Enhancing the Catalytic Performance of PdNPs for Cr(VI) Reduction by Increasing Pd(0) Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungus Culture
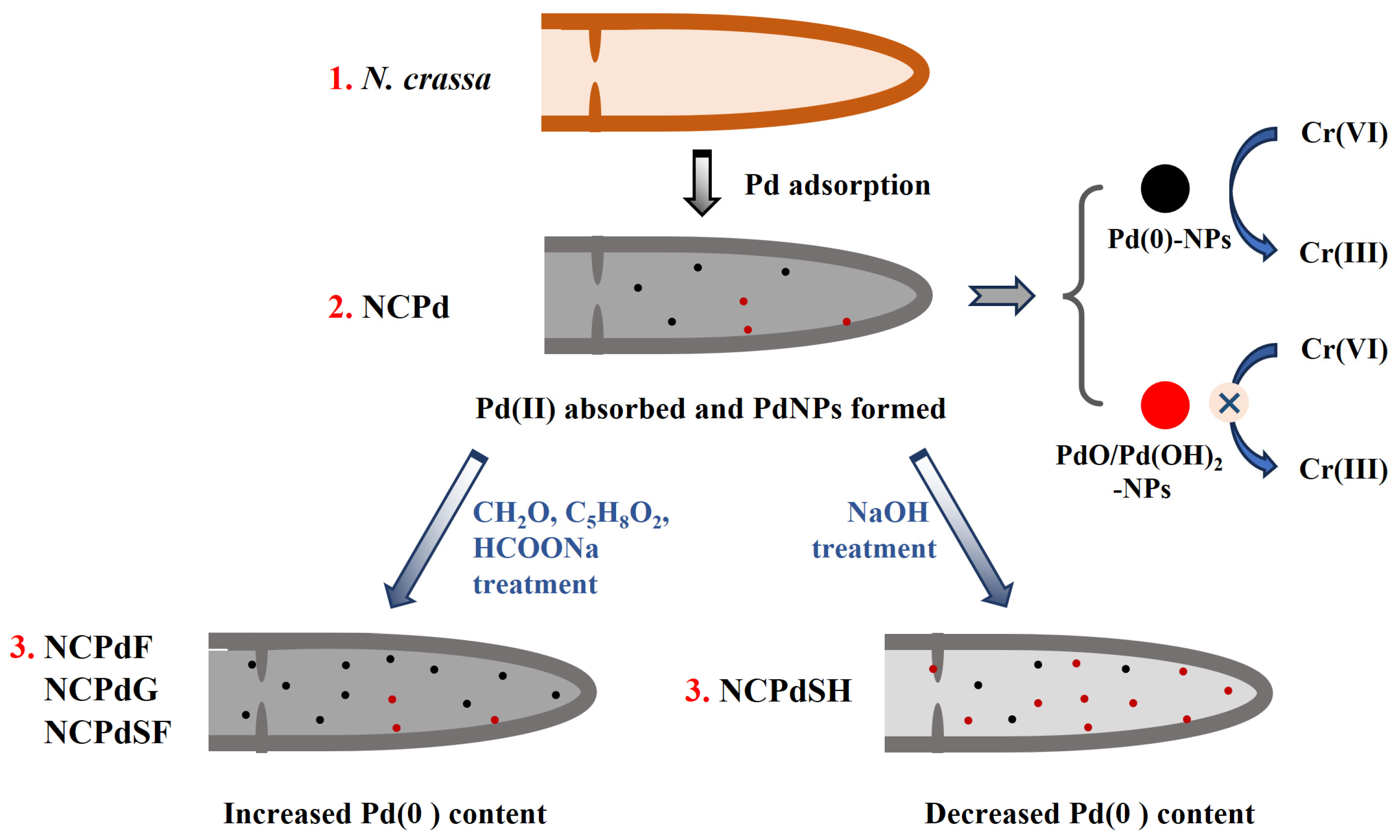
2.2. Pd-Loaded Sample Preparation
- Pd-absorbed N. crassa (NCPd): The biomass was freeze-dried.
- Formaldehyde-treated N. crassa (NCPdF): The biomass was suspended in 50 mL of Milli-Q water, and 12 mL of formaldehyde (37–40% concentration) was added to the suspension. The mixture was incubated in 30 °C with agitation at 220 rpm for 12 h. After incubation, the biomass was filtered, washed twice with Milli-Q water, and freeze-dried for subsequent use.
- Glutaraldehyde-treated N. crassa (NCPdG): The biomass was suspended in 50 mL Milli-Q water, and 2.5 mL of glutaraldehyde (50% concentration) was added. The mixture was incubated in 30 °C with agitation at 220 rpm for 12 h. Afterward, the biomass was filtered, washed twice with Milli-Q water, and freeze-dried for subsequent use.
- Sodium-hydroxide-treated N. crassa (NCPdSH): The biomass was suspended in 50 mL Milli-Q water. Then, 2 mL of sodium hydroxide at concentration of 0.3 M was slowly dropped into the suspension under gentle magnetic stirring conditions. The mixture was incubated in 30 °C with agitation at 220 rpm for 12 h. After incubation, the biomass was filtered, washed twice with Milli-Q water, and freeze-dried for subsequent use.
- Sodium-formate-treated N. crassa (NCPdSF): The biomass was suspended in 50 mL degassed sodium formate (25 mM). The mixture was transferred to a serum bottle and flushed with N2 for 1 h to drive off the dissolved O2 then sealed with butyl rubber stopper. The mixture was incubated in 30 °C with agitation at 220 rpm for 12 h. After incubation, the biomass was filtered, washed twice with Milli-Q water, and freeze-dried for subsequent use.
2.3. Quantitative Analysis of Pd in Catalysts
2.4. SEM, TEM, XPS, and FTIR Analyses
2.5. Catalytic Performance of Pd-Loaded Fungus in Cr(VI) Reduction
3. Results and Discussion
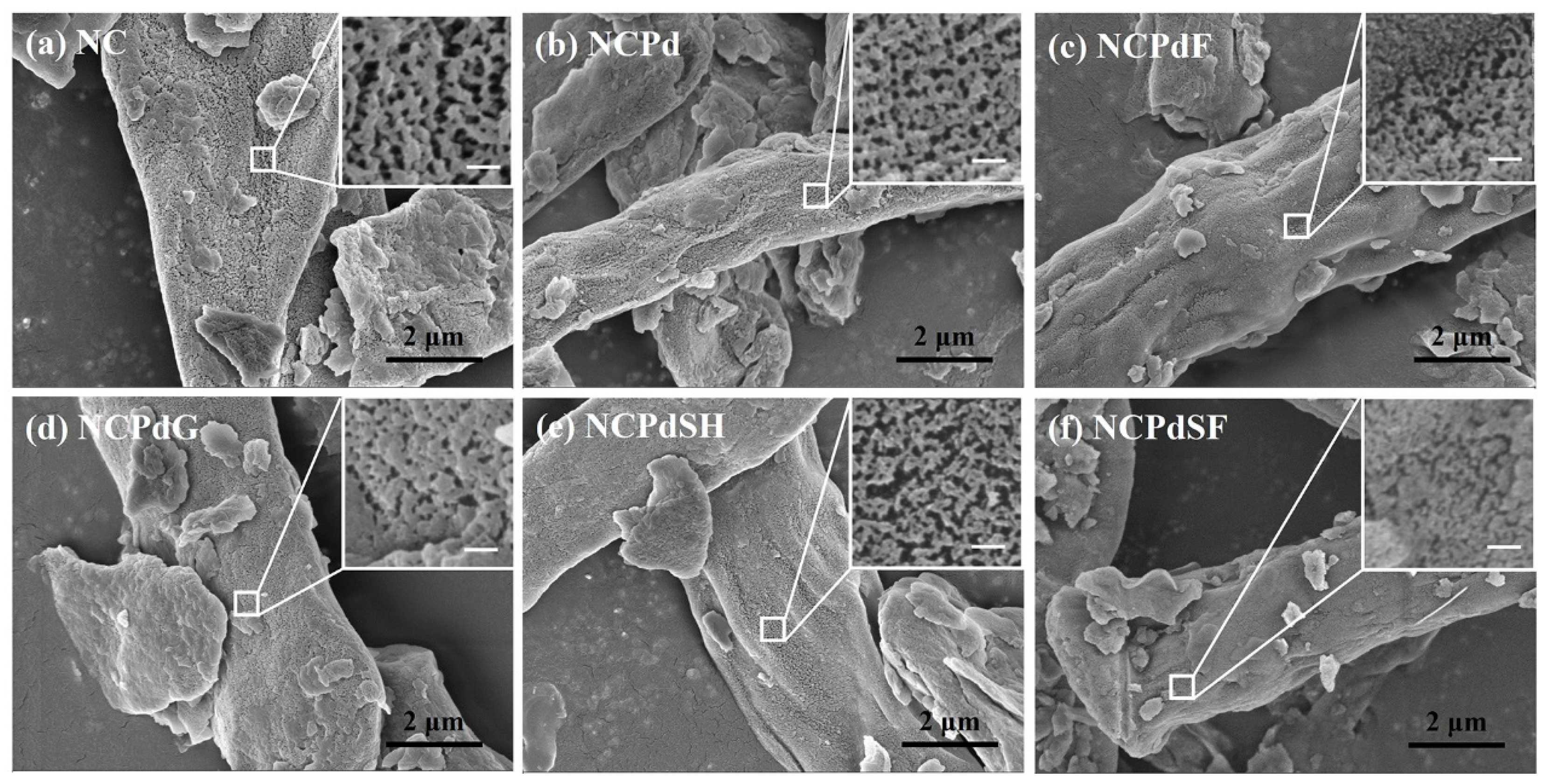
3.1. SEM Analysis—Morphological Characteristics
3.2. TEM—PdNPs in Whole Fungal Cells
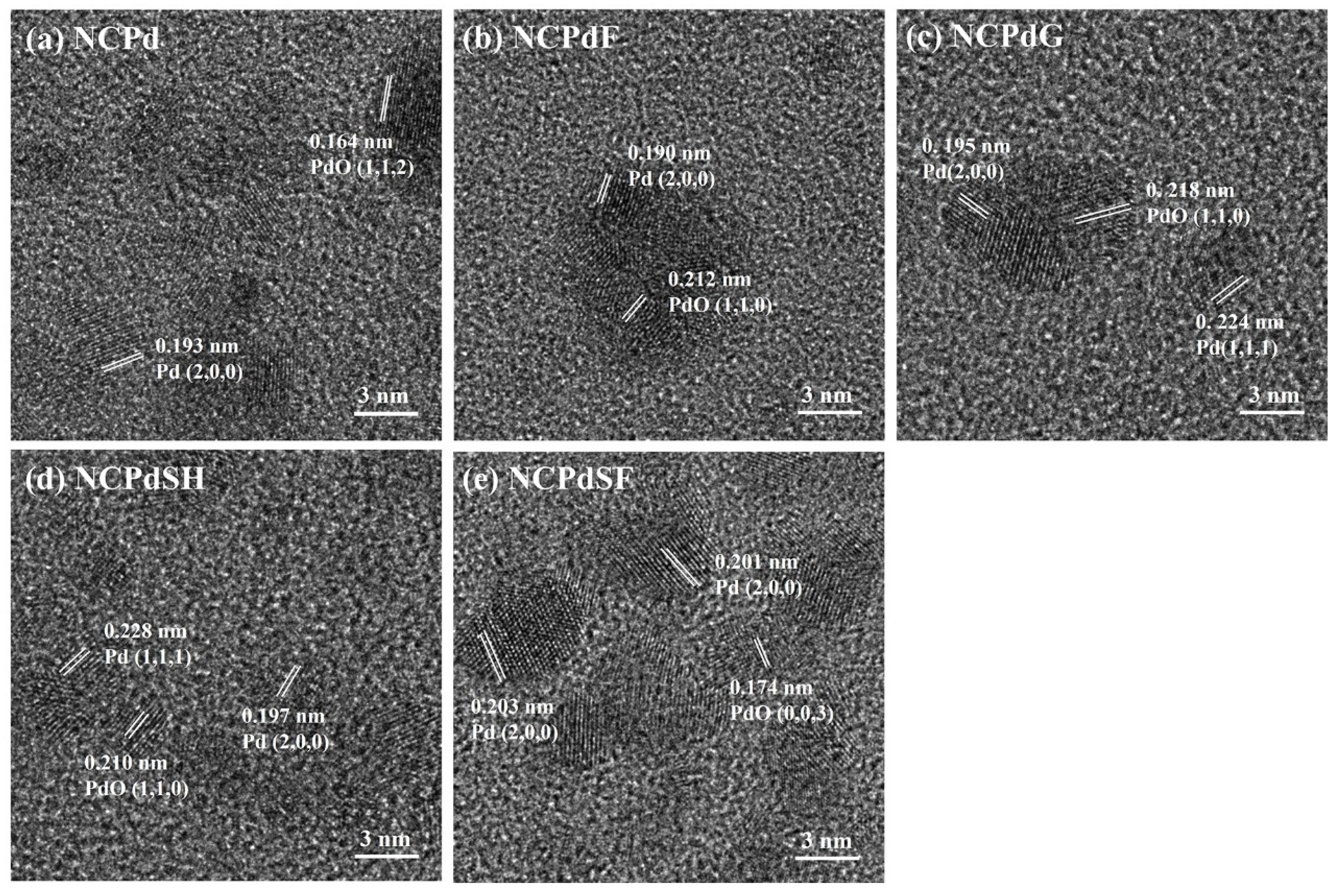
3.3. HRTEM—PdNP Crystal Structure in Whole Fungal Cells
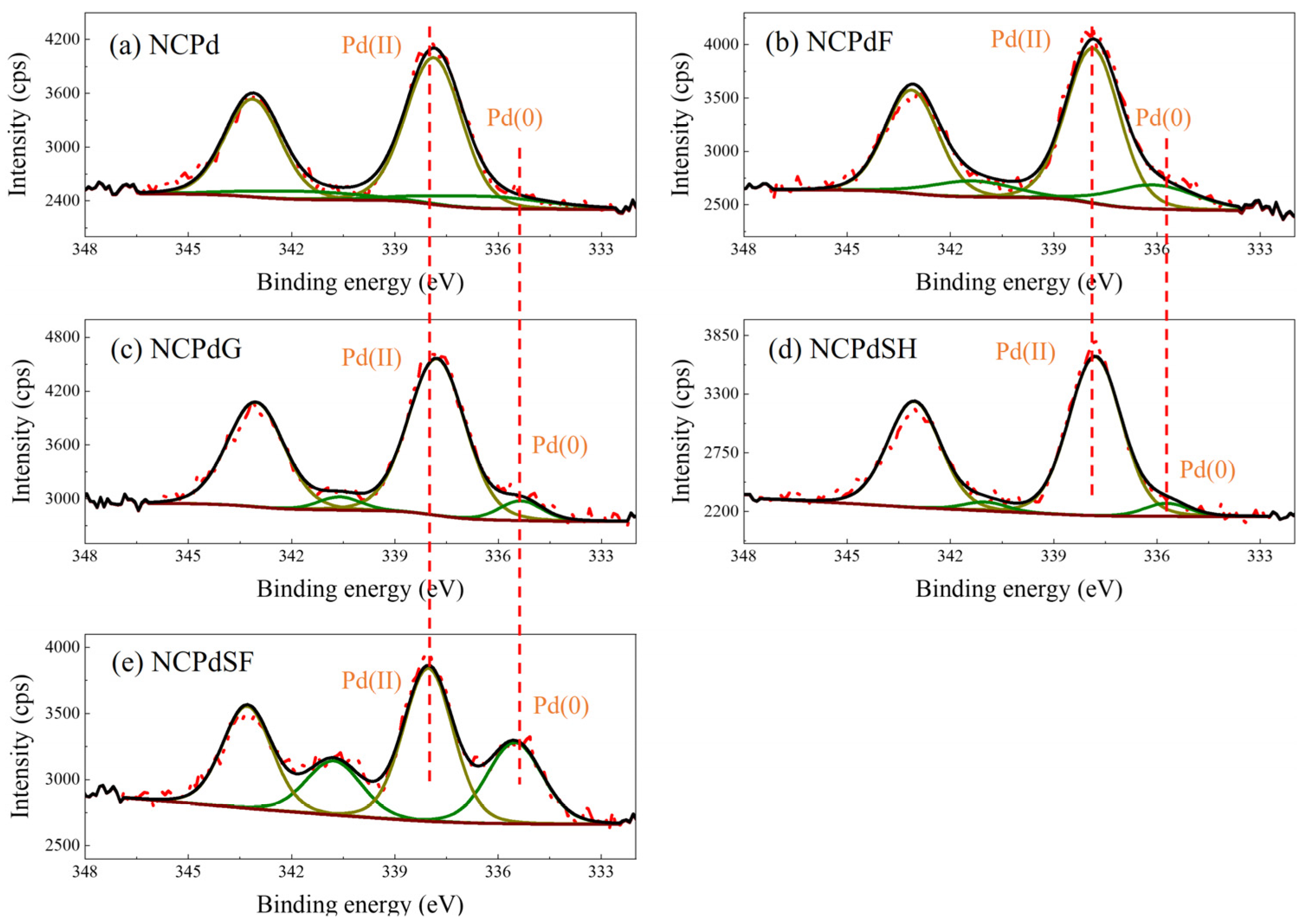
3.4. XPS—Pd Chemical State Analysis
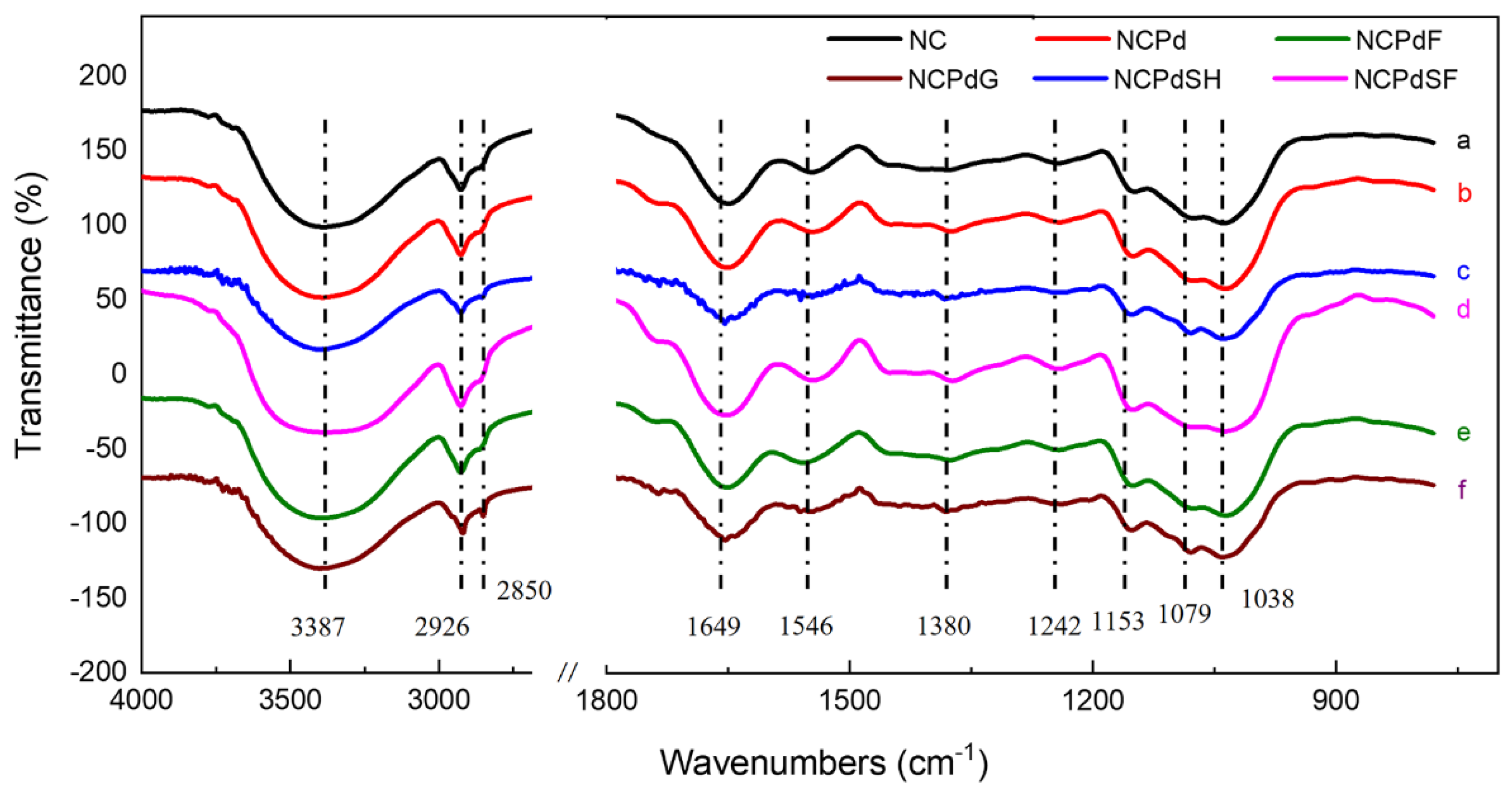
3.5. FTIR—Functional Groups Related to Pd(II) Absorption and Reduction
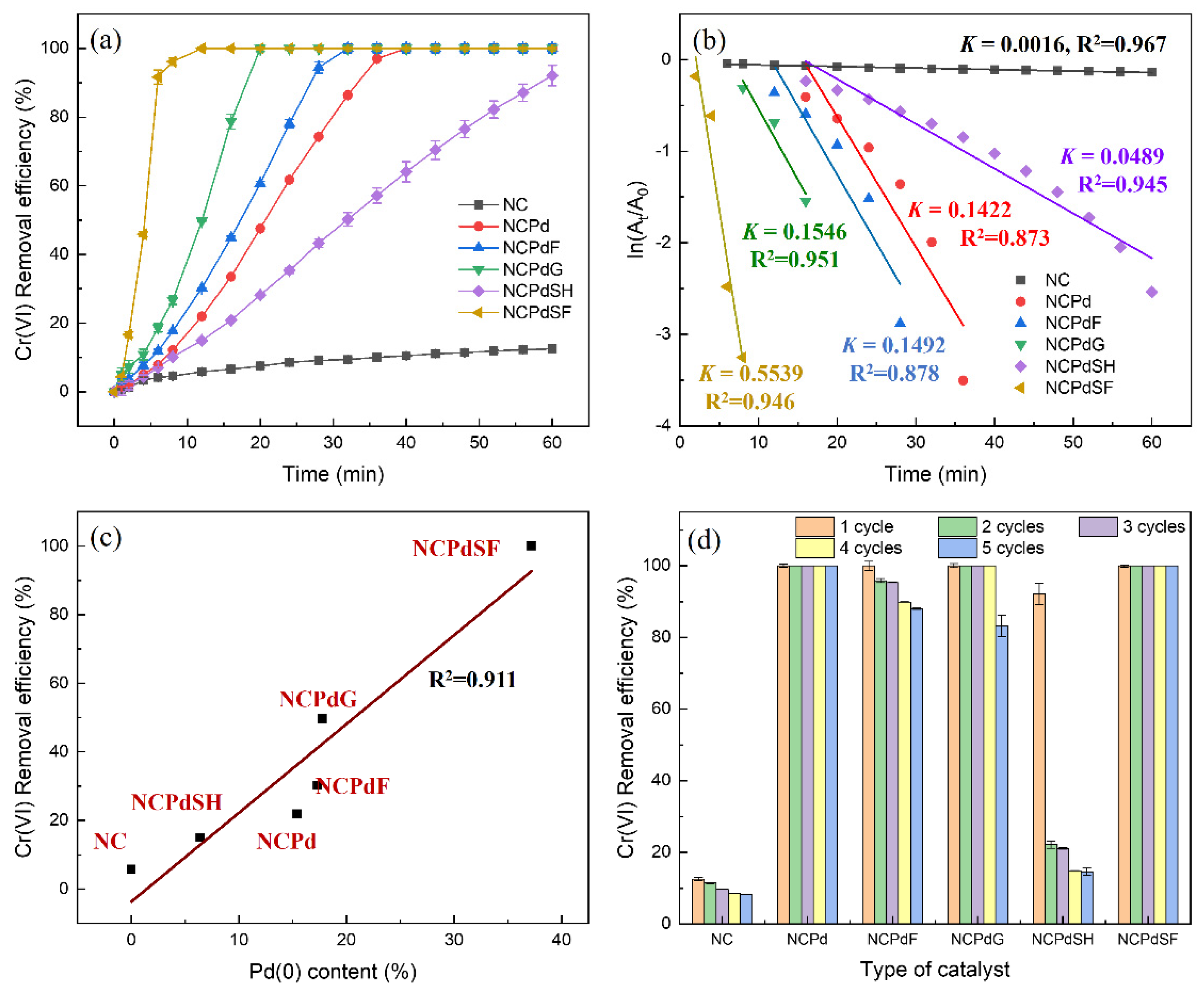
3.6. Catalytic Activity of Pd-Loaded Biomass in Cr(VI) Reduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouhaninezhad, A.A.; Hojati, S.; Masir, M.N. Adsorption of Cr (VI) onto micro- and nanoparticles of palygorskite in aqueous solutions: Effects of pH and humic acid. Ecotoxicol. Environ. Saf. 2020, 206, 111247. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef] [PubMed]
- Maamoun, I.; Falyouna, O.; Eljamal, R.; Bensaida, K.; Tanaka, K.; Tosco, T.; Sugihara, Y.; Eljamal, O. Multi-functional magnesium hydroxide coating for iron nanoparticles towards prolonged reactivity in Cr(VI) removal from aqueous solutions. J. Environ. Chem. Eng. 2022, 10, 107431. [Google Scholar] [CrossRef]
- Bashir, M.S.; Ramzan, N.; Najam, T.; Abbas, G.; Gu, X.; Arif, M.; Qasim, M.; Bashir, H.; Shah, S.S.A.; Sillanpää, M. Metallic nanoparticles for catalytic reduction of toxic hexavalent chromium from aqueous medium: A state-of-the-art review. Sci. Total Environ. 2022, 829, 154475. [Google Scholar] [CrossRef]
- Jawed, A.; Golder, A.K.; Pandey, L.M. Synthesis of iron oxide nanoparticles mediated by Camellia sinensis var. Assamica for Cr(VI) adsorption and detoxification. Bioresour. Technol. 2023, 376, 128816. [Google Scholar] [CrossRef]
- Zhou, N.; Gong, K.; Hu, Q.; Cheng, X.; Zhou, J.; Dong, M.; Wang, N.; Ding, T.; Qiu, B.; Guo, Z. Optimizing nanocarbon shell in zero-valent iron nanoparticles for improved electron utilization in Cr(VI) reduction. Chemosphere 2020, 242, 125235. [Google Scholar] [CrossRef]
- Bahador, F.; Foroutan, R.; Esmaeili, H.; Ramavandi, B. Enhancement of the chromium removal behavior of Moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohydr. Polym. 2021, 251, 117085. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Morales, G.; Castro-Ruiz, A.; Rodríguez-Tobías, H.; Abraham, G.A.; Rivero, G.; Lozano-Morales, S.A. Photoc atalytic reduction of hexavalent chromium ions from aqueous solutions using polymeric microfibers surface modified with ZnO nanoparticles. Fibers Polym. 2021, 22, 3271–3280. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Basaran, S.T.; Kutlar, F.S.; Sahinkaya, E. High concentration hexavalent chromium removal performance of a sulfidogenic activated carbon-bed bioreactor at moderate temperature. J. Water Process Eng. 2021, 42, 102162. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent advances in removal techniques of Cr(VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.C.; Chen, G. Comparative study of various magnetic nanoparticles for Cr(VI) removal. Sep. Purif. Technol. 2007, 56, 249–256. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Self-assembled silver nanoparticles decorated on exfoliated graphitic carbon nitride/carbon sphere nanocomposites as a novel catalyst for catalytic reduction of Cr(VI) to Cr(III) from wastewater and reuse for photocatalytic applications. ACS Omega 2021, 6, 35221–35243. [Google Scholar] [CrossRef]
- Lv, Z.; Tan, X.; Wang, C.; Alsaedi, A.; Hayat, T.; Chen, C. Metal-organic frameworks-derived 3D yolk shell-like structure Ni@carbon as a recyclable catalyst for Cr (VI) reduction. Chem. Eng. J. 2020, 389, 123428. [Google Scholar] [CrossRef]
- Xu, T.; Xue, J.; Zhang, X.; He, G.; Chen, H. Ultrafine cobalt nanoparticles supported on reduced graphene oxide: Efficient catalyst for fast reduction of hexavalent chromium at room temperature. Appl. Surf. Sci. 2017, 402, 294–300. [Google Scholar] [CrossRef]
- Liu, W.J.; Ling, L.L.; Wang, Y.Y.; He, H.; He, Y.R.; Yu, H.Q.; Jiang, H. One-pot high yield synthesis of Ag nanoparticle-embedded biochar hybrid materials from waste biomass for catalytic Cr(VI) reduction. Environ. Sci. Nano 2016, 3, 745–753. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mahmoudi-Gom Yek, S.; Motahharifar, N.; Ghafori Gorab, M. Recent Developments in the Plant-Mediated Green Synthesis of Ag-Based Nanoparticles for Environmental and Catalytic Applications. Chem. Rec. 2019, 19, 2436–2479. [Google Scholar] [CrossRef]
- Tan, L.; Jones, T.R.; Poitras, J.; Xie, J.; Liu, X.; Southam, G. Biochemical synthesis of palladium nanoparticles: The influence of chemical fixatives used in electron microscopy on nanoparticle formation and catalytic performance. J. Hazard. Mater. 2020, 398, 122945. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Liu, R. Flash nanoprecipitation of polymer supported Pt colloids with tunable catalytic chromium reduction property. Colloid Polym. Sci. 2018, 296, 327–333. [Google Scholar] [CrossRef]
- Fu, G.T.; Jiang, X.; Wu, R.; Wei, S.H.; Sun, D.M.; Tang, Y.W.; Lu, T.H.; Chen, Y. Arginine-assisted synthesis and catalytic properties of single-crystalline palladium tetrapods. ACS Appl. Mater. Interfaces 2014, 6, 22790–22795. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, P.; Thanasekaran, P.; Lin, K.C.; Liu, S.B. Biomass derived sheet-like carbon/palladium nanocomposite: An excellent opportunity for reduction of toxic hexavalent chromium. ACS Sustain. Chem. Eng. 2017, 5, 5302–5312. [Google Scholar] [CrossRef]
- Law, C.K.Y.; Bonin, L.; Gusseme, B.D.; Boon, N.; Kundu, K. Biogenic synthesis of palladium nanoparticles: New production methods and applications. Nanotechnol. Rev. 2022, 11, 3104–3124. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Kang, N.; Ha, C.; Shi, C.; Wu, P. Biorecovery mechanism of palladium as nanoparticles by Enterococcus faecalis: From biosorption to bioreduction. Chem. Eng. J. 2017, 328, 1051–1057. [Google Scholar] [CrossRef]
- Tan, L.; Xiao, Y.; Cui, H.; Xu, H.; Xu, M.; Wu, H.; Dong, H.; Qiu, G.; Liu, X.; Xie, J. Influence of sulfhydryl sites on palladium (II) adsorption by displaying EC20 on the surface of Escherichia coli. J. Chem. Technol. Biotechnol. 2018, 93, 3569–3581. [Google Scholar] [CrossRef]
- Matsena, M.T.; Chirwa, E.M.N. Comparative analysis of biological versus chemical synthesis of palladium nanoparticles for catalysis of chromium (VI) reduction. Sci. Rep. 2021, 11, 16674. [Google Scholar] [CrossRef]
- Tan, L.; Dong, H.; Liu, X.; He, J.; Xu, H.; Xie, J. Mechanism of palladium(Ⅱ) biosorption by Providencia vermicola. RSC Adv. 2017, 7, 7060–7072. [Google Scholar] [CrossRef]
- Tan, L.; Liu, X.; Zhang, Y. Glutaraldehyde fixation promotes palladium and gold nanoparticles formation in yeast and enhances their catalytic activity in 4-nitrophenol reduction. J. Hazard. Mater. 2023, 446, 130696. [Google Scholar] [CrossRef]
- Deplanche, K.; Bennett, J.A.; Mikheenko, I.P.; Omajali, J.; Wells, A.S.; Meadows, R.E.; Wood, J.; Macaskie, L.E. Catalytic activity of biomass-supported Pd nanoparticles: Influence of the biological component in catalytic efficacy and potential application in ‘green’ synthesis of fine chemicals and pharmaceuticals. Appl. Catal. B Environ. 2014, 147, 651–665. [Google Scholar] [CrossRef]
- Li, W.B.; Wu, K.; Feng, H.; Wang, N.; Zhang, J.H.; Wang, J.J.; Li, X.F. Atomic layer deposition of ultrafine Pd nanoparticles for enhancing the rate capability of LiNi0.8Co0.1Mn0.1O2 cathode. Tungsten 2022, 4, 346–355. [Google Scholar] [CrossRef]
- Xiong, J.; Mo, S.; Song, L.; Fu, M.; Chen, P.; Wu, J.; Chen, L.; Ye, D. Outstanding stability and highly efficient methane oxidation performance of palladium-embedded ultrathin mesoporous Co2MnO4 spinel catalyst. Appl. Catal. A Gen. 2020, 598, 117571. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, C.; Lei, Z.; Chen, B.; Fang, X. Synthesis of hydrogen peroxide over Pd/SiO2/COR monolith catalysts by anthraquinone method. Catal. Today 2016, 276, 36–45. [Google Scholar] [CrossRef]
- Kim, J.; Reddy, D.A.; Ma, R.; Kim, T.K. The influence of laser wavelength and fluence on palladium nanoparticles produced by pulsed laser ablation in deionized water. Solid State Sci. 2014, 37, 96–102. [Google Scholar] [CrossRef]
- Tan, L.; Long, C.; Lai, H.; Huo, X.; Yu, W.; Wei, G.; Tong, T.; Tian, C. Biochar: Preserving the long-term catalytic activity of biosynthesized PdNPs/AuNPs in Cr(VI) reduction. J. Anal. Appl. Pyrolysis 2024, 183, 106816. [Google Scholar] [CrossRef]
- Lin, H.Q.; Chen, Y.W. Complete oxidation of toluene on Pd/modified-CeO2 catalysts. J. Taiwan Inst. Chem. Eng. 2016, 67, 69–73. [Google Scholar] [CrossRef]
- Altamirano-Gutiérrez, A.; Fernández, A.M.; Aruna, K.K.; Manoharan, R.; Karthikeyan, P.; Siller-Ceniceros, A.; Meléndez-González, P.; Bartolo-Pérez, P.; Rodríguez-Varela, F.J. Evaluation of supported and unsupported Pd–CeO2 nanostructured anode electrocatalysts for the formic acid and the glycerol oxidation reactions in acid media. J. Appl. Electrochem. 2015, 45, 1195–1204. [Google Scholar] [CrossRef]
- Dutt, S.S.; Gangapuram, B.R.; Ahmed, I.; Jakka, S.R.; Mardi, R.K.R.; Seku, K. Ecofriendly synthesis of PdNPs using Eupatorium adenophorum leaf extract and their catalytic properties. Res. Chem. Intermed. 2024, 50, 4447–4464. [Google Scholar] [CrossRef]
- Kumar, N.S.; Min, K. Phenolic compounds biosorption onto Schizophyllum commune fungus: FTIR analysis, kinetics and adsorption isotherms modeling. Chem. Eng. J. 2011, 168, 562–571. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Bhattacharjee, N.; Ganguly, S. Evidences for the augmented Cd (II) biosorption by Cd (II) resistant strain Candida tropicalis XTA1874 from contaminated aqueous medium. Sci. Rep. 2023, 13, 12034. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Varjani, S.; Yaashikaa, P.R.; Jeevanantham, S.; Ramamurthy, R.; Reshma, B. Simultaneous removal of Cu (II) and reactive green 6 dye from wastewater using immobilized mixed fungal biomass and its recovery. Chemosphere 2021, 271, 129519. [Google Scholar] [CrossRef]
- Tunali, S.; Akar, T.; Özcan, A.S.; Kiran, I.; Özcan, A. Equilibrium and kinetics of biosorption of lead(II) from aqueous solutions by Cephalosporium aphidicola. Sep. Purif. Technol. 2006, 47, 105–112. [Google Scholar] [CrossRef]
- Ding, C.; Cheng, W.; Sun, Y.; Wang, X. Novel fungus-Fe3O4 bio-nanocomposites as high performance adsorbents for the removal of radionuclides. J. Hazard. Mater. 2015, 295, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Song, H.P.; Li, X.G.; Sun, J.S.; Xu, S.M.; Han, X. Application of a magnetotactic bacterium, Stenotrophomonas sp. to the removal of Au (III) from contaminated wastewater with a magnetic separator. Chemosphere 2008, 72, 616–621. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Keskin, Z.S.; Dinçer, E.; Ayed, A.B. Influential lead uptake using dried and inactivated-fungal biomass obtained from Panaeolus papilionaceus: Biological activity, equilibrium, and mechanism. Biomass Convers. Biorefinery 2025, 15, 7283–7294. [Google Scholar] [CrossRef]
- Kumar, N.S.; Boddu, V.M.; Krishnaiah, A. Biosorption of phenolic compounds by Trametes Versicolor Polyporus fungus. Adsorpt. Sci. Technol. 2009, 27, 31–46. [Google Scholar] [CrossRef]
- Shin, W.; Kim, Y. Biosorption characteristics of heavy metals (Ni2+, Zn2+, Cd2+, Pb2+) from aqueous solution by Hizikia fusiformis. Environ. Earth Sci. 2014, 71, 4107–4114. [Google Scholar] [CrossRef]
- Thavarajah, R.; Mudimbaimannar, V.K.; Elizabeth, J.; Rao, U.K.; Ranganathan, K. Chemical and physical basics of routine formaldehyde fixation. J. Oral Maxillofac. Pathol. 2012, 16, 400–405. [Google Scholar] [CrossRef]
- Raja, C.P.; Jacob, J.M.; Balakrishnan, R.M. Selenium biosorption and recovery by Marine Aspergillus terreus in an upflow bioreactor. J. Environ. Eng. 2016, 142, C4015008. [Google Scholar] [CrossRef]
- Toptas, A.; Demierege, S.; Mavioglu Ayan, E.; Yanik, J. Spent mushroom compost as biosorbent for dye biosorption. CLEAN-Soil Air Water 2014, 42, 1721–1728. [Google Scholar] [CrossRef]
- Alruwaili, H.A.; Alhumaimess, M.S.; Alsirhani, S.K.; Alsohaimi, I.H.; Alanazi, S.J.; El-Aassar, M.; Hassan, H.M. Bimetallic nanoparticles supported on Ce-BTC for highly efficient and stable reduction of nitroarenes: Towards environmental sustainability. Environ. Res. 2024, 249, 118473. [Google Scholar] [CrossRef]
- Al Khazaleh, M.; Reddy, G.B.; Al-Abri, M.; Seku, K. Biogenic synthesis of palladium nanoparticles with Albizia gum for degradation of Congo red and 4-nitrophenol dyes. Opt. Mater. 2023, 142, 113970. [Google Scholar] [CrossRef]
- Park, J.; Won, S.W.; Mao, J.; Kwak, I.S.; Yun, Y. Recovery of Pd(II) from hydrochloric solution using polyallylamine hydrochloride-modified Escherichia coli biomass. J. Hazard. Mater. 2010, 181, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Kadam, J.; Madiwale, S.; Bashte, B.; Dindorkar, S.; Dhawal, P.; More, P. Green mediated synthesis of palladium nanoparticles using aqueous leaf extract of Gymnema sylvestre for catalytic reduction of Cr (VI). SN Appl. Sci. 2020, 2, 1854. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Shen, Q.; Zheng, X.; Chen, Y. Enhancement of hexavalent chromium reduction by Shewanella oneidensis MR-1 in presence of copper nanoparticles via stimulating bacterial extracellular electron transfer and environmental adaptability. Bioresour. Technol. 2022, 361, 127686. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Liu, Q.; Du, P.; Liu, W.; He, Z. Biosynthesis of palladium nanoparticles using Shewanella loihica PV-4 for excellent catalytic reduction of chromium(Ⅵ). Environ. Sci. Nano 2018, 5, 730–739. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.; Tan, L.; Shi, Z.; Huang, S.; Yu, W.; Wei, G.; Xie, J.; Zhou, S.; Tian, C. Enhancing the Catalytic Performance of PdNPs for Cr(VI) Reduction by Increasing Pd(0) Content. Microorganisms 2025, 13, 1346. https://doi.org/10.3390/microorganisms13061346
Lai H, Tan L, Shi Z, Huang S, Yu W, Wei G, Xie J, Zhou S, Tian C. Enhancing the Catalytic Performance of PdNPs for Cr(VI) Reduction by Increasing Pd(0) Content. Microorganisms. 2025; 13(6):1346. https://doi.org/10.3390/microorganisms13061346
Chicago/Turabian StyleLai, Hongfei, Ling Tan, Zhenkun Shi, Shiyi Huang, Wenjia Yu, Guotong Wei, Jianping Xie, Shuang Zhou, and Chaoyu Tian. 2025. "Enhancing the Catalytic Performance of PdNPs for Cr(VI) Reduction by Increasing Pd(0) Content" Microorganisms 13, no. 6: 1346. https://doi.org/10.3390/microorganisms13061346
APA StyleLai, H., Tan, L., Shi, Z., Huang, S., Yu, W., Wei, G., Xie, J., Zhou, S., & Tian, C. (2025). Enhancing the Catalytic Performance of PdNPs for Cr(VI) Reduction by Increasing Pd(0) Content. Microorganisms, 13(6), 1346. https://doi.org/10.3390/microorganisms13061346




