The Sustainable Production of Terpenoids in Cyanobacterial Chassis
Abstract
1. Introduction
2. Synthetic Pathways of Terpenoids
2.1. The Upstream Module for the Synthesis of IPP and DMAPP

2.2. The Downstream Module for the Synthesis of Various Terpenoids
3. Enhance Terpenoids Production via Metabolic Engineering of Cyanobacteria
3.1. Metabolic Engineering of the MEP Pathway
3.2. Introduction of Exogenous MVA
3.3. Optimization and Regulation of Terpenoid Synthases
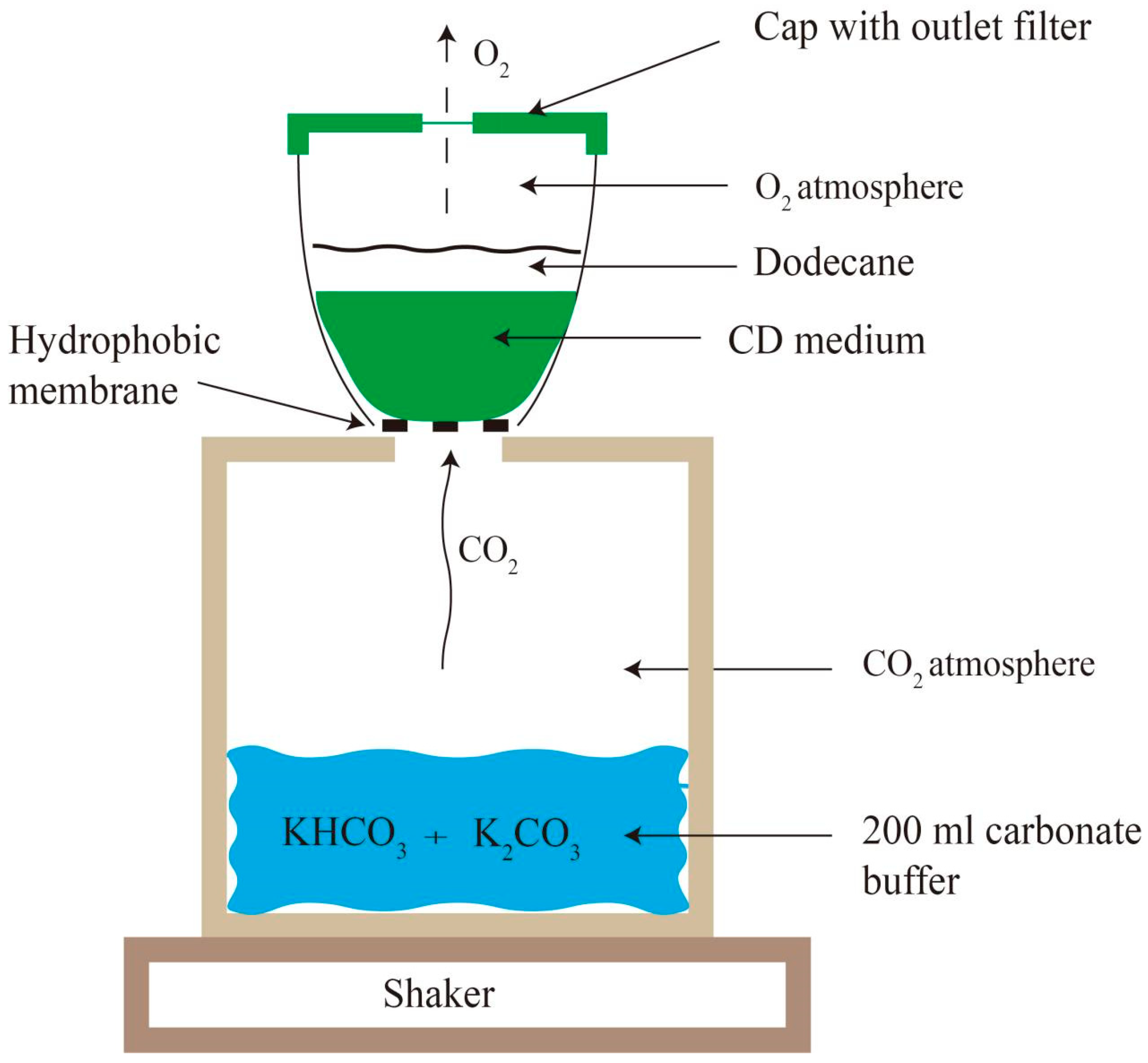
3.4. Optimization of Fermentation Conditions
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Nielsen, J.; Liu, Z. Engineering yeast metabolism for production of terpenoids for use as perfume ingredients, pharmaceuticals and biofuels. FEMS Yeast Res. 2017, 17, fox080. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G.; Lathe, R. Terpenes, hormones and life: Isoprene rule revisited. J. Endocrinol. 2019, 242, R9–R22. [Google Scholar] [CrossRef]
- Karunanithi, P.S.; Zerbe, P. Terpene synthases as metabolic gatekeepers in the evolution of plant terpenoid chemical diversity. Front. Plant Sci. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- David, B.; Wolfender, J.L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.H.; Lu, X.Y.; Zong, H.; Zhuge, B. Tuning geraniol biosynthesis via a novel decane-responsive promoter in Candida glycerinogenes. ACS Synth. Biol. 2022, 11, 1835–1844. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Chen, Z.; Yalikun, Y.; He, L.; Liu, T.; Ma, G. Engineering cyanobacteria as a new platform for producing taxol precursors directly from carbon dioxide. Biotechnol. Biofuels Bioprod. 2024, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, K.; Mutturi, S. Recent advances in the microbial production of squalene. World J. Microbiol. Biotechnol. 2022, 38, 91. [Google Scholar] [CrossRef]
- Zhou, P.; Ye, L.; Xie, W.; Lv, X.; Yu, H. Highly efficient biosynthesis of astaxanthin in Saccharomyces cerevisiae by integration and tuning of algal crtZ and bkt. Appl. Microbiol. Biotechnol. 2015, 99, 8419–8428. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Lin, Y.; Li, W.; Wang, D.; Ruan, S.; Yang, Y.; Liang, S. Metabolic engineering of pichia pastoris for high-level production of lycopene. ACS Synth. Biol. 2023, 12, 2961–2972. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Xiao, W.H.; Tyo, K.E.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef]
- Liu, T.; Khosla, C. Chemistry. A balancing act for Taxol precursor pathways in E. coli. Science 2010, 330, 44–45. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Z.; Ma, W.; Jiang, Y.; Jiang, W.; Wan, Y.; Xin, F.; Zhang, W.; Jiang, M. Metabolic engineering of Yarrowia lipolytica for efficient production of terpenoids. Ind. Eng. Chem. Res. 2024, 63, 14469–14479. [Google Scholar] [CrossRef]
- Einhaus, A.; Steube, J.; Freudenberg, R.A.; Barczyk, J.; Baier, T.; Kruse, O. Engineering a powerful green cell factory for robust photoautotrophic diterpenoid production. Metab. Eng. 2022, 73, 82–90. [Google Scholar] [CrossRef]
- Ezhumalai, G.; Arun, M.; Manavalan, A.; Rajkumar, R.; Heese, K. A Holistic Approach to Circular Bioeconomy Through the Sustainable Utilization of Microalgal Biomass for Biofuel and Other Value-Added Products. Microb Ecol. 2024, 87, 61. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Coman, D.; Beck, G.; Barja, M.V.; Colinas, M.; Graf, A.; Welsch, R.; Rütimann, P.; Bühlmann, P.; Bigler, L. Arabidopsis GERANYLGERANYL DIPHOSPHATE SYNTHASE 11 is a hub isozyme required for the production of most photosynthesis-related isoprenoids. New Phytol. 2016, 209, 252–264. [Google Scholar] [CrossRef]
- Yu, Y.; You, L.; Liu, D.; Hollinshead, W.; Tang, Y.J.; Zhang, F. Development of Synechocystis sp. PCC 6803 as a phototrophic cell factory. Mar. Drugs 2013, 11, 2894–2916. [Google Scholar] [CrossRef]
- Betterle, N.; Melis, A. Photosynthetic generation of heterologous terpenoids in cyanobacteria. Biotechnol. Bioeng. 2019, 116, 2041–2051. [Google Scholar] [CrossRef]
- Berla, B.M.; Saha, R.; Immethun, C.M.; Maranas, C.D.; Moon, T.S.; Pakrasi, H.B. Synthetic biology of cyanobacteria: Unique challenges and opportunities. Front. Microbiol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Branco Dos Santos, F.; Du, W.; Hellingwerf, K.J. Synechocystis: Not just a plug-bug for CO2, but a green E. coli. Front. Bioeng. Biotechnol. 2014, 2, 36. [Google Scholar] [CrossRef]
- Vasudevan, R.; Gale, G.A.R.; Schiavon, A.A.; Puzorjov, A.; Malin, J.; Gillespie, M.D.; Vavitsas, K.; Zulkower, V.; Wang, B.; Howe, C.J.; et al. CyanoGate: A Modular cloning suite for engineering cyanobacteria based on the plant MoClo syntax. Plant Physiol. 2019, 180, 39–55. [Google Scholar] [CrossRef]
- Hunter, W.N. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2007, 282, 21573–21577. [Google Scholar] [CrossRef]
- Lin, P.C.; Pakrasi, H.B. Engineering cyanobacteria for production of terpenoids. Planta 2019, 249, 145–154. [Google Scholar] [CrossRef]
- Kant, G.; Pandey, A.; Hasan, A.; Bux, F.; Kumari, F.; Srivastava, S. Cell factories for methylerythritol phosphate pathway mediated terpenoid biosynthesis: An application of modern engineering towards sustainability. Process Biochem. 2024, 139, 146–164. [Google Scholar] [CrossRef]
- Huang, P.W.; Wang, L.R.; Geng, S.S.; Ye, C.; Sun, X.M.; Huang, H. Strategies for enhancing terpenoids accumulation in microalgae. Appl. Microbiol. Biotechnol. 2021, 105, 4919–4930. [Google Scholar] [CrossRef]
- Chaves, J.E.; Melis, A. Biotechnology of cyanobacterial isoprene production. Appl. Microbiol. Biotechnol. 2018, 102, 6451–6458. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, S.; Li, H.; Xi, H.; Cheng, W.; Yang, C. A Ribulose-5-phosphate Shunt from the Calvin-Benson Cycle to Methylerythritol Phosphate Pathway for Enhancing Photosynthetic Terpenoid Production. ACS Synth. Biol. 2024, 13, 876–887. [Google Scholar] [CrossRef]
- Li, M.; Long, B.; Dai, S.Y.; Golden, J.W.; Wang, X.; Yuan, J.S. Altered Carbon Partitioning Enhances CO2 to Terpene Conversion in Cyanobacteria. BioDes. Res. 2022, 2022, 9897425. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; Lindberg, P. Metabolic engineering of Synechocystis sp. PCC 6803 for improved bisabolene production. Metab. Eng. Commun. 2020, 12, e00159. [Google Scholar] [CrossRef]
- Dienst, D.; Wichmann, J.; Mantovani, O.; Rodrigues, J.S.; Lindberg, P. High density cultivation for efficient sesquiterpenoid biosynthesis in Synechocystis sp. PCC 6803. Sci. Rep. 2020, 10, 5932. [Google Scholar] [CrossRef]
- Li, S.; Sun, T.; Chen, L.; Zhang, W. Light and carbon dioxide-driven synthesis of high-density fuel in Synechococcus elongates UTEX 2973. Chin. J. Biotechnol. 2020, 36, 2126–2138. [Google Scholar] [CrossRef]
- Englund, E.; Andersen-Ranberg, J.; Miao, R.; Hamberger, B.; Lindberg, P. Metabolic engineering of Synechocystis sp. PCC 6803 for production of the plant diterpenoid manoyl oxide. ACS Synth. Biol. 2015, 4, 1270–1278. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Xin, C.; Zheng, Y.; Cheng, Y.; Sun, S.; Li, R.; Zhu, X.G.; Dai, S.Y.; Rentzepis, P.M.; et al. Enhanced limonene production in cyanobacteria reveals photosynthesis limitations. Proc. Natl. Acad. Sci. USA 2016, 113, 14225–14230. [Google Scholar] [CrossRef]
- Gao, X.; Gao, F.; Liu, D.; Zhang, H.; Nie, X.; Yang, C. Engineering the methylerythritol phosphate pathway in cyanobacteria for photosynthetic isoprene production from CO2. Energy Environ. Sci. 2016, 9, 1400–1411. [Google Scholar] [CrossRef]
- Kiyota, H.; Okuda, Y.; Ito, M.; Hirai, M.Y.; Ikeuchi, M. Engineering of cyanobacteria for the photosynthetic production of limonene from CO2. J. Biotechnol. 2014, 185, 1–7. [Google Scholar] [CrossRef]
- Shimada, N.; Okuda, Y.; Maeda, K.; Umeno, D.; Takaichi, S.; Ikeuchi, M. Astaxanthin production in a model cyanobacterium Synechocystis sp. PCC 6803. J. Gen. Appl. Microbiol. 2020, 66, 116–120. [Google Scholar] [CrossRef]
- Davies, F.K.; Jinkerson, R.E.; Posewitz, M.C. Toward a photosynthetic microbial platform for terpenoid engineering. Photosynth. Res. 2015, 123, 265–284. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth. Res. 2016, 130, 123–135. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Heterologous synthesis of geranyllinalool, a diterpenol plant product, in the cyanobacterium Synechocystis. Appl. Microbiol. Biotechnol. 2017, 101, 2791–2800. [Google Scholar] [CrossRef]
- Lin, P.C.; Saha, R.; Zhang, F.; Pakrasi, H.B. Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2017, 7, 17503. [Google Scholar] [CrossRef]
- Germann, A.T.; Nakielski, A.; Dietsch, M.; Petzel, T.; Moser, D.; Triesch, S.; Westhoff, P.; Axmann, I.M. A systematic over-expression approach reveals native targets to increase squalene production in Synechocystis sp. PCC 6803. Front. Plant Sci. 2023, 14, 1024981. [Google Scholar] [CrossRef] [PubMed]
- Sebesta, J.; Peebles, C.A. Improving heterologous protein expression in Synechocystis sp. PCC 6803 for alpha-bisabolene production. Metab. Eng. Commun. 2019, 10, e00117. [Google Scholar] [CrossRef] [PubMed]
- Menin, B.; Lami, A.; Musazzi, S.; Petrova, A.A.; Santabarbara, S.; Casazza, A.P. A comparison of constitutive and inducible non-endogenous keto-carotenoids biosynthesis in Synechocystis sp. PCC 6803. Microorganisms 2019, 7, 501. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Wang, Q. Photoresponse Mechanism in cyanobacteria: Key factor in photoautotrophic chassis. Adv. Exp. Med. Biol. 2018, 1080, 75–96. [Google Scholar] [CrossRef]
- Ciebiada, M.; Kubiak, K.; Daroch, M. Modifying the cyanobacterial metabolism as a key to efficient biopolymer production in photosynthetic microorganisms. Int. J. Mol. Sci. 2020, 21, 7204. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, H.; Zhang, W.; Li, Y. Production of industrial chemicals from CO2 by engineering cyanobacteria. Adv. Exp. Med. Biol. 2018, 1080, 97–116. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total. Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Guljamow, A.; Kreische, M.; Ishida, K.; Liaimer, A.; Altermark, B.; Bähr, L.; Hertweck, C.; Ehwald, R.; Dittmann, E. High-density cultivation of terrestrial nostoc strains leads to reprogramming of secondary metabolome. Appl. Environ. Microbiol. 2017, 83, e01510-17. [Google Scholar] [CrossRef]
- Lippi, L.; Bähr, L.; Wüstenberg, A.; Wilde, A.; Steuer, R. Exploring the potential of high-density cultivation of cyanobacteria for the production of cyanophycin. Algal. Res. 2018, 31, 363–366. [Google Scholar] [CrossRef]
- Cheah, Y.E.; Albers, S.C.; Peebles, C.A. A novel counter-selection method for markerless genetic modification in Synechocystis sp. PCC 6803. Biotechnol. Prog. 2013, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.; Hejazi, M.; Kraft, R.; Ziegler, K.; Lockau, W. Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-L-arginyl-poly-L-aspartic acid (cyanophycin): Molecular cloning of the gene of Synechocystis sp. PCC 6803, expression in Escherichia coli, and biochemical characterization of the purified enzyme. Eur. J. Biochem. 1999, 263, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Krasikov, V.; Aguirre von Wobeser, E.; Dekker, H.L.; Huisman, J.; Matthijs, H.C. Time-series resolution of gradual nitrogen starvation and its impact on photosynthesis in the cyanobacterium Synechocystis PCC 6803. Physiol. Plant. 2012, 145, 426–439. [Google Scholar] [CrossRef]
- Fuszard, M.A.; Ow, S.Y.; Gan, C.S.; Zhou, C. The quantitative proteomic response of Synechocystis sp. PCC6803 to phosphate acclimation. Aquat. Biosyst. 2013, 9, 5. [Google Scholar] [CrossRef]
- Sheng, J.; Kim, H.W.; Badalamenti, J.P.; Sridharakrishnan, S.; Krajmalnik-Brown, R.; Rittmann, B.E.; Vannela, R. Effects of temperature shifts on growth rate and lipid characteristics of Synechocystis sp. PCC6803 in a bench-top photobioreactor. Bioresour. Technol. 2011, 102, 11218–11225. [Google Scholar] [CrossRef] [PubMed]
- van Alphen, P.; Abedini Najafabadi, H.; Branco Dos Santos, F.; Hellingwerf, K.J. Increasing the photoautotrophic growth rate of Synechocystis sp. PCC 6803 by identifying the limitations of its cultivation. Biotechnol. J. 2018, 13, e1700764. [Google Scholar] [CrossRef]
- Ungerer, J.; Lin, P.C.; Chen, H.Y.; Pakrasi, H.B. Adjustments to Photosystem Stoichiometry and Electron Transfer Proteins Are Key to the Remarkably Fast Growth of the Cyanobacterium Synechococcus elongatus UTEX 2973. mBio 2018, 9, e02327-17. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, S.; Sun, J.; Zeng, X.; Duan, Y.; Luan, G.; Lu, X. Rapidly improving high light and high temperature tolerances of cyanobacterial cell factories through the convenient introduction of an AtpA-C252F mutation. Front. Microbiol. 2021, 12, 647164. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Selão, T.T.; Norling, B.; Nixon, P.J. Newly discovered Synechococcus sp. PCC 11901 is a robust cyanobacterial strain for high biomass production. Commun. Biol. 2020, 3, 215. [Google Scholar] [CrossRef]
- Gao, Y.L.; Cournoyer, J.E.; De, B.C.; Wallace, C.L.; Ulanov, A.V.; La Frano, M.R.; Mehta, A.P. Introducing carbon assimilation in yeasts using photosynthetic directed endosymbiosis. Nat. Commun. 2024, 15, 5947. [Google Scholar] [CrossRef]
| Product | Strain | Titer | Time (Days) | Engineering Strategies | References |
|---|---|---|---|---|---|
| Isoprene | Synechocystis 6803 | 12.3 mg/g DCW | 4 | CpcB-IspS | [26] |
| Isoprene | Synechococcus 7942 | 105.2 mg/L | 6 | Ru5P-PP, IspG-IspH | [27] |
| Pinene | Synechococcus 7942 | 5.2 mg/L | 6 | Ru5P-PP + IspG-IspH, LS-GPPS | [28] |
| Limonene | Synechococcus 7942 | 21 mg/L | 5 | LS-GGPPS | [18] |
| β-Phellandrene | Synechocystis 6803 | 24 mg/g DCW | 2 | Exogenous MVA pathway, CpcB-PHLS, Nptl-GPPS | [18] |
| Taxadiene | Synechocystis 6803 | 2.94 mg/L | 7 | GGPPS-TASY, DXS-IspA | [7] |
| Bisabolene | Synechocystis 6803 | 186 mg/L | 12 | AgB-IspA, DXS-IDI | [29] |
| Patchoulol | Synechocystis 6803 | 17.3 mg/L | 8 | PpetE-Ps | [30] |
| β-Caryophyllene | Synechococcus elongatus UTEX 2973 | 212.37 μg/L | 6 | IDI1-GPPS-IspA-TPS21 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, B.; Qiu, L.; Lv, R.; Yu, Z. The Sustainable Production of Terpenoids in Cyanobacterial Chassis. Microorganisms 2025, 13, 1342. https://doi.org/10.3390/microorganisms13061342
Hong B, Qiu L, Lv R, Yu Z. The Sustainable Production of Terpenoids in Cyanobacterial Chassis. Microorganisms. 2025; 13(6):1342. https://doi.org/10.3390/microorganisms13061342
Chicago/Turabian StyleHong, Bo, Ling Qiu, Ruo Lv, and Zongxia Yu. 2025. "The Sustainable Production of Terpenoids in Cyanobacterial Chassis" Microorganisms 13, no. 6: 1342. https://doi.org/10.3390/microorganisms13061342
APA StyleHong, B., Qiu, L., Lv, R., & Yu, Z. (2025). The Sustainable Production of Terpenoids in Cyanobacterial Chassis. Microorganisms, 13(6), 1342. https://doi.org/10.3390/microorganisms13061342







