Abstract
Egypt’s rapid population increase has resulted in higher water demand. It may significantly reduce the amount of water available for agriculture, increasing the chance of using saline water in agriculture. Using saline water certainly poses a major threat to maize growth and may severely affect the growth and productivity of this important crop. Therefore, the aim of this study was to isolate newly native salt-tolerant bacteria from Egyptian saline soils and assess their ability to produce growth-promoting substances under salinity stress, as well as test the mitigating impact of these isolated salt-tolerant bacteria along with arbuscular mycorrhizal fungi (AMF) in maize plants under salinity stress. We isolated ninety-seven salt-tolerant bacterial isolates, and these isolates show a high ability to grow under different concentrations of NaCl. The nine most efficient isolates show a high ability to produce indole acetic acid (IAA), gibberellic acid (GA), P-solubilized exopolysaccharides (EPS), proline, and antioxidants under different NaCl concentrations. Using the 16S rRNA gene, the most effective isolate STB 89 was identified, and its impact, along with AMF, on the growth of salinity-stressed maize was tested in a pot experiment. Our results showed that the growth parameters (shoot length, root length, dry weight, and leaf area), photosynthetic-related pigments (Chlorophyll a, b, and carotenoids), NPK content, and antioxidant enzymes (PPO, POX, and CAT) were improved significantly at p ≤ 0.05 due to the bioinoculant applications, while reduced proline accumulation, Na uptake, and the Na+/K+ ratio in maize plant tissues were observed compared to the control plants. Moreover, the indices of AMF colonization in maize roots and the count of bacteria in the rhizosphere were enhanced due to the bioinoculant applications under salinity stress. In addition, we found that the combined application was more pronounced than the individual application impact. Hence, our results recommended that salt-tolerant bacteria (STB 89) could support salinity, mitigating the impact of AMF in maize plants, as well as allowing better practical techniques for maize cultivation and soil sustainability under salinity stress.
1. Introduction
The high population growth in Egypt has led to an increase in crop cultivation to meet the food gap. This has led to increased water consumption, which accounts for roughly 80–85% of water withdrawals for agricultural practices [1]. This percentage can be reduced by using saline water for irrigation, which can be accomplished by alternating or combining saline water with high-quality water sources [2,3]. However, using saline water certainly poses a major threat to plant growth and productivity, in addition to having negative effects on sustainability by increasing soil salinity [4].
Salinity is a major limiting factor in farming ecosystems, which has adversely impacted food security globally [5]. Irrigation with saline water harms plants in several physiological ways, including increased osmotic tension caused by increased salt concentrations in the root zone of plants, which leads to a reduction in water conductivity, soil aeration, and osmotic potential, resulting in physiological dryness and nutritional imbalance [6,7]. Furthermore, plants under salinity over-absorb toxic ions such as Na+ and Cl+, disrupting ion balance and severely impacting enzyme activity, photosynthesis, and protein synthesis in plant tissues [8]. In addition, salinization increases oxidative stress, which in turn causes severe damage to DNA, protein, and lipids, affecting normal cellular functioning [9].
Several studies have found that inoculation with arbuscular mycorrhizal fungus (AMF) can help plants cope with the effects of salinization [2,10,11,12,13,14,15,16,17]. AMF can form a symbiotic relationship with many terrestrial plant roots, which in turn increase the surface area of the root for nutrient and water uptake [18]. Moreover, AMF can maintain continuous movement of P into the roots under salinity stress, which is critical for vital metabolic processes in plant tissues, by producing phosphatase enzyme, which liberates P from its bound form and increases P availability in the soil, as well as by maintaining an intrinsic phosphate concentration (Pi) within the hyphae through polyphosphate formation [7]. Furthermore, AMF improves K uptake, controls Na+ translocation to aboveground parts, and regulates Na+ concentrations in plant tissues. This is due to mycorrhizal plants’ ability to sequester Na+ into vacuoles or exclude it from the cytosol. AMF also assists the host plant in retracting Na+ from the xylem and redirecting it away from photosynthetic tissues and toward the roots [5,7]. Additionally, under salinity stress, mycorrhizal plants accumulate more osmolytes such as proline, polyamines, sugars, organic acids, and amino acids, as well as regulate the antioxidant defense system [19,20,21,22], which helps in osmotic adjustment and the scavenging of reactive oxygen species (ROS) [2]. However, there are several challenges to using AMF in conventional agriculture, such as chemical fertilizers and other agrochemicals, as well as intensive tillage, which all have a negative impact on arbuscular mycorrhizal symbiosis, decreasing root colonization, the spread of intra- and extraradical mycelia, spore germination, and functions [23]. Hence, the utilization of salt-tolerant bacteria (STB) has become an eco-friendly approach for alleviating salinity stress [24,25,26,27,28], and it may support the mitigating impact of AMF on plants, challenging salinity stress [29]. Several mechanisms aid STB directly and indirectly mitigate the negative impacts of salt stress on plants including the production of phytohormones [30,31], ACC deaminase [32], and exopolysaccharides [33]. In addition, inoculation with STB could modulate the physiological process in plant tissues including osmoprotectant accumulation [34,35] and antioxidant production [34,36,37,38,39,40].
Maize (Zea mays L.) is considered Egypt’s second most important crop [18], yet maize plants are extremely sensitive to salinity, which is one of the leading causes of plant stress [41]. In this context, our aim was to isolate newly native salt-tolerant bacteria from Egyptian saline soils and assess their ability to produce growth-promoting substances under salinity stress. We hypothesized that inoculation of maize plants with STB would increase the mitigating impact of AMF and assist maize plants in tolerating salinity stress. To test our hypothesis, we tested a variety of morphological, biochemical, physiological, and microbiological characteristics to obtain a deeper understanding of the underlying mechanisms of bioinoculants’ ability to reduce salinity stress in maize plants.
2. Materials and Methods
2.1. Soil Sampling and Isolation of Salt-Tolerant Bacteria
Soil samples were collected from several locations in Egyptian saline soils, as shown in Figure S1. The samples were taken from a depth of 0–15 cm, stored in an icebox, transferred to the laboratory, and then air-dried and analyzed for electrical conductivity (EC) and pH [42], as presented in Table 1. Pour-plating method was used for bacterial isolation from soil samples using nutrient agar medium (OXOID, Basingstoke Hampshire, UK), and then the picked colonies were maintained on nutrient agar slopes according to the study of Nader et al. [43]. After that, the bacterial isolates were grown on nutrient broth medium (OXOID, Basingstoke Hampshire, UK) containing different concentrations of NaCl (0%, 3%, 6%, 9%, 12%, and 15%). The bacterial cultures were incubated for 72 h at 30 °C, and the optical density (OD) was measured by a spectrophotometer at 600 nm according to the study of Abdelsattar et al. [44].

Table 1.
Some chemical properties of the collected soil samples.
2.2. Estimating Plant Growth Promotion Traits of Salt-Tolerant Bacteria
Briefly, 1 mL of 24 h old culture of salt-tolerant bacterial isolates was inoculated in 100 mL of nutrient broth medium in conical flasks (250 mL). Incubation of the bacterial cultures was performed at 30 °C on a rotary shaker (150 rpm). To induce salinity stress, different concentrations of NaCl (0%, 3%, 6%, 9%, 12%, and 15%) were supplied to the liquid media. For indole acetic acid (IAA) measurement, 0.1% tryptophan was added to the liquid media, and IAA was assayed calorimetrically in the culture supernatant at 530 nm after 2 days of incubation [45]. Gibberellic acid (GA) was determined calorimetrically at 760 nm [46]. Pikovskaya medium [47] was used to determine the ability of isolated bacteria to solubilize tri-calcium phosphate, and the P-solubilized was determined in the supernatant at 660 nm using a spectrophotometer (Thermo Scientific BioMate 3, Thermo Electron Corporation, Waltham, MA, USA) [48,49]. Exopolysaccharides (EPS) were determined as in the study of Sharath et al. [50] after 3 days of incubation, and calculated as mg/L. Proline was determined using the ninhydrin colorimetric method at 520 nm in the culture supernatant [51]. Free radical scavenging assay was estimated as a non-enzymatic antioxidant assay using the protocol described by Abou-Aly et al. [46].
2.3. Identification of Bacteria
Bacterial identification was performed by the National Research Centre (NRC), Egypt, using 16S rRNA sequencing. The BLAST algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 31 May 2025) was used to compare the acquired sequences to the known sequences in the NCBI database. Aligning 16S rRNA sequences was performed using MEGA 12.0 software. The bacterial accession number for the 16S rRNA sequence was obtained from the GenBank of NCBI.
2.4. Experimental Setup
A pot experiment was carried out during the summer season of 2024 at the experimental farm of the Faculty of Agriculture, Mansoura University, Egypt (27.00° N, 30.00° E). Plastic pots of 25 cm (diameter) and 30 cm (height) were filled with 10 kg pot−1, and the experimental soil’s physical, chemical, and biological characteristics are presented in Table 2. The experiment included 36 pots that were arranged in a complete randomized block design with four experimental settings as follows: (1) Control; (2) AMF; (3) STB; and (4) AMF + STB. Irrigation was performed with three concentrations of saline water (CK, 50 mM NaCl, and 100 mM NaCl). For the AMF application, 5 g of trapped soil (50 spores g−1 soil) and 0.5 g of fresh Sudan grass roots colonized with Glomus clarum, Funneliformis mosseae, and Gigaspora margarita (with 70% colonization) were added to each pot. To produce the mycorrhizal plants, the inoculum was placed roughly 5 cm below the soil surface to encourage fungal colonization of maize roots. An equal amount of autoclaved soil and colonized root inoculum was added to the non-mycorrhizal plants to maintain comparable nutritional conditions. For bacterial application, the seeds were immersed in a liquid culture of Stutzerimonas stutzeri PV248835 (STB 89) previously grown on nutrient broth medium for 2 days at 30 °C (108 cfu/mL) for 30 min before sowing. Arabic gum (16%) was used as an adhesive agent. After 14 days since sowing, each plant was inoculated with an extra 10 mL of the bacterial culture. The non-bacterial treatments received an equal volume of autoclaved inoculum. Maize seeds (Triple Hybrid, Hoth 352) were planted (10 seeds/pot) after sterilization with NaClO 0.5% for five minutes, and the plants were thinned to three plants per pot.

Table 2.
Physicochemical and biological properties of the experimental soil.
2.5. Bacterial Count Determination and Estimation of Mycorrhizal Colonization
The pour-plating method was used for bacterial count in the rhizosphere of maize plants. The total bacterial count (TBC), phosphate-solubilizing bacterial count (PSBC), and potassium-releasing bacterial count (KRBC) were counted according to the study of Gao et al. [18] on nutrient agar medium [52], Pikovskaya’s medium [47], and Alexandrov’s medium [53], respectively. The mycorrhizal colonization was determined in trypan-blue-stained maize roots according to Phillips and Hyman [54], and then the mycorrhizal indices (frequency, F%; intensity, M%; arbuscule frequency, A%) were determined using the Mycocalc program (https://www2.dijon.inrae.fr/mychintec/Mycocalc-prg/download.html, accessed on 15 March 2025) according to Trouvelot et al. [55].
2.6. Growth Measurements and Photosynthetic Pigments Determination
After 60 days, 3 plants from each treatment were randomly selected. Then, shoot length, root length, shoot dry weight, and root dry weight according to the study of Gao et al. [18]. Leaf area was measured in the mature leaf in the middle quadrant of the plant according to the method of Anmaullah et al. [56] by using the following formula: LAI = L × W × K (where L = leaf length, W = leaf width, and K = constant ≈ 0.75). Photosynthetic pigments were determined according to the method of Lichtenthaler and Wellburn [57], briefly, fresh leaf samples (0.2 g) were homogenized in 3 mL of ethanol (95%, v/v), and leaf chlorophylls a, b, and carotenoids were determined spectrophotometry at 665, 649, and 470 nm, then their concentrations were calculated in mgL−1.
2.7. Determination of Nutrients, Proline, and Enzyme Activities
Total N and P contents were determined using the methods of Kjeldhal (Behr distillation unit, Germany) [42] and Snell and Snell [58], respectively. K and Na contents were determined by atomic absorption spectroscopy (GBC Scientific Equipment model SENSAA Dual, Australia) [59]. Proline was determined in the fresh leaf samples using the ninhydrin colorimetric method, and its concentration was calculated and expressed as µg−1 FW according to Bates et al. [51]. Polyphenol oxidase (PPO), Peroxidase (POX), and Catalase (CAT) activities were measured at 495 nm, 470 nm, and 240 nm by tracking the changes in absorbance every 30 s intervals for 3 min, and the enzyme activities were expressed as U g−1 FW, following [60,61,62], respectively.
2.8. Statistical Analysis
The data presented are the means ± standard deviation (SD). Using SPSS software version 27.0, a one-way analysis of variance ANOVA and Duncan’s multiple range test at the 0.05 level was performed. The software of CoStat was used to evaluate the interaction between bio-inoculation and salinity stress using two-way ANOVA, and * p < 0.05, ** p < 0.001, and *** p < 0.0001 indicate significant differences between factors. RStudio Version 4.5.0 was used to perform the principal component analysis and the heatmap of correlation. The presentation of graphs was performed using OriginPro 2024 software.
3. Results
3.1. Isolation and Screening of Salt-Tolerant Bacteria
Data regarding some chemical properties of the collected soil samples from different locations, including Klabsho, North Delta, Bahariya Oasis, El-Senbellawein, and Gamasa, are presented in Table 1. The electrical conductivity (dSm−1) of the studied soil samples S1, S2, S3, S4, S5, S6, S7, S8, S9, with S10 being 10.25, 2.63, 3.38, 2.38, 9.90, 10.71, 7.19, 9.35, 7.36, and 2.11, respectively. However, pH values ranged from 7.98 to 8.79. Ninety-seven bacterial isolates were isolated from nutrient agar plates and evaluated for growth in various NaCl concentrations (0%, 3%, 6%, 9%, 12%, and 15%), as shown in Tables S1A–D. Nine bacterial isolates (STB 8, STB 30, STB 38, STB 41, STB 59, STB 60, STB 77, STB 88, and STB 89) were chosen for further studies due to their high ability to grow under salinity stress as calculated by (increase/decrease) percent (%) of growth compared to the control (Tables S2A–D).
3.2. Plant Growth Promotion Traits of Salt-Tolerant Bacteria
The high-tolerant nine isolates were tested to produce IAA, GA, P-solubilization, EPS, proline, and antioxidants with various concentrations of NaCl (0%, 3%, 6%, 9%, 12%, and 15%). The results in Figure 1A showed that all the tested isolates could produce IAA at various concentrations of NaCl (0%, 3%, 6%, 9%, 12%, and 15%); however, the IAA production decreased as NaCl concentration increased in the culture. The maximum production of IAA was recorded for isolate STB 89 (269.56 mg/100 mL), which steadily reduced as the NaCl concentration increased until reaching 61.12 mg/100 mL at 15% NaCl. A similar trend was observed in GA production, which decreased as NaCl concentration increased in the culture (Figure 1B). The maximum production of GA was recorded for isolate STB 89 (122.02 mg/100 mL), which steadily reduced as the NaCl concentration increased until reaching (45.12 mg/100 mL) at 15% NaCl, followed by isolate STB 88 (98.81 mg/100 mL), which steadily reduced as the NaCl concentration increased until reaching (55.57 mg/100 mL) at 15% NaCl. For P-solubilization, all the tested isolates have the ability to solubilize P at various concentrations of NaCl (0%, 3%, 6%, 9%, 12%, and 15%). The highest P-solubilized amounts (11.48, 10.47, 9.51, and 9.40 mg/100 mL) were recorded for isolates STB 77, STB 8, STB 59, and STB 89, respectively (Figure 1C).
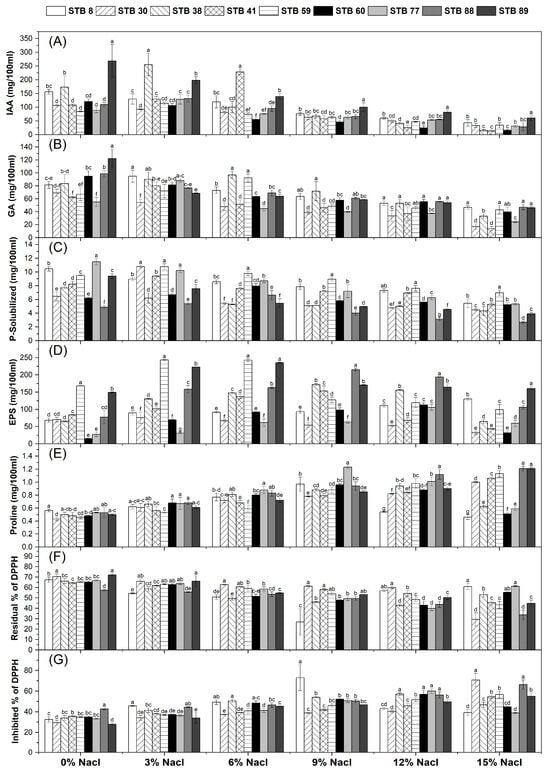
Figure 1.
Plant growth promotion traits of salt-tolerant bacterial isolates. (A), IAA; (B), GA; (C), P-Solubilized; (D), EPS; (E), Proline; (F), Residual % of DPPH; (G), Inhibited % of DPPH. Data are means ± standard deviation; different letters within the same group indicate significant differences between means at p ≤ 0.05. IAA, Indole acetic acid; GA, Gibberellic acid; EPS, Exopolysaccharides.
Regarding exopolysaccharides production (EPS), all the tested isolates have the ability to produce EPS at various concentrations of NaCl (0%, 3%, 6%, 9%, 12%, and 15%); however, the EPS production increased as NaCl concentration increased in the culture (Figure 1D). The highest EPS production was recorded for isolates STB 59 and STB 89 (168.20 and 149.27 mg/100 mL). The same pattern was observed for proline production, with the highest amounts (0.56, 0.53, 0.53, 0.50, and 0.50 mg/100 mL) for isolates STB 8, STB 77, STB 88, STB 38, and STB 89, respectively (Figure 1E).
Data in Figure 1F indicate that the maximum residual DPPH (minimum inhibition) by the bacterial isolates was observed in cultures without NaCl. However, the inhibition of DPPH by the bacterial isolates generally increased with the increasing concentration. The maximum inhibition of DPPH was recorded in the culture at 9% NaCl (73.09%) by STB 8, followed by isolates STB 30 and STB 88 at 15% NaCl (70.73 and 66.24%), respectively (Figure 1G).
3.3. Identification of the Most Effective Salt-Tolerant Bacterial Isolate
Salt-tolerant bacterial isolate (STB 89) was chosen for identification because it could produce substantial amounts of plant growth-promoting substances under salinity stress conditions, as previously reported. The bacterial isolate was identified as Stutzerimonas stutzeri according to the NCBI database. Accession number PV248835 was deposited for the bacterial isolate in NCBI GenBank (Figure S2).
3.4. Bacterial Count and Mycorrhizal Colonization Under Salinity Stress
The effect of bioinoculant applications on the bacterial count in the rhizosphere of maize plants under either control or salinity stress conditions is presented in Table 3. Under both unstressed and stressed plants, the bacterial count in the rhizosphere of bioinoculant-inoculated treatments was higher than that of uninoculated treatments. Furthermore, as the concentration of salinity increased, the bacterial count (TCB, PSBC, and KRBC) decreased. Considering the two salinity levels (50 mM NaCl and 100 mM NaCl), the average decrease in the TCB, PSBC, and KRBC was 5.73, 2.18, and 1.44%, respectively, as compared with the control. However, the bioinoculant applications increased the bacterial counts under saline stress compared with their corresponding control. Also, the combined treatment at different salinity concentrations gave the highest bacterial counts. In addition, it was observed that inoculation with STB enhanced total bacterial count more than AMF. The total bacterial count was improved with the application of AMF, STB 89, and combination treatments by (3.24, 5.32, and 7.47%) in the rhizosphere of treated plants compared with the untreated control plants. A similar direction was noted for phosphate-solubilizing bacterial count and potassium-releasing bacterial count (Table 2). Phosphate-solubilizing bacterial count and potassium-releasing bacterial count were improved with the application of AMF, STB 89, and combination treatments by (2.40, 4.04, and 6.07%) and (0.97, 2.64, and 3.17%), respectively, in the rhizosphere of treated plants compared with the untreated control plants.

Table 3.
Counts of bacteria in the rhizosphere of maize and levels of AMF colonization (%) in the roots of maize plants grown under either control or salinity stress conditions.
The frequency (F%), intensity (M%), and arbuscular development (A%) of mycorrhizal colonization in the roots of maize plants were measured (Table 2). Regardless of salinity stress, the data revealed that the mycorrhizal colonization in maize roots was increased with the combined application when compared to the single application of AMF. However, salinity stress significantly altered the indices (F, M, and A%) of AMF colonization in maize roots, and there were significant interactions between salinity stress and mycorrhizal colonization. Also, the mycorrhizal colonization gradually declined as the concentrations of salinity increased. In addition, there was no mycorrhizal colonization of non-mycorrhizal plant roots. Figure 2 shows the mycorrhizal structures in the stained roots of maize plants. These findings support AMF’s ability to colonize maize roots at all salinity levels.

Figure 2.
Photomicrographs of maize root cells (Scale bars represent = 20 µm): (A), control without colonization; (B–D), AMF-inoculated maize roots showing mycorrhizal structure; Arb, Arbscules; Ves, Vesicles; Hyph, Hyphae.
3.5. Morphological Traits of Maize Plants Under Salinity Stress
Mean data regarding the morphological response of maize plants grown under either control or salinity stress conditions are presented in Table 4. The data reveal that salinity stress significantly reduced plant growth and biomass accumulation compared with the corresponding control maize plants. Considering the two salinity levels (50 mM NaCl and 100 mM NaCl), the average decrease in the shoot length, root length, SDW, RDW, and leaf area was 27.50, 22.21, 45.46, 52.98, and 31.11%, respectively, as compared with the control plants. Irrespective of the salinity stress effects, AMF, STB, and their combination treatments improved plant growth and biomass of maize plants compared with control plants. In general, the highest values of plant growth and biomass were observed in plants treated with the combined application. Shoot and root lengths improved with the application of AMF, STB 89, and their combination treatments by (18.03, 11.24, and 23.31%) and (26.02, 19.55, and 35.39%), respectively, compared with the control plants. Furthermore, the dry weight of shoots and roots was improved with the application of AMF, STB 89, and their combination treatments by (38.58, 24.67, and 50.18%) and (63.60, 45.33, and 74.45%), respectively, compared with the control plants. In addition, leaf area was improved with the application of AMF, STB 89, and their combination treatments by (32.64, 24.33, and 42.72%), respectively, compared with the control plants. These results indicated that the morphological traits of maize plants were enhanced significantly under salinity stress due to the bioinoculant applications.

Table 4.
Morphological response of maize plants grown under either control or salinity stress conditions.
3.6. Photosynthetic-Related Pigments of Maize Plants Under Salinity Stress
Although chlorophyll is essential for keeping plants green and healthy, salinity reduces chlorophyll content in salt-sensitive plants. Bioinoculants could be a promising strategy to improve photosynthetic apparatus under salinity stress. Mean data regarding chlorophyll contents in the leaves of maize plants grown under either control or salinity stress conditions are presented in Figure 3. Chlorophyll content was decreased as the concentration of salinity level increased. Considering the two salinity levels (50 mM NaCl and 100 mM NaCl), the average decrease in chlorophyll a, b, total, and carotenoid content in the leaves was 55.92, 51.49, 54.21, and 36.17%, respectively, as compared with the control plants. On the other side, plants treated with the combined application had the highest chlorophyll content, followed by AMF and STB 89 applications, under either control or salinity stress conditions. Inoculation with AMF, STB 89, and their combination resulted in an increase in chlorophyll a, b, total, and carotenoids content in the leaves by (40.35, 31.14, and 52.37%), (46.61, 31.93, and 60.50%), (43.01, 31.41, and 55.96%), and (41.84, 35.15, and 50.91%), respectively, compared with the control plants. The obtained results reported that the photosynthetic-related pigments in the leaves of maize plants were significantly enhanced under salinity stress due to the bioinoculants application.
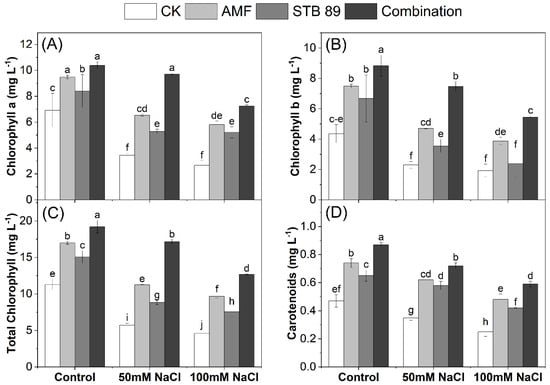
Figure 3.
Changes in photosynthetic-related pigments in the leaves of maize plants grown under either control or salinity stress conditions: (A): chlorophyll a, (B): chlorophyll b, (C): total chlorophyll, (D): carotenoids. Data are means ± standard deviation; different letters indicate significant differences between means at p ≤ 0.05.
3.7. Nutrient Content and Determination of the Na+/K+ Ratio
Mean data regarding N, P, K, Na contents, and the Na+/K+ ratio in the leaves and roots of maize plants grown under either control or salinity stress conditions, are presented in Figure 4. The contents of N, P, and K decreased significantly as the concentration of salinity increased. Considering the two salinity levels (50 mM NaCl and 100 mM NaCl), the average decrease in N, P, and K in the leaves and roots was (30.88, 6.86, and 13.58%) and (12.92, 20.53, and 8.69%), respectively, as compared with the control plants. Compared with the corresponding control plants, inoculation with AMF, STB 89, and their combination increased N content in maize shoots and roots by (25.66, 15.40, and 33.77%) and (39.89, 23.97, and 46.71%), respectively (Figure 4A,B). Furthermore, P content was increased by (34.52, 24.35, and 43.19%) and (19.88, 11.04, and 38.81%), respectively (Figure 4C,D). In addition, K content was increased by (20.00, 12.04, and 28.07%) and (22.14, 12.46, and 32.98%), respectively (Figure 4E,F). On the contrary, there was an increase in Na content and the Na+/K+ ratio as the concentration of salinity increased. Considering the two salinity levels (50 mM NaCl and 100 mM NaCl), the average increase in Na content and the Na+/K+ ratio in the leaves and roots was (46.47 and 52.54%) and (30.18 and 36.16%), respectively, as compared with the control plants. However, inoculation with AMF, STB 89, and their combination could decrease Na uptake by (27.17, 16.61, and 33.62%) and (16.90, 9.56, and 28.12%) in the shoots and roots, respectively (Figure 4G,H). In addition, the Na+/K+ ratio was increased in the shoots and roots by (43.85, 28.27, and 53.68%) and (35.17, 21.15, and 51.07%), respectively, after inoculation with AMF, STB 89, and their combination. Such results indicate the potential impact of bioinoculants to enhance nutrient uptake and to reduce Na uptake under salinity stress.
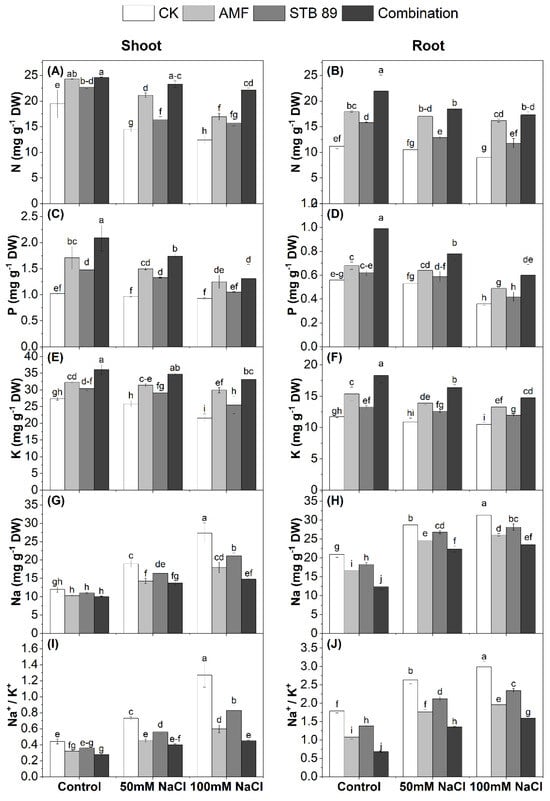
Figure 4.
N, P, K, and Na contents (mg g−1 DW) and Na+/K+ ratio in the shoots and roots of maize plants grown under either control or salinity stress conditions. (A), N-shoot; (B), N-root; (C), P-shoot; (D), P-root; (E), K-shoot; (F), K-root; (G), Na-shoot; (H), Na-root; (I) Na+/K+-shoot; (J), Na+/K+-root. Data are means ± standard deviation; different letters indicate significant differences between means at p ≤ 0.05. Ck, Check; AMF, Arbuscular mycorrhizal fungi; STB, Salinity tolerant bacteria “Stutzerimonas stutzeri PV248835”.
3.8. Proline Content and Determination of Antioxidant Enzyme Activities
Mean data regarding proline contents and the activity of antioxidant enzymes in the leaves of maize plants grown under either control or salinity stress conditions are presented in Figure 5. As expected, the contents of proline were increased as the concentration of salinity increased. Considering the two salinity levels (50 mM NaCl and 100 mM NaCl), the average increase in proline content in maize leaves was 50.28% as compared with the control plants. However, inoculation with AMF, STB 89, and their combination decreased the percentages of proline by (20.42, 14.39, and 26.99%), respectively. Results showed that the combined application reduced proline content more pronouncedly than the sole application of AMF and STB 89. Regardless of the bioinoculants application, the PPO activity increased in the leaves of maize plants as the concentration of salinity increased. However, inoculation with AMF, STB 89, and their combination resulted in a significant increase in PPO activity, and the enzyme activity was increased along with the increase in the concentration of salinity. Maximum PPO activity was observed in the combined application under 100 mM NaCl (Figure 5B). For the activities of POX and CAT, regardless of the bioinoculants application, a slight insignificant increase was observed as the concentration of salinity increased. However, inoculation with AMF, STB 89, and their combination resulted in a significant increase in POX and CAT activities, and the enzyme activities were increased along with the increase in the concentration of salinity. Maximum POX and CAT activities were observed in the combined application under 100 mM NaCl (Figure 5C,D). The results showed that the bioinoculant application had the ability to reduce proline content while also activating the antioxidant enzyme defense system in maize plants under salinity stress, and this response was more pronounced in the combined application than in the individual AMF and STB 89 applications.
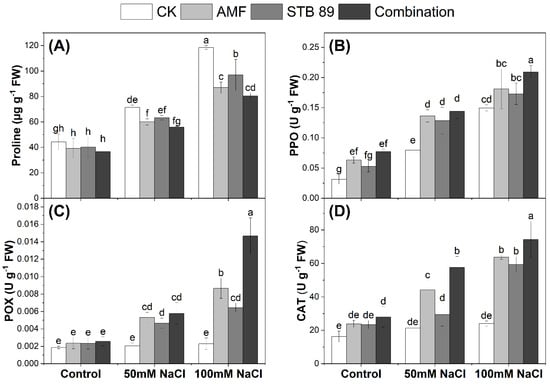
Figure 5.
Proline content (µg g−1 FW) and antioxidant enzymes activity (U g−1 FW) in the leaves of maize plants grown under either control or salinity stress conditions. (A) Proline content; (B) PPO activity; (C) POX activity; (D) CAT activity. Data are means ± standard deviation; different letters indicate significant differences between means at p ≤ 0.05. Ck, Check; AMF, Arbuscular mycorrhizal fungi; STB, Salinity tolerant bacteria “Stutzerimonas stutzeri PV248835”.
3.9. Evaluation of the Impact of AMF, STB, and Their Combination by Principal Component Analysis and Heatmap of Correlation Under Salinity Stress
The maize growth-related parameters were analyzed by PCA (Figure 6A). Two components (PC1 and PC2) were shown from the PCA of the studied traits that explained 77.20% and 13.9% of the data variability, respectively. In addition, hierarchical clustering analysis showed differences in growth-related parameter values in bioinoculants-treated maize plants under control and salinity stress conditions (Figure 6B). These results indicate that bioinoculants contributed significantly to mitigating the detrimental impacts of salt stress on maize plants.
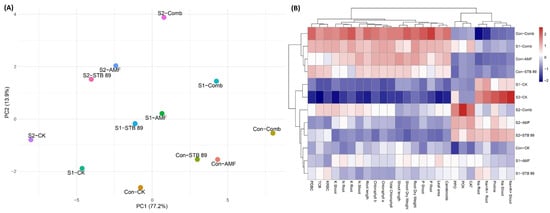
Figure 6.
Changes in maize growth-related parameters as affected by AMF, STB 89, and their combination under both normal and salinity stress conditions using multivariate statistical analysis: (A) Principal component analysis (PCA). (B) Heatmap of correlation.
4. Discussion
4.1. Isolated Salt-Tolerant Bacteria Produce Plant Growth Substances Actively
We isolated a total of 97 bacterial isolates from Egyptian saline soils. All of them could grow in different concentrations of NaCl actively (Table S1A–D). The results showed that all isolates could grow at all salinity concentrations at varying degrees, with nine potent isolates, which were selected due to their high ability to grow under salinity stress conditions (Table S2A–D). The nine most tolerant isolates were screened to produce IAA, GA, P-solubilization, EPS, proline, and antioxidants at various concentrations of NaCl (0%, 3%, 6%, 9%, 12%, and 15%), and all of them could produce these growth substances under both conditions. These compounds may provide additional benefits to plant growth, quality, stress tolerance, and plant health against pathogens [63,64,65,66,67]. Under the salinity stress conditions, the production of IAA, GA, and P-solubilized was decreased as NaCl concentration increased in the culture (Figure 1A–C). However, the production of proline, EPS, and antioxidants was increased as NaCl concentration increased in the culture (Figure 1D–G). The isolate STB 89 was chosen due to high production of plant growth promotion substances under both unstressed and stressed conditions, and this isolate was identified as Stutzerimonas stutzeri PV248835. Similarly, Abdelsattar et al. [44] isolated several salt-tolerant bacteria from Egyptian saline soils and found that these bacteria showed a high production of PGP, such as high salt tolerance, N-fixation, P-solubilization, and IAA production. Also, Kumar et al. [68] found that the amount of IAA and P-solubilized produced by salt-tolerant Bacillus pumilus JPVS11 gradually decreased as NaCl concentrations increased; however, the proline content was increased with increasing NaCl concentration. Also, Shahid et al. [69] found a higher number of EPS under salt-stress conditions compared with the control. This increase might suggest that bacteria produce proline, EPS, and antioxidants in response to salinity stress. Therefore, the isolated bacteria’s ability to produce growth-promoting traits could help maize plants withstand salt stress.
4.2. AMF and STB 89 Improve Bacterial Counts and Mycorrhizal Colonization
We observed an increase in bacterial counts (TCB, PSBC, and KRBC) due to the applications of bioinoculants when compared to the control treatments under both unstressed and stressed plants (Table 3). Such an increase in bacterial count may be attributed to the survivability of nutrients and organic matter, which operate as an energy source for bacterial reproduction, as well as the heat that is produced during OM decomposition, which promotes bacterial growth [18]. Similar observations by Gao et al. [18] found an increase in TCB, PSBC, and KRBC in maize rhizosphere after inoculation with bioinoculants. These results show that the bioinoculants could contribute to helping maize plants absorb more nutrients, particularly P and K, from soil under salinity stress.
Mycorrhizal colonization levels (F%, M%, and A%) were decreased due to salinity stress; however, the used mycorrhizal species could still colonize maize roots. The decrease in fungal development and structure as salinity concentration increased could be ascribed to salt stress, which limits fungal growth and structure. Similarly, both [70] and [2] observed a decrease in fungal growth and spore germination. However, maize plant development improved across all salinity levels, despite the decrease in root colonization, indicating the beneficial impacts of AMF on the host plant growth. The combined treatment had the highest mycorrhizal colonization values under both unstressed and stressed plants (Table 3). Our findings indicated that the AMF strains used can tolerate and form a symbiotic relationship with maize plants and modify the root architecture under high salinity stress, potentially allowing maize plants to absorb more water and nutrients, in particular phosphorus.
4.3. AMF and STB 89 Improve the Morphological Traits of Maize Plants
The morphological traits of maize plants have been negatively affected by salinity stress; however, using bioinoculants improved maize growth (shoot length, root length, dry weight, and leaf area) under both unstressed and stressed plants (Table 4, Figure 6A,B). This could be attributed to the capability of Stutzerimonas stutzeri PV248835 to produce high amounts of growth-promoting substances (Figure 1), which could explain the improvement in the growth of maize plants. In addition, AMF symbiosis improves plant growth by increasing water availability using hyphae and the concentration of nutrients, particularly P, in plant tissues under salinity. The highest values of morphological traits were observed in the combined treatment under both unstressed and stressed plants (Table 4); this could be attributed to the synergistic effect between bacteria and AMF [10,15,71]. Our results are in line with recent findings, which report that the use of Funneliformis constrictum significantly increased the growth of maize under salinity stress [2]. As well as this, de Carvalho Neta et al. [15] found that co-inoculation with AMF and PGPB was more pronounced in improving maize growth under salinity stress rather than individual inoculation. Our findings indicate that inoculation with Stutzerimonas stutzeri PV248835 could help improve the impact of AMF on maize growth under salinity stress.
4.4. AMF and STB 89 Improve Photosynthetic-Related Pigments Content in Maize Plants
Although chlorophyll is essential for keeping plants green and healthy, salinity reduces chlorophyll content in salt-sensitive plants. Such a decrease may be attributed to salt stress, which accelerated the degradation of chlorophyll, especially Chlorophyll b, which is an important component of the PSII light-harvesting pigment protein complex, and its reduction would directly damage the structure and function of this complex. Our results indicate that bioinoculants could be a promising strategy to improve photosynthetic apparatus under salinity stress (Figure 3), as the used bioinoculants (AMF, STB 89, and their combination) could aid maize plants in increasing chlorophyll content under all levels of salinity stress. Previous studies reported the beneficial impact of AMF on enhancing photosynthetic pigments under salinity stress; likewise, El-Sawah et al. [2] found a significant increase in photosynthetic pigment contents in the leaves of maize under salinity stress after inoculation with Funneliformis constrictum. However, our results indicate that inoculation with Stutzerimonas stutzeri PV248835 in combination with AMF could strengthen their impact on photosynthetic pigment contents, and thus maize growth.
4.5. AMF and STB 89 Enhance Nutrient Content and Reduce the Na+/K+ Ratio in Maize Plants
Salinity increased the absorption of Na+, whereas it decreased N, P, and K uptake, and also increased the Na+/K+ ratio (Figure 4). However, the obtained results showed that AMF, STB 89, and their combination could enhance N, P, and K uptake; also, a decrease was observed in Na+ and the Na+/K+ ratio under salinity stress compared to the control plants. That impact could be attributable to the AMF and beneficial bacteria providing P nutrition to host plants [18] and compartmentalization of Na+ ions [16]. Also, AMF can restrict the transport of Na from roots to shoots in plants, which decreases Na+/K+ in leaves and stems, in addition to protecting the photosynthetic organs from damage [16,72]. Similar studies found that inoculation with AMF and/or PGPR reduces the accumulation of Na in plant tissues, especially in shoots; however, an increase in P and K was observed [15,17]. Our results indicate the potential impact of Stutzerimonas stutzeri PV248835, along with AMF, to enhance nutrient uptake and to reduce Na uptake under salinity stress.
4.6. AMF and STB 89 Reduce Proline Content and Activate Antioxidant Defense System in Maize Plants
Plants employ amino acids such as proline to maintain cellular osmotic and antioxidant balance during abiotic stress. In addition to being an osmoprotectant, proline can operate as a stress signal; hence, plants may acquire more proline due to a biotic stress or less proline as a result of decreased stress [43]. Our results showed a decrease in proline accumulation due to bioinoculants, and this decrease was more pronounced in the combined application (Figure 5A). The accumulation of low proline in the treated plants indicated that these plants were less exposed to salinity and did not require excessive proline accumulation to cope with salinity stress. Similarly, Echeverria et al. ref. [73] shows that AMF significantly decreased proline accumulation in lotus plants under salinity stress. In contrast, Elhindi et al. [74] observed an increase in proline accumulation in sweet basil plants under salinity stress. While Borde et al. [75] observed a decrease in proline accumulation in the shoots of the bajra plant due to AMF inoculation, proline accumulation in the roots was higher in mycorrhizal plants at all levels of salinity stress. Hence, these patterns may depend on several factors such as plant species, plant organs, and salinity level. On the other hand, we observed a significant increase in the activities of antioxidant enzymes (PPO, POX, and CAT) due to bioinoculants application (Figure 5B–D). Elevated enzyme activity indicates that maize plants engage their defensive systems of antioxidant enzymes to scavenge those producing ROS during salt stress. These results are consistent with those obtained by [2,76,77] in maize plants. These results indicate that the elevated enzyme activities resulting from bioinoculants application can protect maize plants from membrane damage caused by salinity stress.
5. Conclusions
Pressure on freshwater resources due to population growth is a major problem, resulting in a scarcity of water available for irrigation, which may necessitate the use of saline water for irrigation, which is problematic owing to the existence of plants sensitive to salinity, such as maize. Therefore, in this study, we isolated ninety-seven salt-tolerant bacterial isolates that may aid maize plants tolerate salinity stress. These bacteria could grow under different concentrations of NaCl, and nine isolates of them could produce growth-promoting substances effectively under salinity stress, in vitro. The most potent isolate (STB 89) was identified as Stutzerimonas stutzeri PV248835, and it was tested along with AMF on its ability to mitigate salinity stress in maize plants in a pot experiment. Our results showed that the inoculation of Stutzerimonas stutzeri PV248835 with AMF improved growth parameters, photosynthetic-related pigments, NPK content, and antioxidant enzymes while reducing proline accumulation, Na uptake, and the Na+/K+ ratio in maize plant tissues. In addition, bio-inoculation improved bacterial counts and mycorrhizal colonization under salinity stress. Taken together, inoculation of Stutzerimonas stutzeri PV248835 could support salinity stress mitigating the impact of AMF, and a cost-effective and easy-applicable technique could help farmers to mitigate salinity stress on maize plants. Future research should focus on signaling and molecular approaches to plant–microbe interactions, as well as expanding field experiments to study maize productivity under the combined effect of bio-inoculation and salinity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13061345/s1, Table S1(A–D): Effect of NaCl concentrations on the growth of bacterial isolates; Table S2(A–D): (increase/decrease) percent (%) of growth compared to the control; Figure S1: A map showing the locations from where soil samples were collected; Figure S2: Phylogenetic tree of Stutzerimonas stutzeri PV248835.
Author Contributions
Conceptualization, R.M.Z., A.H.A., E.H.A. and A.M.E.-S.; methodology, R.M.Z. and A.M.E.-S.; software, R.M.Z. and A.M.E.-S.; validation, R.M.Z. and A.M.E.-S.; formal analysis, R.M.Z. and A.M.E.-S.; investigation, R.M.Z. and A.M.E.-S.; resources, R.M.Z. and A.M.E.-S.; data curation, R.M.Z. and A.M.E.-S.; writing—original draft preparation, R.M.Z. and A.M.E.-S.; writing—review and editing, R.M.Z., A.H.A., E.H.A. and A.M.E.-S.; visualization, R.M.Z., A.H.A., E.H.A. and A.M.E.-S.; supervision, A.H.A., E.H.A. and A.M.E.-S.; project administration, A.M.E.-S.; funding acquisition, R.M.Z. and A.M.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Mansoura University Research Fund, General Administration of Research, Graduate Studies and Research Sector, Mansoura University, Egypt.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the Agricultural Microbiology Lab; the Soil, Water, Fertilizer, and Plant Analysis Lab; and the Seed Pathology and Tissue Culture Lab, in the Faculty of Agriculture, Mansoura University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Negm, A.M.; Omran, E.-S.E.; Mahmoud, M.A.; Abdel-Fattah, S. Update, Conclusions, and Recommendations for Conventional Water Resources and Agriculture in Egypt. In Conventional Water Resources and Agriculture in Egypt; Negm, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 659–681. [Google Scholar]
- El-Sawah, A.M.; Abdel-Fattah, G.G.; Holford, P.; Korany, S.M.; Alsherif, E.A.; AbdElgawad, H.; Ulhassan, Z.; Jośko, I.; Ali, B.; Sheteiwy, M.S. Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L. to salinity. Microbiol. Res. 2023, 266, 127254. [Google Scholar] [CrossRef]
- Sang, H.; Guo, W.; Gao, Y.; Jiao, X.; Pan, X. Effects of Alternating Fresh and Saline Water Irrigation on Soil Salinity and Chlorophyll Fluorescence of Summer Maize. Water 2020, 12, 3054. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Yuan, Z. Plant Responses and Adaptations to Salt Stress: A Review. Horticulturae 2024, 10, 1221. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Lackóová, L. Arbuscular mycorrhizal fungi and salinity stress mitigation in plants. Front. Plant Sci. 2025, 15, 1504970. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Z.; Feng, S.; Guo, P.; Wang, Y.; Hao, B.; Guo, W.; Li, F.Y. Synergistic effects of AMF and PGPR on improving saline-alkaline tolerance of Leymus chinensis by strengthening the link between rhizosphere metabolites and microbiomes. Environ. Technol. Innov. 2024, 36, 103900. [Google Scholar] [CrossRef]
- Wen, Y.; Wu, R.; Qi, D.; Xu, T.; Chang, W.; Li, K.; Fang, X.; Song, F. The effect of AMF combined with biochar on plant growth and soil quality under saline-alkali stress: Insights from microbial community analysis. Ecotoxicol. Environ. Saf. 2024, 281, 116592. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martínez-Andújar, C.; Karray-Bouraouib, N.; Pérez-Alfocea, F.; Albacete, A. The crosstalk interaction of ethylene, gibberellins, and arbuscular mycorrhiza improves growth in salinized tomato plants by modulating the hormonal balance. J. Plant Physiol. 2024, 303, 154336. [Google Scholar] [CrossRef]
- Huang, P.; Huang, S.; Ma, Y.; Danish, S.; Hareem, M.; Syed, A.; Elgorban, A.M.; Eswaramoorthy, R.; Wong, L.S. Alleviation of salinity stress by EDTA chelated-biochar and arbuscular mycorrhizal fungi on maize via modulation of antioxidants activity and biochemical attributes. BMC Plant Biol. 2024, 24, 63. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, P.; Cao, F.; Liu, X.; Ji, M.; Xie, M. Effects of AMF on Maize Yield and Soil Microbial Community in Sandy and Saline Soils. Plants 2024, 13, 2056. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Neta, S.J.; Araújo, V.L.V.P.; Fracetto, F.J.C.; da Silva, C.C.G.; de Souza, E.R.; Silva, W.R.; Lumini, E.; Fracetto, G.G.M. Growth-promoting bacteria and arbuscular mycorrhizal fungus enhance maize tolerance to saline stress. Microbiol. Res. 2024, 284, 127708. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; An, T.; Huang, D.; Liu, R.; Xu, B.; Zhang, S.; Deng, X.; Siddique, K.H.M.; Chen, Y. Arbuscular mycorrhizal symbioses alleviating salt stress in maize is associated with a decline in root-to-leaf gradient of Na+/K+ ratio. BMC Plant Biol. 2021, 21, 457. [Google Scholar] [CrossRef]
- Chandra, P.; Singh, A.; Prajapat, K.; Rai, A.K.; Yadav, R.K. Native arbuscular mycorrhizal fungi improve growth, biomass yield, and phosphorus nutrition of sorghum in saline and sodic soils of the semi–arid region. Environ. Exp. Bot. 2022, 201, 104982. [Google Scholar] [CrossRef]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Q.; Wang, B.; Zhang, N.; Qiu, R.; Yuan, Y.; Yang, M.; Wang, F.; Mei, L.; Cui, G. Arbuscular mycorrhizal fungi communities and promoting the growth of alfalfa in saline ecosystems of northern China. Front. Plant Sci. 2024, 15, 1438771. [Google Scholar] [CrossRef]
- Malik, J.A.; Alqarawi, A.A.; Alotaibi, F.; Habib, M.M.; Sorrori, S.N.; Almutairi, M.B.R.; Dar, B.A. Alleviation of NaCl Stress on Growth and Biochemical Traits of Cenchrus ciliaris L. via Arbuscular Mycorrhizal Fungi Symbiosis. Life 2024, 14, 1276. [Google Scholar] [CrossRef]
- Kakabouki, I.; Stavropoulos, P.; Roussis, I.; Mavroeidis, A.; Bilalis, D. Contribution of Arbuscular Mycorrhizal Fungi (AMF) in Improving the Growth and Yield Performances of Flax (Linum usitatissimum L.) to Salinity Stress. Agronomy 2023, 13, 2416. [Google Scholar] [CrossRef]
- Qin, W.; Yan, H.; Zou, B.; Guo, R.; Ci, D.; Tang, Z.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; et al. Arbuscular mycorrhizal fungi alleviate salinity stress in peanut: Evidence from pot-grown and field experiments. Food Energy Secur. 2021, 10, e314. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhowmik, S.; Dutta, S.S. Challenges in Application of Arbuscular Mycorrhizal Inocula in Conventional Agriculture. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Inoculum Production and Application; Parihar, M., Rakshit, A., Adholeya, A., Chen, Y., Eds.; Springer Nature: Singapore, 2024; pp. 229–252. [Google Scholar]
- Szymańska, S.; Lis, M.I.; Piernik, A.; Hrynkiewicz, K. Pseudomonas stutzeri and Kushneria marisflavi Alleviate Salinity Stress-Associated Damages in Barley, Lettuce, and Sunflower. Front. Microbiol. 2022, 13, 788893. [Google Scholar] [CrossRef]
- Lami, M.J.; Adler, C.; Caram-Di Santo, M.C.; Zenoff, A.M.; de Cristóbal, R.E.; Espinosa-Urgel, M.; Vincent, P.A. Pseudomonas stutzeri MJL19, a rhizosphere-colonizing bacterium that promotes plant growth under saline stress. J. Appl. Microbiol. 2020, 129, 1321–1336. [Google Scholar] [CrossRef]
- Szymańska, S.; Dąbrowska, G.B.; Tyburski, J.; Niedojadło, K.; Piernik, A.; Hrynkiewicz, K. Boosting the Brassica napus L. tolerance to salinity by the halotolerant strain Pseudomonas stutzeri ISE12. Environ. Exp. Bot. 2019, 163, 55–68. [Google Scholar] [CrossRef]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of inoculation with nitrogen-fixing bacterium Pseudomonas stutzeri A1501 on maize plant growth and the microbiome indigenous to the rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Bacilio, M.; Moreno, M.; Bashan, Y. Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Appl. Soil Ecol. 2016, 107, 394–404. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Patel, T.; Saraf, M. Biosynthesis of phytohormones from novel rhizobacterial isolates and their in vitro plant growth-promoting efficacy. J. Plant Interact. 2017, 12, 480–487. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef]
- Tiwari, G.; Duraivadivel, P.; Sharma, S.; Hariprasad, P. 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. by mitigating drought and salt stress. Sci. Rep. 2018, 8, 17513. [Google Scholar] [CrossRef]
- Qurashi, A.; Sabri, A. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 11183–11191. [Google Scholar] [CrossRef]
- Saum, S.H.; Müller, V. Salinity-Dependent Switching of Osmolyte Strategies in a Moderately Halophilic Bacterium: Glutamate Induces Proline Biosynthesis in Halobacillus halophilus. J. Bacteriol. 2007, 189, 6968–6975. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Patel, D.; Saraf, M. Influence of soil ameliorants and microflora on induction of antioxidant enzymes and growth promotion of Jatropha curcas L. under saline condition. Eur. J. Soil Biol. 2013, 55, 47–54. [Google Scholar] [CrossRef]
- Tu, Q.; Tang, S.; Huang, S. Mitigation of salinity stress via improving growth, chlorophyll contents and antioxidants defense in sunflower with Bacillus pumilis and biochar. Sci. Rep. 2025, 15, 9641. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, D.; Alheswairini, S.S.; Barasarathi, J.; Enshasy, H.A.E.; Lalitha, S.; Mir, S.H.; Nithyapriya, S.; Sayyed, R. Halophilic rhizobacteria promote growth, physiology and salinity tolerance in Sesamum indicum L. grown under salt stress. Front. Microbiol. 2025, 16, 1590854. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Wang, B.; Li, H.; Li, S.; Zhang, H.; Haider, F.U.; Li, X. Harnessing the role of rhizo-bacteria to mitigate salinity stress in rice (Orzya sativa); focus on antioxidant defense system, photosynthesis response, and rhizosphere microbial diversity. Rhizosphere 2025, 33, 101043. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course: A Manual of Methods Useful for Instruction and Research in Soil Chemistry, Physical Chemistry of Soils, Soil Fertility, and Soil Genesis; UW-Madison Libraries parallel press: Madison, WI, USA, 2005. [Google Scholar]
- Nader, A.A.; Hauka, F.I.A.; Afify, A.H.; El-Sawah, A.M. Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max, L.). Microorganisms 2024, 12, 1123. [Google Scholar] [CrossRef]
- Abdelsattar, M.; El-Sawah, A.M.; El-Kady, S.; Hauka, F.I.A. Evaluation of Plant Growth Promoting of Salt-tolerant Rhizobacteria Isolated from Egyptian Saline Soils. J. Agric. Chem. Biotechnol. 2022, 13, 95–100. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; KHAN, M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Abou-Aly, H.E.; Youssef, A.M.; El-Meihy, R.M.; Tawfik, T.A.; El-Akshar, E.A. Evaluation of heavy metals tolerant bacterial strains as antioxidant agents and plant growth promoters. Biocatal. Agric. Biotechnol. 2019, 19, 101110. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Boltz, D.F.; Mellon, M.G. Spectrophotometric Determination of Phosphorus as Molybdiphosphoric Acid. Anal. Chem. 1948, 20, 749–751. [Google Scholar] [CrossRef]
- Hemalatha, N.; Raja, N.; Jayachitra, A.; Rajalakshmi, A.; Valarmathi, N. Isolation and characterization of phosphate solubilizing bacteria and analyzing their effect on Capsicum annum L. Inter. J. Biol. Pharm. Res 2013, 4, 159–167. [Google Scholar]
- Sharath, S.; Triveni, S.; Nagaraju, Y.; Latha, P.C.; Vidyasagar, B. The Role of Phyllosphere Bacteria in Improving Cotton Growth and Yield Under Drought Conditions. Front. Agron. 2021, 3, 680466. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Skerman, V.B. A Guide to the Identification of the Genera of Bacteria, 2nd ed.; The Williams & Wilkins Co.: Baltimore, MD, USA, 1967. [Google Scholar]
- Setiawati, T.C.; Mutmainnah, L. Solubilization of potassium containing mineral by microorganisms from sugarcane rhizosphere. Agric. Agric. Sci. Procedia 2016, 9, 108–117. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN118. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Measure du taux de mycorrhization d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Amanullah, H.; Marwat, K.; Shah, P.; Maula, N.; Arifullah, S. Nitrogen levels and its time of application influence leaf area, height and biomass of maize planted at low and high density. Pak. J. Bot 2009, 41, 761–768. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Snell, F.D.; Snell, C.T. Colorimetric Methods of Analysis; D. van Nostrand Company, Inc.: New York, NY, USA, 1961. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo Enzymology; Kalyani Publishers: New Delhi, India, 1980; p. 286. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinhem, Germany, 1983; pp. 273–286. [Google Scholar]
- Alonazi, M.A.; Alwathnani, H.A.; AL-Barakah, F.N.I.; Alotaibi, F. Native Plant Growth-Promoting Rhizobacteria Containing ACC Deaminase Promote Plant Growth and Alleviate Salinity and Heat Stress in Maize (Zea mays L.) Plants in Saudi Arabia. Plants 2025, 14, 1107. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.; Ashour, A. Use of Cyanobacteria for Controlling Flax Seedling Blight. J. Agric. Chem. Biotechnol. 2018, 9, 259–261. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Reyad, A.M.; Khalaf, M.H.; Sheteiwy, M.S.; Dawood, M.F.A.; El-Sawah, A.M.; Shaban Ahmed, E.; Malik, A.; Al-Qahtani, W.H.; Abdel-Maksoud, M.A.; et al. Investigating the Endophyte Actinomycetota sp. JW0824 Strain as a Potential Bioinoculant to Enhance the Yield, Nutritive Value, and Chemical Composition of Different Cultivars of Anise (Pimpinella anisum L.) Seeds. Biology 2024, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- El-Amriti, F.A.; Ouf, S.A.; Abu-Elghait, M.; Desouky, S.E.; Mohamed, M.S.M. Alleviation of salt stress on Zea mays L. plant by PGPR isolates as an effective sustainable strategy. Biocatal. Agric. Biotechnol. 2024, 61, 103346. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Sheteiwy, M.S.; El-Keblawy, A.; Ulhassan, Z.; Khalaf, M.H.; Mohamed, H.S.; Okla, M.K.; AlGarawi, A.M.; El-Sawah, A.M.; Ahmed, E.S.; et al. The potential biofortification role of Actinopolyspora sp. JTT-01 in enhancing the yield and tissue chemical composition of caraway plants. BMC Plant Biol. 2025, 25, 540. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Shahid, M.; Altaf, M.; Ali, S.; Tyagi, A. Isolation and assessment of the beneficial effect of exopolysaccharide-producing PGPR in Triticum aestivum (L.) plants grown under NaCl and Cd-stressed conditions. Plant Physiol. Biochem. 2024, 215, 108973. [Google Scholar] [CrossRef] [PubMed]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Huang, C.; Peng, F.; Wang, T.; Liao, J.; Ma, S.; You, Q.; Xue, X. Synergistic combination of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria modulates morpho-physiological characteristics and soil structure in Nitraria tangutorum bobr. Under saline soil conditions. Res. Cold Arid Reg. 2022, 14, 393–402. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Boopathi, T.; Manivannan, P. Comprehensive Assessment of Ameliorative Effects of AMF in Alleviating Abiotic Stress in Tomato Plants. J. Fungi 2021, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, M.; Sannazzaro, A.I.; Ruiz, O.A.; Menéndez, A.B. Modulatory effects of Mesorhizobium tianshanense and Glomus intraradices on plant proline and polyamine levels during early plant response of Lotus tenuis to salinity. Plant Soil 2013, 364, 69–79. [Google Scholar] [CrossRef]
- Elhindi, K.M.; El-Din, A.S.; Elgorban, A.M. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 2017, 24, 170–179. [Google Scholar] [CrossRef]
- Borde, M.; Dudhane, M.; Jite, P. Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition. Crop Prot. 2011, 30, 265–271. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Meddich, A.; Ikan, C.; Lahlali, R.; Ait Barka, E.; Hajirezaei, M.-R.; Duponnois, R.; Baslam, M. Enhancing Maize Productivity and Soil Health under Salt Stress through Physiological Adaptation and Metabolic Regulation Using Indigenous Biostimulants. Plants 2023, 12, 3703. [Google Scholar] [CrossRef]
- Shabaan, M.; Asghar, H.N.; Zahir, Z.A.; Zhang, X.; Sardar, M.F.; Li, H. Salt-Tolerant PGPR Confer Salt Tolerance to Maize Through Enhanced Soil Biological Health, Enzymatic Activities, Nutrient Uptake and Antioxidant Defense. Front. Microbiol. 2022, 13, 901865. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).