Comparative Analysis of Nano-Bactericides and Thiodiazole–Copper on Tomato Rhizosphere Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tomato Seedling Growth
2.3. Tomato Treatment
2.4. Rhizosphere Soil Sample Collection
2.5. DNA Extraction and Amplification
2.6. Bioinformatic Analysis
3. Results
3.1. Changes in Microbial Members After Treatment with Nano-Bactericide and Bactericide
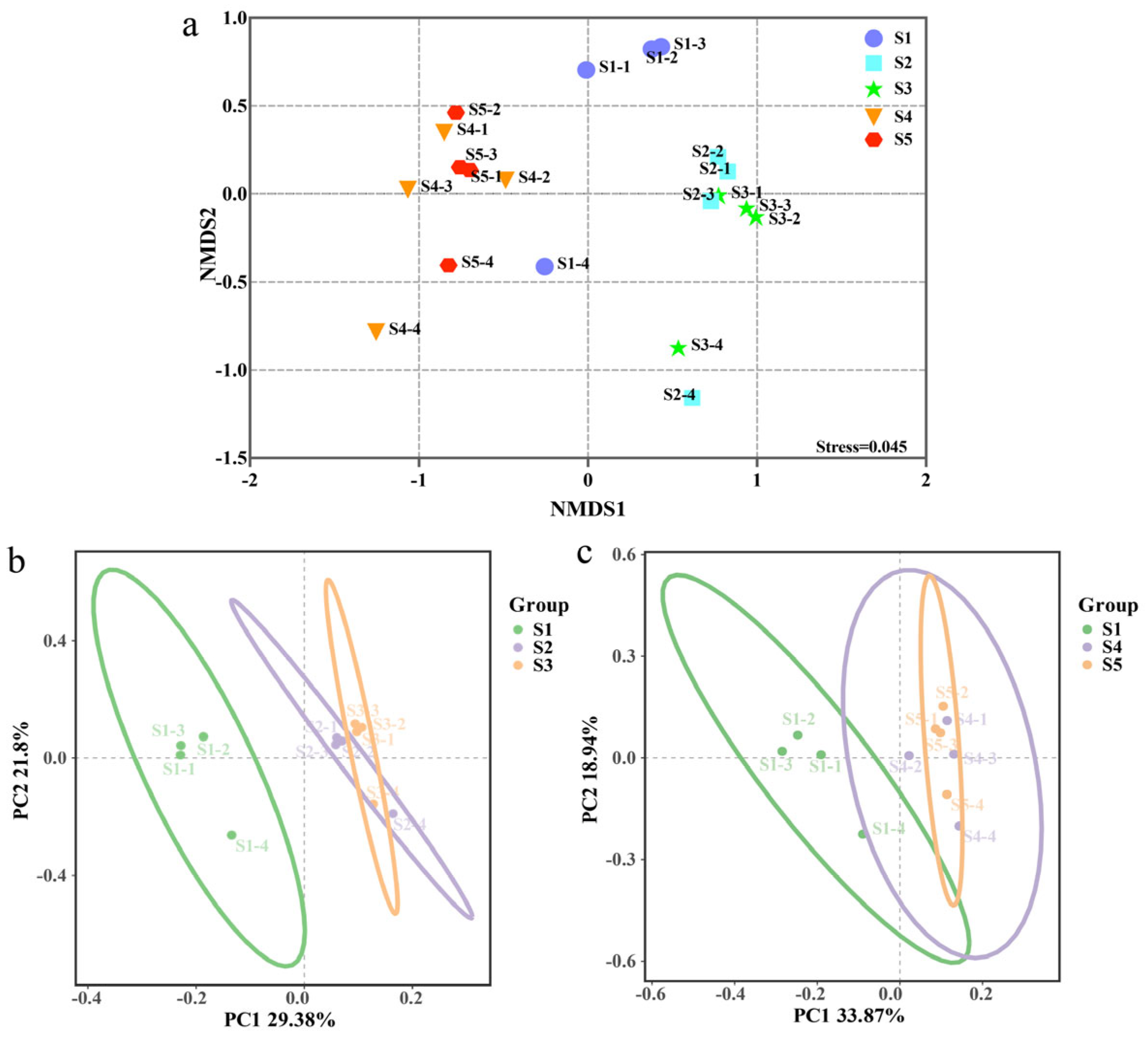
3.2. Microbial Alpha Diversity and Beta Diversity
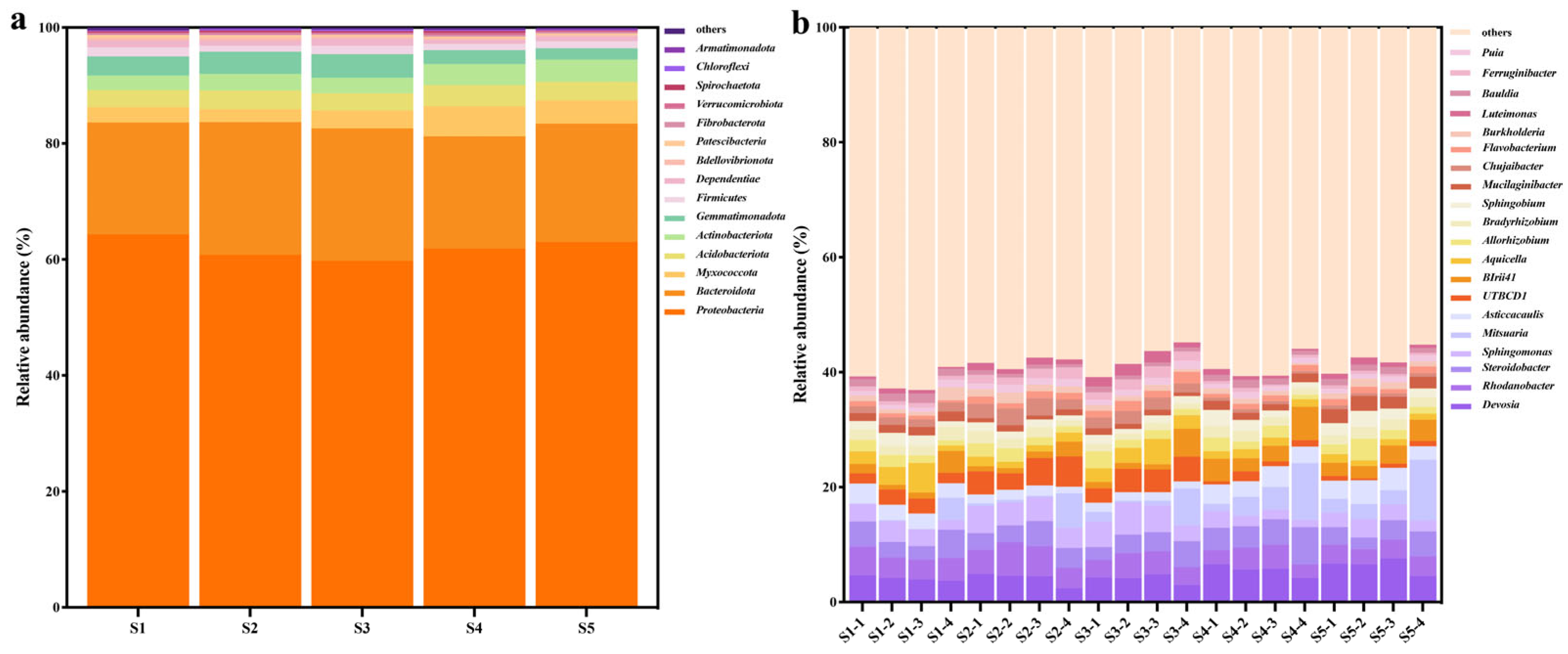
3.3. Tomato Bacterial Composition Affected by Nano-Bactericide and Bactericide
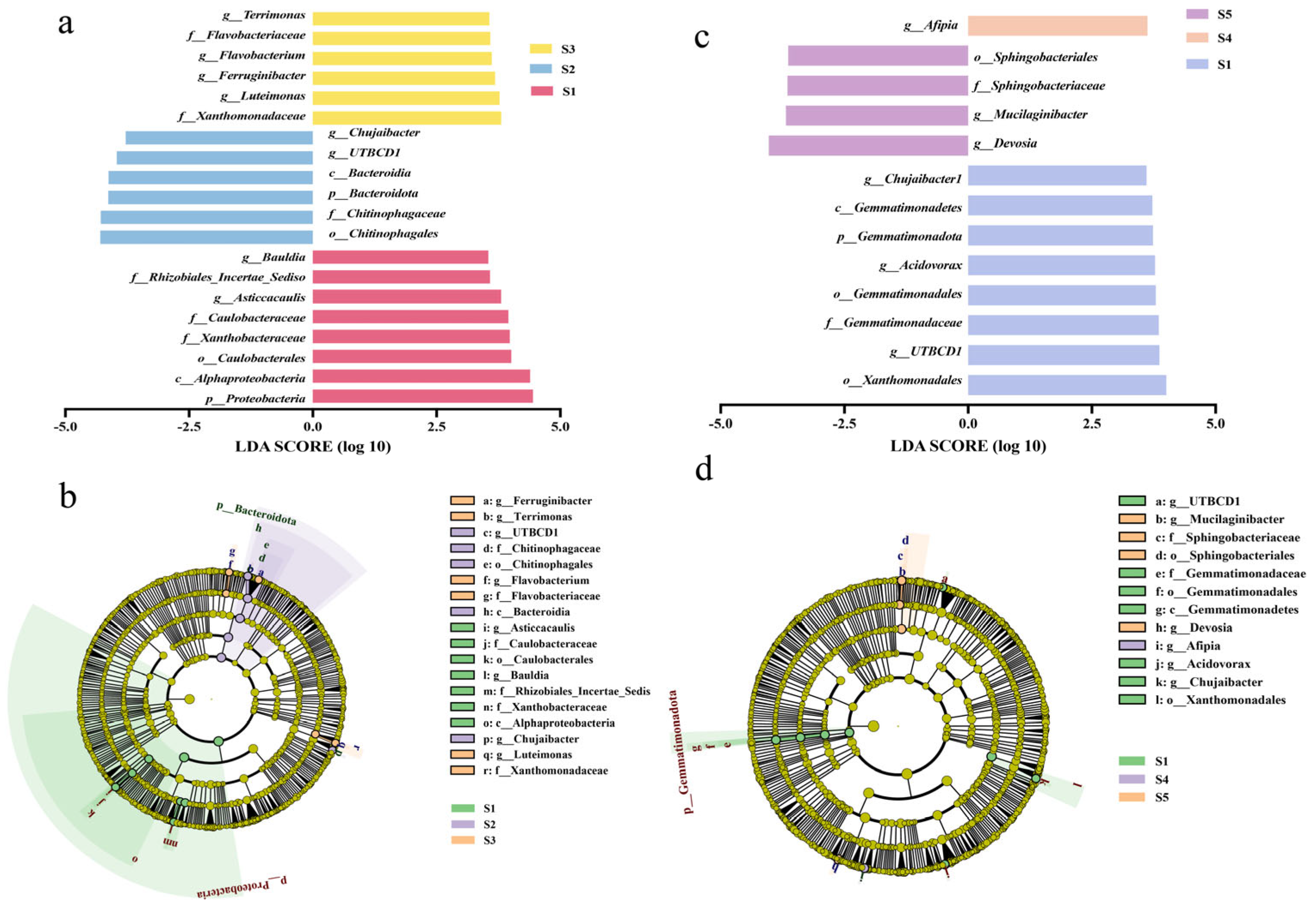
3.4. LEfSe Analysis of the Rhizosphere Bacteria
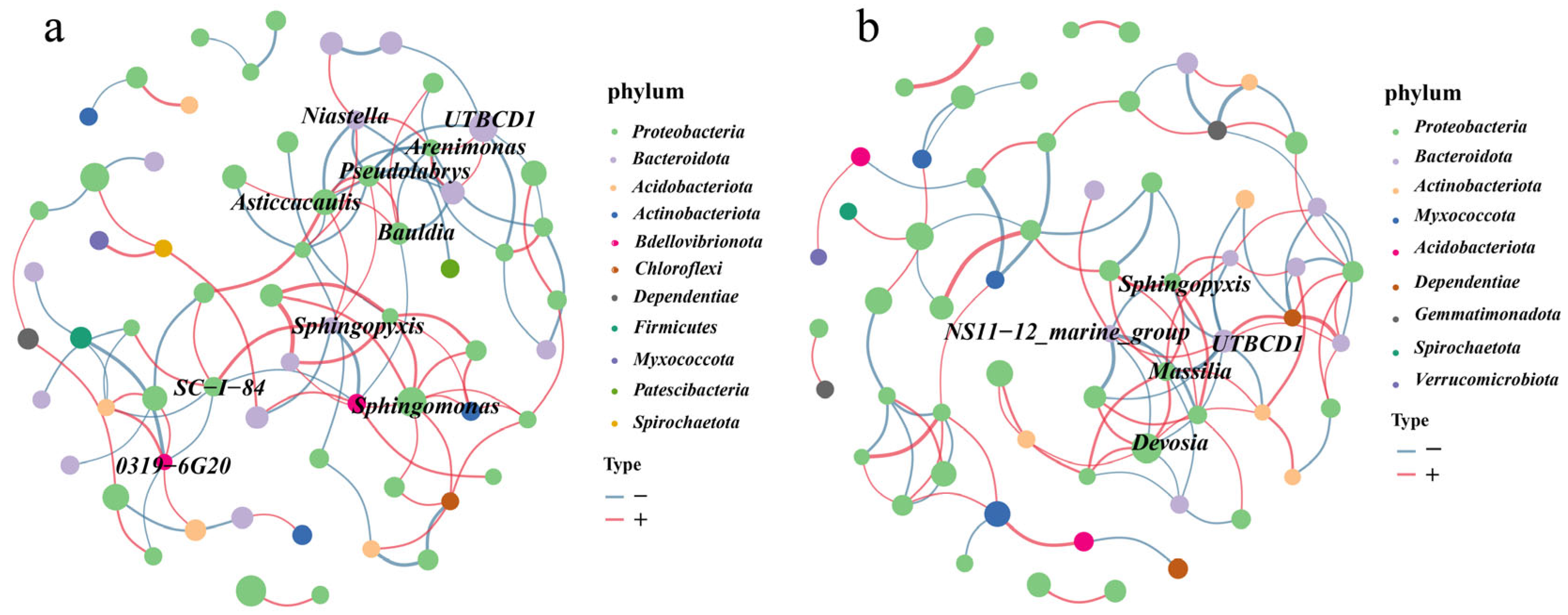
3.5. Co-Occurrence Networks Analysis
3.6. The Functional Prediction of Microbial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathore, A.C.; Mehta, H.; Jayaprakash, J.; Singh, C.; Gupta, A.K.; Kumar, P.; Islam, S.; Kar, S.K.; Patra, S.; Chand, L.; et al. Modified plant architecture integrated with liquid fertilizers improves fruit productivity and quality of tomato in North West Himalaya, India. Sci. Rep. 2021, 11, 18664. [Google Scholar] [CrossRef] [PubMed]
- Bhoomika, S.; Shalunkhe, S.R.; Sakthi, A.R.; Saraswathi, T.; Manonmani, S.; Raveendran, M.; Sudha, M. CRISPR-Cas9: Unraveling genetic secrets to enhance floral and fruit traits in tomato. Mol. Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019, 47, 13–23. [Google Scholar] [CrossRef]
- Van Eck, J.; Keen, P.; Tjahjadi, M. Agrobacterium tumefaciens-mediated transformation of tomato. Methods Mol. Biol. 2019, 1864, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhang, Y.; Cheng, Y.; Tian, Y.; Luo, J.; Hu, Z.; Chen, G. The role of melatonin in tomato stress response, growth and development. Plant Cell Rep. 2022, 41, 1631–1650. [Google Scholar] [CrossRef]
- Sun, C.; Yao, G.; Zhao, J.; Chen, R.; Hu, K.; He, G.; Zhang, H. SlERF109-like and SlNAC1 coordinately regulated tomato ripening by inhibiting ACO1 transcription. Int. J. Mol. Sci. 2024, 25, 1873. [Google Scholar] [CrossRef]
- Prasad, A.; Sharma, N.; Hari-Gowthem, G.; Muthamilarasan, M.; Prasad, M. Tomato yellow leaf curl virus: Impact, challenges, and management. Trends Plant Sci. 2020, 25, 897–911. [Google Scholar] [CrossRef]
- Potnis, N. Harnessing eco-evolutionary dynamics of Xanthomonads on tomato and pepper to tackle new problems of an old disease. Annu. Rev. Phytopathol. 2021, 59, 289–310. [Google Scholar] [CrossRef]
- Olowe, O.M.; Nicola, L.; Asemoloye, M.D.; Akanmu, A.O.; Babalola, O.O. Trichoderma: Potential bio-resource for the management of tomato root rot diseases in Africa. Microbiol. Res. 2022, 257, 126978. [Google Scholar] [CrossRef]
- Hussain, M.A.; Nijabat, A.; Rehman, M.M.U.; Qurashi, R.; Siddiqui, M.H.; Alamri, S.; Mashwani, Z.-U.; Leghari, S.U.K.; Shah, M.A.; Zaman, Q.U. Management of tomato bacterial canker disease by the green fabricated silver nanoparticles. BMC Plant Biol. 2024, 24, 597. [Google Scholar] [CrossRef]
- Tripathi, Y.N.; Singh, V.K.; Kumar, S.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Identification of hub genes and potential networks by centrality network analysis of PCR amplified Fusarium oxysporum f. sp. lycopersici EF1a gene. BMC Microbiol. 2024, 24, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the fruit metabolome in tomato breeding. Cell 2018, 172, 249–261.e12. [Google Scholar] [CrossRef] [PubMed]
- Chitwood-Brown, J.; Vallad, G.E.; Lee, T.G.; Hutton, S.F. Breeding for resistance to Fusarium wilt of tomato: A review. Genes 2021, 12, 1673. [Google Scholar] [CrossRef] [PubMed]
- García-Machado, F.J.; García-García, A.L.; Borges, A.A.; Jiménez-Arias, D. Root treatment with a vitamin K3 derivative: A promising alternative to synthetic fungicides against Botrytis cinerea in tomato plants. Pest Manag. Sci. 2022, 78, 974–981. [Google Scholar] [CrossRef]
- Karimi, P.; Sadeghi, S.; Kariminejad, F.; Sadani, M.; Asadi, A.M.S.; Oghazyan, A.; Bay, A.; Mahmudiono, T.; Fakhri, Y. The concentration of pesticides in tomato: A global systematic review, meta-analysis, and health risk assessment. Environ. Sci. Pollut. Res. Int. 2023, 30, 103390–103404. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bacteria, fungi, and enzymes in soil treated with sulcotrione and terbuthylazine. Int. J. Mol. Sci. 2023, 24, 14469. [Google Scholar] [CrossRef]
- Zheng, X.; Jahn, M.T.; Sun, M.; Friman, V.-P.; Balcazar, J.L.; Wang, J.; Shi, Y.; Gong, X.; Hu, F.; Zhu, Y.-G. Organochlorine contamination enriches virus-encoded metabolism and pesticide degradation associated auxiliary genes in soil microbiomes. ISME J. 2022, 16, 1397–1408. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Hannula, S.E.; Hundscheid, M.P.J.; Gunnewiek, P.J.A.K.; de Boer, W. Stimulated saprotrophic fungi in arable soil extend their activity to the rhizosphere and root microbiomes of crop seedlings. Environ. Microbiol. 2021, 23, 6056–6073. [Google Scholar] [CrossRef]
- Kalwani, M.; Chakdar, H.; Srivastava, A.; Pabbi, S.; Shukla, P. Effects of nanofertilizers on soil and plant-associated microbial communities: Emerging trends and perspectives. Chemosphere 2022, 287, 132107. [Google Scholar] [CrossRef]
- Wu, L.X.; Wang, Y.; Lyu, H.; Chen, X.D. Effects of a compound Trichoderma agent on Coptis chinensis growth, nutrients, enzyme activity, and microbial community of rhizosphere soil. PeerJ 2023, 11, e15652. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Song, B.; Li, X.; Huang, W. Long-term cultivation of sugar beet: Effect on rhizosphere micro-flora, soil fertility and beet productivity. Sugar Tech 2022, 24, 1821–1831. [Google Scholar] [CrossRef]
- Lopes, L.D.; Wang, P.; Futrell, S.L.; Schachtman, D.P. Sugars and jasmonic acid concentration in root exudates affect maize rhizosphere bacterial communities. Appl. Environ. Microbiol. 2022, 88, e0097122. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kim, Y.-H.; Kang, S.-M.; Lee, J.-H.; Lee, I.-J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Process. Biochem. 2011, 46, 440–447. [Google Scholar] [CrossRef]
- Wang, T.; Gao, M.; Shao, W.; Wang, L.; Yang, C.; Wang, X.; Yao, S.; Zhang, B. Dissecting the role of soybean rhizosphere-enriched bacterial taxa in modulating nitrogen-cycling functions. Appl. Microbiol. Biotechnol. 2024, 108, 347. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Ghoulam, C.; Barakat, A.; Zeroual, Y.; Bargaz, A. Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher puse efficiency and crop productivity. J. Adv. Res. 2021, 38, 13–28. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Rahman, M.U.; Fakhar, A.; Rafique, M.; et al. Plant growth-promoting rhizobacteria eliminate the effect of drought stress in plants: A review. Front. Plant Sci. 2022, 13, 875774. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Dukare, A.; Mhatre, P.; Maheshwari, H.S.; Bagul, S.; Manjunatha, B.S.; Khade, Y.; Kamble, U. Delineation of mechanistic approaches of rhizosphere microorganisms facilitated plant health and resilience under challenging conditions. 3 Biotech 2022, 12, 57. [Google Scholar] [CrossRef]
- Yin, C.; Vargas, J.M.C.; Schlatter, D.C.; Hagerty, C.H.; Hulbert, S.H.; Paulitz, T.C. Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome 2021, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, J.; Liu, M.; Li, H.; Sun, Q.; Nechitaylo, G.S.; Bogoslovskaya, O.A.; Olkhovskaya, I.P.; Glushchenko, N.N. Tomato response to metal nanoparticles introduction into the nutrient medium. IET Nanobiotechnol. 2020, 14, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Kumari, A.; Mahanta, M.; Upamanya, G.K.; Heisnam, P.; Borua, S.; Kaman, P.K.; Mishra, A.K.; Mallik, M.; Muthukrishnan, G.; et al. Nanotechnological approaches for management of soil-borne plant pathogens. Front. Plant Sci. 2023, 14, 1136233. [Google Scholar] [CrossRef]
- Wang, L.; Pan, T.; Gao, X.; An, J.; Ning, C.; Li, S.; Cai, K. Silica nanoparticles activate defense responses by reducing reactive oxygen species under Ralstonia solanacearum infection in tomato plants. NanoImpact 2022, 28, 100418. [Google Scholar] [CrossRef]
- Rashid, M.I.; Shah, G.A.; Sadiq, M.; Amin, N.U.; Ali, A.M.; Ondrasek, G.; Shahzad, K. Nanobiochar and copper oxide nanoparticles mixture synergistically increases soil nutrient availability and improves wheat production. Plants 2023, 12, 1312. [Google Scholar] [CrossRef]
- Liang, W.; Cheng, J.; Zhang, J.; Xiong, Q.; Jin, M.; Zhao, J. pH-responsive on-demand alkaloids release from core-shell ZnO@ZIF-8 nanosphere for synergistic control of bacterial wilt disease. ACS Nano 2022, 16, 2762–2773. [Google Scholar] [CrossRef]
- Abd-Ellatif, S.; Ibrahim, A.A.; Safhi, F.A.; Razik, E.S.A.; Kabeil, S.S.A.; Aloufi, S.; Alyamani, A.A.; Basuoni, M.M.; Alshamrani, S.M.; Elshafie, H.S. Green synthesized of Thymus vulgaris chitosan nanoparticles induce relative WRKY-genes expression in Solanum lycopersicum against Fusarium solani, the causal agent of root rot disease. Plants 2022, 11, 3129. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Khader, A.; Mahmoud, E. Green iron oxide nanoparticles and magnetic nanobiochar: Enhancing tomato performance, phytochemicals, and root-knot nematode resistance. BMC Plant Biol. 2024, 24, 469. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, E.; Singh, N.; Khandelwal, N.; Ganie, Z.A.; Choudhary, A.; Monikh, F.A.; Darbha, G.K. Impact of nanoplastic debris on the stability and transport of metal oxide nanoparticles: Role of varying soil solution chemistry. Chemosphere 2022, 308, 136091. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Q.; Zheng, T.; Mo, F.; Ouyang, S. Iron oxide nanoparticles in the soil environment: Adsorption, transformation, and environmental risk. J. Hazard. Mater. 2023, 459, 132107. [Google Scholar] [CrossRef]
- Ali, S.; Mir, R.A.; Tyagi, A.; Manzar, N.; Kashyap, A.S.; Mushtaq, M.; Raina, A.; Park, S.; Sharma, S.; Mir, Z.A.; et al. Chromium toxicity in plants: Signaling, mitigation, and future perspectives. Plants 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Komatsu, S. Plant proteomic research for improvement of food crops under stresses: A review. Mol. Omics 2021, 17, 860–880. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-Y.; Huang, Y.; Carvalho, R.; Choudhary, M.; Da Silva, S.; Colee, J.; Huerta, A.; Vallad, G.E.; Freeman, J.H.; Jones, J.B.; et al. Magnesium oxide nanomaterial, an alternative for commercial copper bactericides: Field-scale tomato bacterial spot disease management and total and bioavailable metal accumulation in soil. Environ. Sci. Technol. 2021, 55, 13561–13570. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic nanoparticles for antimicrobial applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef]
- Berta, L.; Coman, N.A.; Rusu, A.; Tanase, C. A review on plant-mediated synthesis of bimetallic nanoparticles, characterisation and their biological applications. Materials 2021, 14, 7677. [Google Scholar] [CrossRef]
- Ning, W.; Luo, X.; Zhang, Y.; Tian, P.; Xiao, Y.; Li, S.; Yang, X.; Li, F.; Zhang, D.; Zhang, S.; et al. Broad-spectrum nano-bactericide utilizing antimicrobial peptides and bimetallic Cu-Ag nanoparticles anchored onto multiwalled carbon nanotubes for sustained protection against persistent bacterial pathogens in crops. Int. J. Biol. Macromol. 2024, 265, 131042. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhu, G.N.; Su, L. Pubertal exposure to thiodiazole copper inhibits thyroid function in juvenile female rats. Exp. Toxicol. Pathol. 2010, 62, 163–169. [Google Scholar] [CrossRef]
- Dai, A.; Zheng, Z.; Huang, Y.; Yu, L.; Wang, Z.; Jian, W. Hydrazone modification of non-food natural product sclareolide as potential agents for plant disease. Heliyon 2022, 8, e12391. [Google Scholar] [CrossRef]
- Nossa, C.W.; Oberdorf, W.E.; Yang, L.; Aas, J.A.; Paster, B.J.; DeSantis, T.Z.; Brodie, E.L.; Malamud, D.; Poles, M.A.; Pei, Z. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 2010, 16, 4135–4144. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Chao, A.; Bunge, J. Estimating the number of species in a stochastic abundance model. Biometrics 2002, 58, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.C.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef]
- Galeano-Castañeda, Y.; Bascuñán, P.; Serre, D.; Correa, M.M. Trans-stadial fate of the gut bacterial microbiota in Anopheles albimanus. Acta Trop. 2020, 201, 105204. [Google Scholar] [CrossRef] [PubMed]
- Galeano-Castañeda, Y.; Bascuñán, P.; Serre, D.; Correa, M.M. Taxonomic hierarchy of the phylum Firmicutes and novel Firmicutes species originated from various environments in Korea. J. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef]
- Chen, M.; Cui, Y.; Liu, C.; Tong, X.; Wang, M.; Wu, C.; Liu, Y.; Zhao, Y.; Chen, X. Characteristics of the microbiome in lung adenocarcinoma tissue from patients in Kunming city of southwestern China. Environ. Sci. Pollut. Res. Int. 2023, 30, 49992–50001. [Google Scholar] [CrossRef]
- Li, J.; Meng, F.; Jiang, M.; Zhang, H.; Chu, G.; Tao, R. Assembly and co-occurrence patterns of rhizosphere bacterial communities are closely linked to soil fertility during continuous cropping of cut chrysanthemum (Chrysanthemum morifolium Ramat). J. Appl. Microbiol. 2023, 134, lxad175. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.Y.; Ma, J.; Li, X.D.; Li, Y.H. Moss habitats distinctly affect their associated bacterial community structures as revealed by the high-throughput sequencing method. World J. Microbiol. Biotechnol. 2018, 34, 58. [Google Scholar] [CrossRef]
- Deng, J.; Yin, Y.; Zhu, W.; Zhou, Y. Variations in soil bacterial community diversity and structures among different revegetation types in the baishilazi nature reserve. Front. Microbiol. 2018, 9, 2874. [Google Scholar] [CrossRef]
- Wei, H.; Peng, C.; Yang, B.; Song, H.; Li, Q.; Jiang, L.; Wei, G.; Wang, K.; Wang, H.; Liu, S.; et al. Contrasting soil bacterial community, diversity, and function in two forests in China. Front. Microbiol. 2018, 9, 1693. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, S.H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, P.; Wang, Y.; Li, W.; Cui, X.; Zhou, J.; Peng, F.; Dai, L. Earthworm activity optimized the rhizosphere bacterial community structure and further alleviated the yield loss in continuous cropping lily (Lilium lancifolium Thunb.). Sci. Rep. 2021, 11, 20840. [Google Scholar] [CrossRef]
- Huang, F.; Deng, X.; Gao, L.; Cai, X.; Yan, D.; Cai, Y.; Chen, X.; Yang, M.; Tong, W.; Yu, L. Effect of marigold (Tagetes erecta L.) on soil microbial communities in continuously cropped tobacco fields. Sci. Rep. 2022, 12, 19632. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O.; Ayangbenro, A. Metagenomic profiling of the community structure, diversity, and nutrient pathways of bacterial endophytes in maize plant. Antonie Van Leeuwenhoek 2020, 113, 1559–1571. [Google Scholar] [CrossRef]
- Martin, H.; Rogers, L.A.; Moushtaq, L.; Brindley, A.A.; Forbes, P.; Quinton, A.R.; Murphy, A.R.J.; Hipperson, H.; Daniell, T.J.; Ndeh, D.; et al. Metabolism of hemicelluloses by root-associated Bacteroidota species. ISME J. 2025, 19, wraf022. [Google Scholar] [CrossRef]
- Pan, X.; Raaijmakers, J.M.; Carrión, V.J. Importance of Bacteroidetes in host-microbe interactions and ecosystem functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar] [CrossRef]
- Orelle, C.; Mathieu, K.; Jault, J.-M. Multidrug ABC transporters in bacteria. Res. Microbiol. 2019, 170, 381–391. [Google Scholar] [CrossRef]
- de Oliveira, M.C.B.; Balan, A. The ATP-Binding Cassette (ABC) transport systems in Mycobacterium Tuberculosis: Structure, function, and possible targets for therapeutics. Biology 2020, 9, 443. [Google Scholar] [CrossRef]
- Agostini, M.; Traldi, P.; Hamdan, M. Mass spectrometry investigation of some ATP-Binding Cassette (ABC) proteins. Medicina 2024, 60, 200. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Ogunniyi, A.D.; Diallo, N.; Huet, Y.; Desnottes, J.-F.; Paton, J.C.; Escaich, S.; Trombe, M.-C. RegR, a global LacI/GalR family regulator, modulates virulence and competence in Streptococcus pneumoniae. Infect. Immun. 2003, 71, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.L.; Parente, D.J.; Shis, D.L.; Hessman, J.A.; Chazelle, A.; Bennett, M.R.; Teichmann, S.A.; Swint-Kruse, L. Allorep: A repository of sequence, structural and mutagenesis data for the LacI/GalR transcription regulators. J. Mol. Biol. 2016, 428, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Andargie, Y.E.; Lee, G.; Jeong, M.; Tagele, S.B.; Shin, J.H. Deciphering key factors in pathogen-suppressive microbiome assembly in the rhizosphere. Front. Plant Sci. 2023, 14, 1301698. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, W.; Luo, X.; Zhang, Y.; Li, S.; Yang, X.; Wang, X.; Chen, Y.; Xu, Y.; Zhang, D.; Zhang, S.; et al. Comparative Analysis of Nano-Bactericides and Thiodiazole–Copper on Tomato Rhizosphere Microbiome. Microorganisms 2025, 13, 1327. https://doi.org/10.3390/microorganisms13061327
Ning W, Luo X, Zhang Y, Li S, Yang X, Wang X, Chen Y, Xu Y, Zhang D, Zhang S, et al. Comparative Analysis of Nano-Bactericides and Thiodiazole–Copper on Tomato Rhizosphere Microbiome. Microorganisms. 2025; 13(6):1327. https://doi.org/10.3390/microorganisms13061327
Chicago/Turabian StyleNing, Weimin, Xiangwen Luo, Yu Zhang, Shijun Li, Xiao Yang, Xin Wang, Yueyue Chen, Yashuang Xu, Deyong Zhang, Songbai Zhang, and et al. 2025. "Comparative Analysis of Nano-Bactericides and Thiodiazole–Copper on Tomato Rhizosphere Microbiome" Microorganisms 13, no. 6: 1327. https://doi.org/10.3390/microorganisms13061327
APA StyleNing, W., Luo, X., Zhang, Y., Li, S., Yang, X., Wang, X., Chen, Y., Xu, Y., Zhang, D., Zhang, S., & Liu, Y. (2025). Comparative Analysis of Nano-Bactericides and Thiodiazole–Copper on Tomato Rhizosphere Microbiome. Microorganisms, 13(6), 1327. https://doi.org/10.3390/microorganisms13061327






