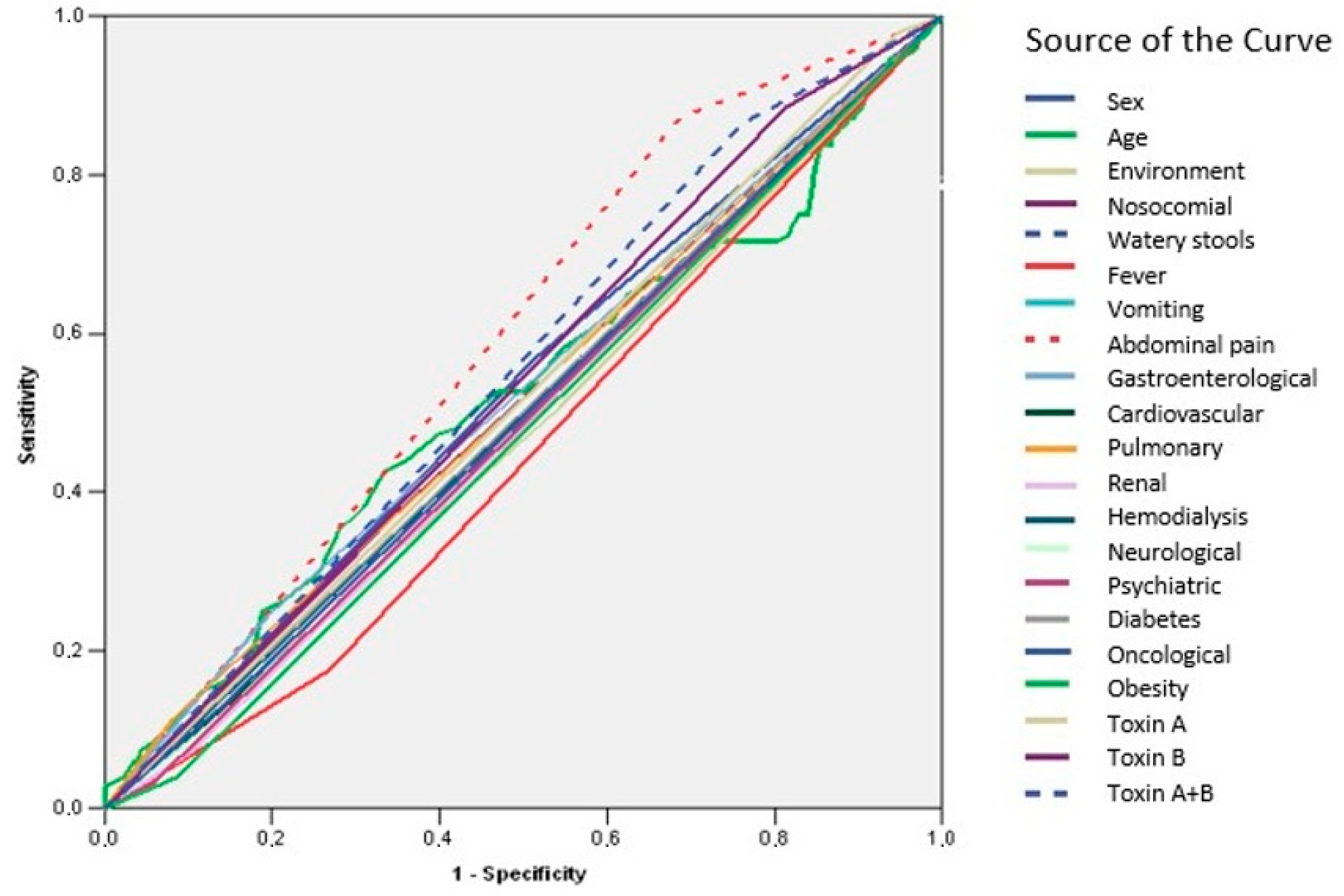
| Test Result Variable(s) | Area | Std. Error | Asymptotic Sig. | Lower Bound | Upper Bound |
| Sex | 0.527 | 0.028 | 0.328 | 0.473 | 0.582 |
| Age | 0.519 | 0.029 | 0.507 | 0.462 | 0.576 |
| Environment | 0.481 | 0.028 | 0.505 | 0.427 | 0.536 |
| Nosocomial | 0.513 | 0.028 | 0.654 | 0.458 | 0.567 |
| Watery stools | 0.501 | 0.028 | 0.963 | 0.447 | 0.556 |
| Fever | 0.453 | 0.027 | 0.093 | 0.400 | 0.506 |
| Vomiting | 0.490 | 0.028 | 0.719 | 0.435 | 0.544 |
| Abdominal pain | 0.593 | 0.026 | 0.001 | 0.541 | 0.644 |
| Gastroenterological | 0.524 | 0.028 | 0.381 | 0.469 | 0.580 |
| Cardiovascular | 0.488 | 0.028 | 0.658 | 0.433 | 0.542 |
| Pulmonary | 0.516 | 0.028 | 0.567 | 0.461 | 0.571 |
| Renal | 0.484 | 0.028 | 0.568 | 0.430 | 0.538 |
| Hemodialysis | 0.498 | 0.028 | 0.957 | 0.444 | 0.553 |
| Neurological | 0.489 | 0.028 | 0.688 | 0.434 | 0.543 |
| Psychiatrics | 0.487 | 0.028 | 0.644 | 0.433 | 0.541 |
| Diabetes | 0.501 | 0.028 | 0.964 | 0.446 | 0.556 |
| Oncological | 0.494 | 0.028 | 0.837 | 0.440 | 0.549 |
| Obesity | 0.476 | 0.027 | 0.395 | 0.423 | 0.530 |
| Toxin A | 0.516 | 0.028 | 0.572 | 0.462 | 0.570 |
| Toxin B | 0.536 | 0.027 | 0.200 | 0.482 | 0.589 |
| Toxin A + B | 0.552 | 0.027 | 0.065 | 0.499 | 0.604 |
| Asymptotic 95% Confidence Interval | |||||
|---|---|---|---|---|---|
| Test Result Variable(s) | Area | Std. Error | Asymptotic Sig. | Lower Bound | Upper Bound |
| Sex | 0.451 | 0.043 | 0.258 | 0.368 | 0.535 |
| Age | 0.798 | 0.027 | 0.000 | 0.745 | 0.852 |
| Environment | 0.492 | 0.043 | 0.860 | 0.408 | 0.577 |
| Nosocomial | 0.562 | 0.043 | 0.147 | 0.477 | 0.647 |
| Watery stools | 0.501 | 0.043 | 0.981 | 0.417 | 0.585 |
| Fever | 0.476 | 0.042 | 0.577 | 0.394 | 0.558 |
| Vomiting | 0.435 | 0.039 | 0.132 | 0.358 | 0.513 |
| Abdominal pain | 0.457 | 0.044 | 0.317 | 0.371 | 0.543 |
| Gastroenterological | 0.485 | 0.042 | 0.720 | 0.402 | 0.567 |
| Cardiovascular | 0.547 | 0.044 | 0.269 | 0.461 | 0.634 |
| Pulmonary | 0.549 | 0.045 | 0.257 | 0.460 | 0.637 |
| Renal | 0.579 | 0.046 | 0.067 | 0.489 | 0.669 |
| Hemodialysis | 0.510 | 0.044 | 0.822 | 0.424 | 0.595 |
| Neurological | 0.505 | 0.043 | 0.909 | 0.420 | 0.590 |
| Psychiatrics | 0.493 | 0.042 | 0.873 | 0.410 | 0.576 |
| Diabetes | 0.581 | 0.045 | 0.060 | 0.492 | 0.670 |
| Oncological | 0.535 | 0.044 | 0.416 | 0.448 | 0.622 |
| Obesity | 0.459 | 0.040 | 0.336 | 0.380 | 0.537 |
| Toxin A | 0.513 | 0.042 | 0.767 | 0.430 | 0.595 |
| Toxin B | 0.548 | 0.040 | 0.265 | 0.469 | 0.627 |
| Toxin A + B | 0.561 | 0.040 | 0.158 | 0.483 | 0.638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stămăteanu, L.O.; Miftode, I.L.; Pleşca, C.E.; Hurmuzache, M.E.; Manciuc, D.C.; Leca, D.; Miftode, E.G. Healthcare-Associated Clostridioides difficile Infection: A Hospital-Based Retrospective Study in North Eastern Romania. Microorganisms 2025, 13, 1377. https://doi.org/10.3390/microorganisms13061377
Stămăteanu LO, Miftode IL, Pleşca CE, Hurmuzache ME, Manciuc DC, Leca D, Miftode EG. Healthcare-Associated Clostridioides difficile Infection: A Hospital-Based Retrospective Study in North Eastern Romania. Microorganisms. 2025; 13(6):1377. https://doi.org/10.3390/microorganisms13061377
Chicago/Turabian StyleStămăteanu, Lidia Oana, Ionela Larisa Miftode, Claudia Elena Pleşca, Mihnea Eudoxiu Hurmuzache, Doina Carmen Manciuc, Daniela Leca, and Egidia Gabriela Miftode. 2025. "Healthcare-Associated Clostridioides difficile Infection: A Hospital-Based Retrospective Study in North Eastern Romania" Microorganisms 13, no. 6: 1377. https://doi.org/10.3390/microorganisms13061377
APA StyleStămăteanu, L. O., Miftode, I. L., Pleşca, C. E., Hurmuzache, M. E., Manciuc, D. C., Leca, D., & Miftode, E. G. (2025). Healthcare-Associated Clostridioides difficile Infection: A Hospital-Based Retrospective Study in North Eastern Romania. Microorganisms, 13(6), 1377. https://doi.org/10.3390/microorganisms13061377








