The Vaginal Microbiome: Associations with Vaginal pH, Menopause and Metabolic Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Collection
2.2.1. Physiological Measurements, Questionnaire Collection, and Biochemical Tests
2.2.2. Vaginal Secretion Sample Collection and Analysis
2.3. Common Vaginal Pathogens Identification
2.4. Full-Length 16S rRNA Gene Sequencing and Library Preparation
2.5. Bioinformatics Analysis
2.5.1. Sequence Processing
2.5.2. Microbiome Analysis
2.6. Statistics Analysis
3. Results
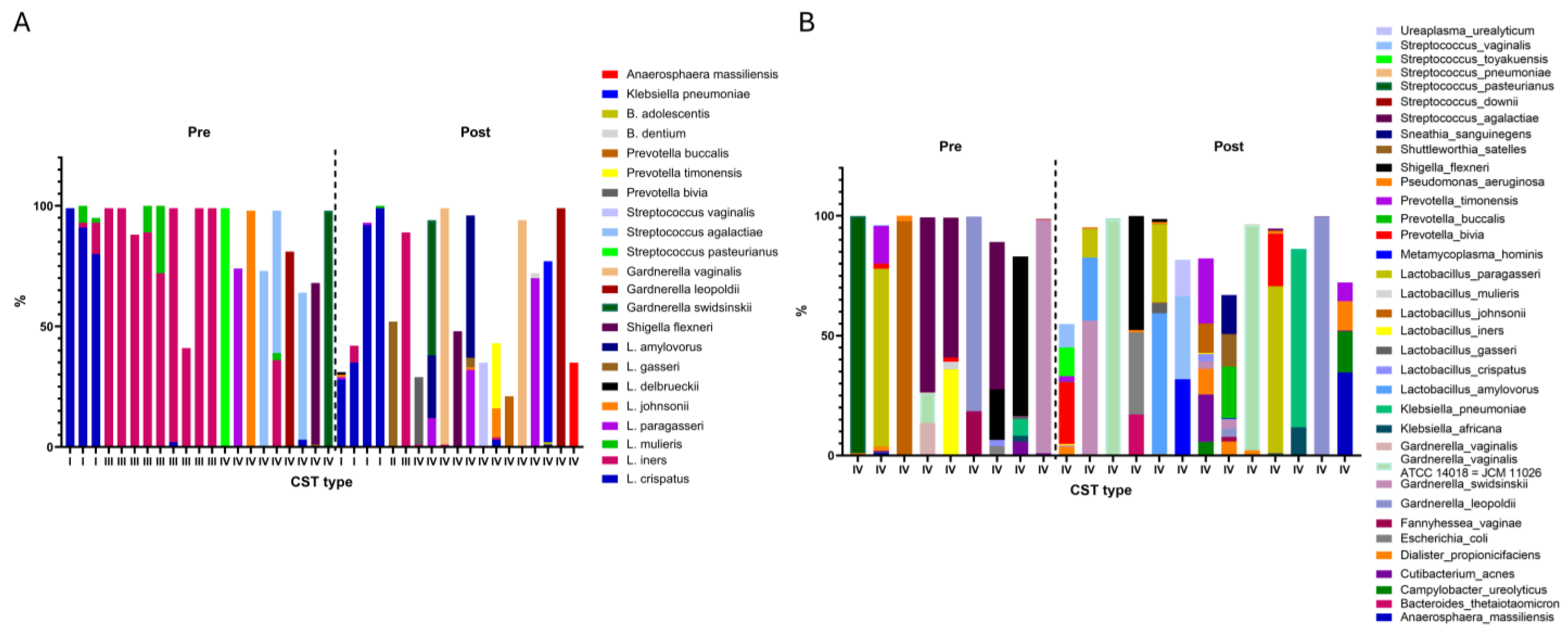
3.1. Distribution Among Community State Types (CST)
3.2. Community State Types (CST) Classification
3.2.1. Vaginal pH
3.2.2. Menopausal Status
3.2.3. Use of Oral Antibiotics
3.3. Postmenopausal Group and Premenopausal Group
3.4. Pathogens and Their Association with Vaginal pH, Menstrual Status, and Glycemic Control Medications
4. Discussion
4.1. Vaginal pH Level
4.2. Menopausal Status
4.3. Antibiotics
4.4. Diabetes Mellitus
4.5. Vitamin D
4.6. Diversity in Vaginal Microbiota
4.7. Specific Pathogens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef] [PubMed]
- France, M.; Mendes-Soares, H.; Forney, L.J.; Snyder, L.; Putonti, C.; Mueller, M.G.; Wolfe, A.J.; Brubaker, L.; Ravel, J.; Fettweis, J.M.; et al. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Romero-Figueroa, M.S.; García-Contreras, R.; Prado-Audelo, M.L.; Urbán-Morlán, Z.; Ortega-Peña, S.; Magaña, J.J.; Piña-Barba, M.C.; González-Torres, M.; Florán, B.; et al. Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities. Pharmaceutics 2019, 11, 217. [Google Scholar] [CrossRef]
- Colonna, C.; Steelman, M. Amsel Criteria; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Abou Chacra, L.; Fenollar, F.; Diop, K. Bacterial vaginosis: What do we currently know? Front. Cell. Infect. Microbiol. 2022, 11, 672429. [Google Scholar] [CrossRef]
- Alonzo Martínez, M.C.; Álvarez, F.J.; González, R.; Martínez, L.; Orio, M.; Pérez, R.; Suárez, A.; Gutiérrez, J.P.; Iglesias, L.; Sánchez, M.; et al. Study of the Vaginal Microbiota in Healthy Women of Reproductive Age. Microorganisms 2021, 9, 1069. [Google Scholar] [CrossRef]
- De Seta, F.; Campisciano, G.; Zanotta, N.; Ricci, G.; Comar, M.; Cason, C.; Donders, G.; Marangoni, A.; Restaino, S.; Raimondi, F.; et al. The Vaginal Community State Types Microbiome-Immune Network as Key Factor for Bacterial Vaginosis and Aerobic Vaginitis. Front. Microbiol. 2019, 10, 2451. [Google Scholar] [CrossRef]
- Gatenby, C.; Simpson, P. Menopause: Physiology, definitions, and symptoms. Best Pract. Res. Clin. Endocrinol. Metab. 2024, 38, 101855. [Google Scholar] [CrossRef]
- Läsche, M.; Baumann, E.; Hussain, M.; Schulze-Rothe, S.; Schulze, M.; Reuschenbach, M.; Grässer, F.A.; Wickenhauser, C.; Fotopoulou, C.; Meinhardt, M.; et al. HPV and other microbiota; who’s good and who’s bad: Effects of the microbial environment on the development of cervical cancer—A non-systematic review. Cells 2021, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P. The Mighty Microbiota: Regulator of the Human Body. Clin. Res. Clin. Trials 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Vazquez, F.; Fernández-Blázquez, A.; García, B. Vaginosis. Vaginal microbiota. Enferm. Infecc. Microbiol. Clin. 2019, 37, 592–601. [Google Scholar] [CrossRef]
- Gasparyan, K.; Balykov, I.; Ilyashenko, V.; Zueva, E.; Zborovskaya, I.; Sinitsyna, M.; Polonskaya, Y.; Dudina, A.; Avdeev, A.; Elchaninov, A.; et al. Features of vaginal microbiocenosis in women of reproductive age with overweight and obesity. Rep. Morphol. 2021, 27, 50–57. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.I.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E.; et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014, 21, 450–458. [Google Scholar] [CrossRef]
- Lykke, M.R.; Helweg-Larsen, J.; Jørgensen, A.C.; Fich, O.L.; Nielsen, A.K.; Johannesson, M.; Arpi, M.; Skovlund, C.W.; Bräuner, H.; Thorsen, J.; et al. Vaginal, cervical and uterine ph in women with normal and abnormal vaginal microbiota. Pathogens 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A.; Alberts, S.C.; Roche, K.; Kohn, J.; Akinyi, M.Y.; Otieno, J.O.; Tung, J.; et al. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol. 2016, 7, 224527. [Google Scholar] [CrossRef]
- Mayer, B.T.; Srinivasan, S.; Fiedler, T.L.; Marrazzo, J.M.; Fredricks, D.N.; Schiffer, J.T.; Liu, C.M.; Gajer, P.; Zhai, J.; Forney, L.J.; et al. Rapid and Profound Shifts in the Vaginal Microbiota Following Antibiotic Treatment for Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 793–802. [Google Scholar] [CrossRef]
- Taddei, C.R.; Cortez, R.V.; Mattar, R.; Torloni, M.R.; Daher, S.; Rizzo, L.V.; França, E.L.; Moron, A.F.; Camargo, R.S.; Sandrim, V.C.; et al. Microbiome in normal and pathological pregnancies: A literature overview. Am. J. Reprod. Immunol. 2018, 80, e12993. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, Y.; Cui, Y. Urinary tract infections and genital mycotic infections associated with SGLT-2 inhibitors: An analysis of the FDA Adverse event Reporting system. Expert Opin. Drug Saf. 2024, 23, 1035–1040. [Google Scholar] [CrossRef]
- Kusunoki, M.; Inoue, A.; Akazawa, N.; Okita, M.; Tanaka, Y.; Ohigashi, M.; Shimazu, S.; Iida, M.; Katsuno, T.; Nishigaki, N.; et al. Influence of Luseogliflozin on Vaginal Bacterial and Fungal Populations in Japanese Patients With Type 2 Diabetes. J. Clin. Med. Res. 2021, 13, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Caufield, P.; Schön, C.N.; Saraithong, P.; Li, Y.; Argimón, S.; Hill, G.B.; Collins, M.; Nasidze, I.; Li, Y.; Moser, S.A.; et al. Oral lactobacilli and dental caries: A model for niche adaptation in humans. J. Dent. Res. 2015, 94 (Suppl. S9), 110S–118S. [Google Scholar] [CrossRef]
- Shim, J.; Choe, Y.J.; Kim, M.; Park, Y.; Lee, J.; Lee, J.K.; Lee, S.A.; Bae, G.R.; Cho, H.W.; Kim, S.S.; et al. Association Between Serum 25-Hydroxyvitamin D Level and Human Papillomavirus Cervicovaginal Infection in Women in the United States. J. Infect. Dis. 2016, 213, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhang, Y.; Wang, Q.; Li, X.; Liu, Y.; Chen, H.; Zhao, Y.; Zhou, J.; Sun, L.; Yang, Q.; et al. Effects of Soluble and Insoluble Fibre on Glycolipid Metabolism and Gut Microbiota in High-Fat-Diet-Induced Obese Mice. Nutrients 2024, 16, 3822. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, T.; Yang, H.; Yang, W.; Zhang, C.; Gao, G. Effect of Vitamin D on the Proliferation and Barrier of Atrophic Vaginal Epithelial Cells. Molecules 2023, 28, 6605. [Google Scholar] [CrossRef]
- Ma, L.; Liu, L.; Wang, L.; Zhang, Y.; Gao, H.; Li, Y.; Zhou, Q.; Xu, Y.; Huang, Y.; Chen, D.; et al. Vitamin D deficiency increases the risk of bacterial vaginosis during pregnancy: Evidence from a meta-analysis based on observational studies. Front. Nutr. 2022, 9, 1016592. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, Y.; Liu, X.; Wang, L.; Chen, Y.; Zhao, H.; Li, M.; Huang, R.; Feng, Y.; Yang, Q.; et al. Effect of Vitamin D Level on Female Vaginitis in Xi’an, China. Int. J. Women’s Health 2024, 16, 2103–2112. [Google Scholar] [CrossRef]
- Turner, A.N.; Carr Reese, P.; Fields, K.S.; Anderson, J.; Ervin, M.; Clark, E.; Austin, M.N.; Panzer, J.; Gopalan, P.; Herbst-Kralovetz, M.M.; et al. A blinded, randomized controlled trial of high-dose vitamin D supplementation to reduce recurrence of bacterial vaginosis. Am. J. Obs. Gynecol. 2014, 211, 479.e1–479.e13. [Google Scholar] [CrossRef]
- Jefferson, K.K.; Edwards, J.M.; Bruno, V.; Williams, C.J.; Brown, S.M.; Laham, N.; Detrick, B.; Goggins, E.R.; Gravett, M.G.; Goldenberg, R.L.; et al. Relationship between vitamin D status and the vaginal microbiome during pregnancy. J. Perinatol. 2019, 39, 824–836. [Google Scholar] [CrossRef]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Hong, K.H.; Ko, D.H.; Kim, H.J.; Kim, S.Y.; Kim, T.Y.; Chong, Y.; Shin, J.H.; Lee, H.; Kim, J.W.; Park, K.U.; et al. Analysis of the vaginal microbiome by next-generation sequencing and evaluation of its performance as a clinical diagnostic tool in vaginitis. Ann. Lab. Med. 2016, 36, 441. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; Macklaim, J.M.; Bisanz, J.E.; Zhou, G.; McGee, F.; Wong, E.; Winsor, G.; Cribby, S.; Stearns, J.C.; Boon, M.E.; et al. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS ONE 2011, 6, e26602. [Google Scholar] [CrossRef] [PubMed]

| All (n = 40) | Postmenopausal (n = 20) | Premenopausal (n = 20) | p | ||
|---|---|---|---|---|---|
| Age, m (sd) | 51(8) | 58(6) | 43(7) | 7.068 | <0.001 *** |
| BMI, m (sd) | 25(4) | 26(4) | 24(4) | 1.513 | 0.139 |
| Smoke, n (%) | 2.421 | 0.298 | |||
| Never | 29(73) | 13(65) | 16(80) | ||
| Ex | 2(5) | 2(10) | 0(0) | ||
| Current | 9(23) | 5(25) | 4(20) | ||
| DM, n (%) | 8.901 | 0.003 ** | |||
| NO | 26(65) | 8(40) | 18(90) | ||
| Yes | 14(35) | 12(60) | 2(10) | ||
| CST, n (%) | 8.536 | 0.014 * | |||
| I + II | 8(20) | 5(25) | 3(15) | ||
| III | 10(25) | 1(5) | 9(45) | ||
| IV | 22(55) | 14(70) | 8(40) |
| CST | Microbiome |
|---|---|
| I | 99% L. crispatus |
| I | 91% L. crispatus, 2% L. iners, 7% L. mulieris |
| I | 28% L. crispatus, 1% L. paragasseri, 1% L. johnsonii, 1% L. delbrueckii |
| I | 80% L. crispatus, 13% L. iners, 2% L. mulieris |
| I | 35% L. crispatus, 7% L. iners |
| I | 92% L. crispatus, 1% L. paragasseri |
| I | 99% L. crispatus, 1% L. mulieris |
| II | 52% L. gasseri |
| III | 99% L. iners |
| III | 99% L. iners |
| III | 88% L. iners |
| III | 89% L. iners, 11% L. mulieris |
| III | 72% L. iners, 28% L. mulieris |
| III | 97% L. iners, 2% L. crispatus |
| III | 89% L. iners |
| III | 41% L. iners |
| III | 99% L. iners |
| III | 99% L. iners |
| IV | 28% Prevotella bivia, 1% L. iners |
| IV | 56% Gardnerella swidsinskii, 12% L. paragasseri, 26% L. amylovorus |
| IV | 98% Gardnerella vaginalis, 1% L. iners |
| IV | 99% Streptococcus pasteurianus |
| IV | 48% Shigella flexneri |
| IV | 59% L. amylovorus, 4% L. gasseri, 32% L. paragasseri, 1% L. johnsonii |
| IV | 74% L. paragasseri |
| IV | 35% Streptococcus vaginalis |
| IV | 27% Prevotella timonensis, 3% L. crispatus, 1% L. iners, 12% L. johnsonii |
| IV | 21% Prevotella buccalis |
| IV | 94% Gardnerella vaginalis |
| IV | 70% L. paragasseri, 2% B. dentium |
| IV | 98% L. johnsonii |
| IV | 73% Streptococcus agalactiae |
| IV | 75% Klebsiella pneumoniae, 1% L. amylovorus, 1% B. adolescentis |
| IV | 59% Streptococcus agalactiae, 36% L. iners, 3% L. mulieris |
| IV | 81% Gardnerella leopoldii |
| IV | 61% Streptococcus agalactiae, 3% L. crispatus |
| IV | 67% Shigella flexneri, 1% L. gasseri |
| IV | 99% Gardnerella leopoldii |
| IV | 98% Gardnerella swidsinskii |
| IV | 35% Anaerosphaera massiliensis |
| CST I + CST II (n = 8) | CST III (n = 10) | CST IV (n = 22) | p a | Post Hoc b | ||||
|---|---|---|---|---|---|---|---|---|
| Median/n | IQR/% | Median/n | IQR/% | Median/n | IQR/% | |||
| Vaginal tract pH | 5.00 | 1.88 | 4.50 | 1.00 | 6.00 | 1.63 | 0.026 | III < IV |
| HbA1c | 5.55 | 2.13 | 5.65 | 1.05 | 5.65 | 1.13 | 0.612 | |
| Glucose | 98.00 | 32.00 | 95.50 | 13.75 | 92.00 | 30.00 | 0.737 | |
| BMI | 24.35 | 5.56 | 25.99 | 7.93 | 24.00 | 6.05 | 0.684 | |
| Vitamin D (25(OH)D) | 21.65 | 16.70 | 19.80 | 8.10 | 20.95 | 8.35 | 0.362 | |
| Waist | 87.25 | 14.25 | 85.75 | 15.13 | 81.00 | 11.63 | 0.244 | |
| Average Body Fat | 35.20 | 5.30 | 34.90 | 16.13 | 34.25 | 8.93 | 0.793 | |
| Trunk Fat | 34.80 | 6.32 | 33.75 | 19.43 | 34.80 | 11.20 | 0.811 | |
| Visceral Fat | 8.00 | 5.00 | 7.00 | 7.50 | 7.00 | 4.25 | 0.771 | |
| Menopause | 0.014 | III, IV | ||||||
| Postmenopause | 5 | 25.00 | 1 | 5.00 | 14 | 70.00 | ||
| Premenopause | 3 | 15.00 | 9 | 45.00 | 8 | 40.00 | ||
| Smoking history | 0.972 | |||||||
| No | 6 | 20.69 | 7 | 24.14 | 16 | 55.17 | ||
| Yes | 2 | 18.18 | 3 | 27.27 | 6 | 54.55 | ||
| BMI | 0.492 | |||||||
| Normal | 2 | 11.76 | 4 | 23.53 | 11 | 64.71 | ||
| Overweight | 3 | 33.33 | 1 | 11.11 | 5 | 55.56 | ||
| Obese | 3 | 21.43 | 5 | 35.71 | 6 | 42.86 | ||
| Vitamin D | 0.158 | |||||||
| <20 | 2 | 10.50 | 7 | 36.8 | 10 | 52.60 | ||
| ≥20 | 6 | 28.60 | 3 | 14.30 | 12 | 57.10 | ||
| HbA1c | 0.121 | |||||||
| <6.5 | 5 | 16.10 | 10 | 32.30 | 16 | 51.60 | ||
| ≥6.5 | 3 | 33.30 | 0 | 0.00 | 6 | 66.70 | ||
| OAD | 0.400 | |||||||
| No | 4 | 14.80 | 8 | 29.60 | 15 | 55.60 | ||
| Yes | 4 | 30.80 | 2 | 15.40 | 7 | 53.80 | ||
| SGLT2 inhibitors | 0.100 | |||||||
| No | 4 | 12.90 | 9 | 29.00 | 18 | 58.10 | ||
| Yes | 4 | 44.40 | 1 | 11.10 | 4 | 44.40 | ||
| Antibiotics (Vaginal) | 0.423 | |||||||
| No | 8 | 21.10 | 10 | 26.30 | 20 | 52.60 | ||
| Yes | 0 | 0.00 | 0 | 0.00 | 2 | 100.00 | ||
| Antibiotics (oral) | 0.048 | |||||||
| No | 8 | 22.20 | 7 | 19.40 | 21 | 58.30 | ||
| Yes | 0 | 0.00 | 3 | 75.00 | 1 | 25.00 | ||
| DM | 0.407 | |||||||
| No | 4 | 15.38 | 8 | 30.77 | 14 | 53.85 | ||
| Yes | 4 | 28.57 | 2 | 14.29 | 8 | 57.14 | ||
| CST I + CST II (n = 5) | CST IV (n = 14) | p a | |||
|---|---|---|---|---|---|
| Median/n | IQR/% | Median/n | IQR/% | ||
| Vaginal tract PH | 6.00 | 1.75 | 6.00 | 1.50 | 0.391 |
| HbA1c | 7.10 | 2.25 | 6.05 | 1.22 | 0.754 |
| Glucose | 114.00 | 31.50 | 102.50 | 40.25 | 0.559 |
| BMI | 27.55 | 5.69 | 25.52 | 5.21 | 0.622 |
| Vitamin D | 20.80 | 13.00 | 21.90 | 10.13 | 0.559 |
| Waist | 94.00 | 11.75 | 85.00 | 11.38 | 0.056 |
| Average Body Fat | 36.30 | 8.05 | 37.55 | 10.70 | 0.893 |
| Trunk Fat | 36.10 | 10.50 | 37.75 | 12.98 | 0.964 |
| Visceral Fat | 8.00 | 5.00 | 9.00 | 7.00 | 0.893 |
| Smoking history | 0.999 | ||||
| No | 3 | 25.00 | 9 | 75.00 | |
| Yes | 2 | 28.57 | 5 | 71.43 | |
| BMI | 0.405 | ||||
| Normal | 0 | 0.00 | 4 | 100.00 | |
| Overweight | 2 | 33.33 | 4 | 66.67 | |
| Obese | 3 | 33.33 | 6 | 66.67 | |
| Vitamin D | 0.603 | ||||
| <20 | 1 | 14.29 | 6 | 85.71 | |
| ≥20 | 4 | 33.33 | 8 | 66.67 | |
| HbA1c | 0.603 | ||||
| <6.5 | 2 | 18.18 | 9 | 81.82 | |
| ≥6.5 | 3 | 37.50 | 5 | 62.50 | |
| OAD | 0.303 | ||||
| No | 1 | 11.11 | 8 | 88.89 | |
| Yes | 4 | 40.00 | 6 | 60.00 | |
| SGLT2 inhibitors | 0.038 | ||||
| No | 1 | 8.33 | 11 | 91.67 | |
| Yes | 4 | 57.14 | 3 | 42.86 | |
| Antibiotics (Vaginal) | 0.999 | ||||
| No | 5 | 27.78 | 13 | 72.22 | |
| Yes | 0 | 0.00 | 1 | 100.00 | |
| Antibiotics (oral) | 0.999 | ||||
| No | 5 | 27.78 | 13 | 72.22 | |
| Yes | 0 | 0.00 | 1 | 100.00 | |
| DM | 0.338 | ||||
| No | 1 | 12.50 | 7 | 87.50 | |
| Yes | 4 | 36.36 | 7 | 63.64 | |
| CST I + CST II (n = 3) | CST III (n = 9) | CST IV (n = 8) | p a | ||||
|---|---|---|---|---|---|---|---|
| Median/n | IQR/% | Median/n | IQR/% | Median/n | IQR/% | ||
| Vaginal pH | 4.50 | - | 4.50 | 1.50 | 5.50 | 1.38 | 0.177 |
| HbA1c | 5.10 | - | 5.60 | 1.00 | 5.45 | 0.63 | 0.465 |
| Glucose | 88.00 | - | 95.00 | 11.50 | 85.00 | 11.25 | 0.160 |
| BMI | 22.31 | - | 27.42 | 7.71 | 21.90 | 2.16 | 0.051 |
| Vitamin D | 22.50 | - | 19.80 | 9.70 | 20.40 | 7.53 | 0.623 |
| Waist | 79.00 | - | 88.50 | 13.25 | 78.00 | 15.75 | 0.105 |
| Average Body Fat | 34.30 | - | 35.80 | 14.35 | 31.35 | 7.05 | 0.264 |
| Trunk Fat | 34.50 | - | 34.20 | 17.40 | 30.90 | 8.25 | 0.466 |
| Visceral Fat | 6.00 | - | 7.00 | 7.50 | 5.50 | 2.75 | 0.327 |
| Smoking history | 0.362 | ||||||
| No | 3 | 18.75 | 6 | 37.50 | 7 | 43.75 | |
| Yes | 0 | 0.00 | 3 | 75.00 | 1 | 25.00 | |
| BMI | 0.061 | ||||||
| Normal | 2 | 16.67 | 3 | 25.00 | 7 | 58.33 | |
| Overweight | 1 | 33.33 | 1 | 33.33 | 1 | 33.33 | |
| Obese | 0 | 0.00 | 5 | 100.00 | 0 | 0.00 | |
| Vitamin D | 0.564 | ||||||
| <20 | 1 | 9.09 | 6 | 54.55 | 4 | 36.36 | |
| ≥20 | 2 | 22.22 | 3 | 33.33 | 4 | 44.44 | |
| HbA1c | 0.454 | ||||||
| <6.5 | 3 | 15.79 | 9 | 47.37 | 7 | 36.84 | |
| ≥6.5 | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 | |
| OAD | 0.818 | ||||||
| No | 3 | 16.67 | 8 | 44.44 | 7 | 38.89 | |
| Yes | 0 | 0.00 | 1 | 50.00 | 1 | 50.00 | |
| SGLT2 | 0.818 | ||||||
| No | 3 | 16.67 | 8 | 44.44 | 7 | 38.89 | |
| Yes | 0 | 0.00 | 1 | 50.00 | 1 | 50.00 | |
| Antibiotics (Vaginal) | 0.454 | ||||||
| No | 3 | 15.79 | 9 | 47.37 | 7 | 36.84 | |
| Yes | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 | |
| Antibiotics (oral) | 0.116 | ||||||
| No | 3 | 17.65 | 6 | 35.29 | 8 | 47.06 | |
| Yes | 0 | 0.00 | 3 | 100.00 | 0 | 0.00 | |
| DM | 0.818 | ||||||
| No | 3 | 16.67 | 8 | 44.44 | 7 | 39.89 | |
| Yes | 0 | 0.00 | 1 | 50.00 | 1 | 50.00 | |
| Pathogen | n | Vaginal pH Level | Cases % Among | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Cases | Post-Menopause | Pre-Menopause | Post-Menopause (%) / Pre-Menopause (%) | SGLT2 (−) (%)/SGLT2 (+) (%) | |||||||||
| Post-Menopause | Pre-Menopause | ||||||||||||
| r | p | r | p | r | p | %/% | p | %/% | p | %/% | p | ||
| Acinetobacter | 1 | 5.0/0.0 | 7.0/0.0 | - | |||||||||
| positive | 0.148 | 0.361 | 0.161 | 0.479 | - | - | 1.000 | 1.000 | - | ||||
| abundance | |||||||||||||
| Actinomyces | 6 | 20.0/10.0 | 23.1/14.3 | 11.1/0.0 | |||||||||
| positive | 0.256 | 0.111 | −0.099 | 0.679 | 0.531 | 0.016 * | 0.658 | 1.000 | 1.000 | ||||
| abundance | 0.231 | 0.151 | −0.149 | 0.532 | 0.532 | 0.016 * | 0.602 | 0.699 | 0.853 | ||||
| Atopobium | 1 | 5.0/0.0 | 7.7/0.0 | - | |||||||||
| positive | 0.071 | 0.665 | 0.000 | 1.000 | - | - | 1.000 | 1.000 | - | ||||
| abundance | |||||||||||||
| Anaerococcus | 14 | 45.0/25.0 | 38.5/57.1 | 27.8/0.0 | |||||||||
| positive | 0.090 | 0.580 | −0.132 | 0.578 | 0.074 | 0.758 | 0.320 | 0.642 | 1.000 | ||||
| abundance | 0.115 | 0.479 | 0.100 | 0.676 | −0.094 | 0.693 | 0.221 | 0.757 | 0.674 | ||||
| Bacteroides | 7 | 25.0/10.0 | 23.1/28.6 | 11.1/0.0 | |||||||||
| positive | 0.313 | 0.049 * | 0.375 | 0.103 | 0.106 | 0.656 | 0.405 | 1.000 | 1.000 | ||||
| abundance | 0.317 * | 0.047 * | 0.347 | 0.134 | 0.129 | 0.587 | 0.398 | 0.938 | 0.853 | ||||
| Clostridium | 2 | 10.0/0.0 | 0.0/28.6 | - | |||||||||
| positive | 0.212 | 0.188 | 0.234 | 0.320 | - | - | 0.468 | 0.111 | - | ||||
| abundance | |||||||||||||
| Dialister | 15 | 55.0/20.0 | 53.8/57.1 | 16.7/50.0 | |||||||||
| positive | 0.198 | 0.221 | 0.079 | 0.739 | 0.023 | 0.924 | 0.050 | 1.000 | 0.368 | ||||
| abundance | 0.248 | 0.122 | 0.213 | 0.367 | 0.936 | 0.035 * | |||||||
| Escherichia | 2 | 5.0/5.0 | 7.7/0.0 | 5.6/0.0 | |||||||||
| positive | 0.101 | 0.535 | 0.302 | 0.195 | 0.042 | 0.861 | 1.000 | 1.000 | 1.000 | ||||
| abundance | |||||||||||||
| Finegoldia | 11 | 40.0/15.0 | 46.2/28.6 | 16.7/0.0 | |||||||||
| positive | 0.274 | 0.087 | 0.045 | 0.851 | 0.293 | 0.210 | 0.157 | 0.642 | 1.000 | ||||
| abundance | 0.247 | 0.124 | 0.106 | 0.656 | 0.283 | 0.114 | |||||||
| Fusobacterium | 9 | 35.0/10.0 | 38.5/28.6 | 11.1/0.0 | |||||||||
| positive | 0.354 * | 0.025 * | 0.074 | 0.758 | 0.531 * | 0.016 * | 0.130 | 1.000 | 1.000 | ||||
| abundance | 0.376 * | 0.017 * | 0.099 | 0.677 | 0.017 * | 0.231 | |||||||
| Gardnerella | 17 | 55.0/30.0 | 61.5/42.9 | 33.3/0.0 | |||||||||
| positive | 0.096 | 0.556 | −0.371 | 0.108 | 0.447 * | 0.048 | 0.201 | 0.642 | 1.000 | ||||
| abundance | 0.094 | 0.566 | −0.295 | 0.206 | 0.060 | 0.165 | |||||||
| Gemella | 8 | 30.0/10.0 | 30.8/28.6 | 11.1/0.0 | |||||||||
| positive | 0.091 | 0.577 | −0.230 | 0.329 | 0.273 | 0.244 | 0.235 | 1.000 | 1.000 | ||||
| abundance | 0.092 | 0.570 | −0.245 | 0.298 | 0.227 | 0.341 | |||||||
| Leptotrichia | 4 | 15.0/5.0 | 15.4/14.3 | 5.6/0.0 | |||||||||
| positive | 0.044 | 0.787 | −0.307 | 0.187 | 0.334 | 0.150 | 0.605 | 1.000 | 1.000 | ||||
| abundance | 0.028 | 0.864 | −0.320 | 0.169 | 0.150 | 0.565 | |||||||
| Mycoplasma | 2 | 10.0/0.0 | 15.4/0.0 | - | |||||||||
| positive | 0.207 | 0.199 | 0.220 | 0.352 | - | - | 0.468 | 0.521 | - | ||||
| abundance | |||||||||||||
| Megasphaera | 2 | 5.0/5.0 | 7.7/0.0 | 5.6/0.0 | |||||||||
| positive | 0.207 | 0.199 | 0.000 | 1.000 | 0.334 | 0.150 | 1.000 | 1.000 | 1.000 | ||||
| abundance | |||||||||||||
| Mobiluncus | 4 | 15.0/5.0 | 15.4/14.3 | 5.6/0.0 | |||||||||
| positive | 0.118 | 0.470 | 0.283 | 0.227 | −0.250 | 0.287 | 0.605 | 1.000 | 1.000 | ||||
| abundance | 0.133 | 0.412 | 0.291 | 0.212 | 0.287 | 0.565 | |||||||
| Porphyromonas | 13 | 50.0/15.0 | 53.8/42.9 | 16.7/0.0 | |||||||||
| positive | 0.454 | 0.003 * | 0.421 | 0.064 | 0.293 | 0.210 | 0.043 * | 1.000 | 1.000 | ||||
| abundance | 0.417 | 0.007 * | 0.339 | 0.143 | 0.218 | 0.060 | |||||||
| Parvimonas | 3 | 10.0/5.0 | 7.7/14.3 | 5.6/0.0 | |||||||||
| positive | 0.213 | 0.186 | 0.000 | 1.000 | 0.334 | 0.150 | 1.000 | 1.000 | 1.000 | ||||
| abundance | |||||||||||||
| Peptostreptococcus | 2 | 10.0/0.0 | 7.7/14.3 | - | |||||||||
| positive | 0.111 | 0.494 | 0.015 | 0.951 | - | - | 0.468 | 1.000 | - | ||||
| abundance | |||||||||||||
| Peptococcus | 2 | 10.0/0.0 | 7.7/14.3 | - | |||||||||
| positive | 0.207 | 0.199 | 0.220 | 0.352 | - | - | 0.468 | 1.000 | - | ||||
| abundance | |||||||||||||
| Peptoniphilus | 12 | 50.0/10.0 | 46.2/57.1 | 11.1/0.0 | |||||||||
| positive | 0.245 | 0.127 | 0.176 | 0.459 | 0.015 | 0.949 | 0.016 * | 1.000 | 1.000 | ||||
| abundance | 0.256 | 0.111 | 0.266 | 0.257 | 0.987 | 0.023 * | |||||||
| Prevotella | 20 | 65.0/35.0 | 69.2/57.1 | 33.4/50.0 | |||||||||
| positive | 0.379 | 0.016 * | 0.138 | 0.562 | 0.372 | 0.106 | 0.114 | 0.651 | 1.000 | ||||
| abundance | 0.332 | 0.036 * | 0.140 | 0.556 | 0.201 | 0.056 | |||||||
| Sneathia | 1 | 5.0/0.0 | 7.7/0.0 | - | |||||||||
| positive | 0.071 | 0.665 | 0.000 | 1.000 | - | - | 1.000 | 1.000 | - | ||||
| abundance | |||||||||||||
| Shuttleworthia | 1 | 5.0/0.0 | 7.7/0.0 | - | |||||||||
| positive | 0.071 | 0.665 | 0.000 | 1.000 | - | - | 1.000 | 1.000 | - | ||||
| abundance | |||||||||||||
| Streptococcus | 25 | 65.0/60.0 | 61.5/71.4 | 61.1/50.0 | |||||||||
| positive | 0.346 | 0.029 * | 0.396 | 0.084 | 0.297 | 0.203 | 1.000 | 1.000 | 1.000 | ||||
| abundance | 0.420 | 0.007 * | 0.263 | 0.263 | 0.020 * | 0.989 | |||||||
| Ureaplasma | 11 | 35.0/20.0 | 38.5/28.6 | 16.7/50.0 | |||||||||
| positive | −0.079 | 0.628 | −0.497 | 0.026 * | 0.216 | 0.360 | 0.479 | 1.000 | 0.368 | ||||
| abundance | −0.020 | 0.901 | −0.405 | 0.077 | 0.264 | 0.398 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chiang, Y.-F.; Huang, K.-C.; Wang, K.-L.; Huang, Y.-J.; Shieh, T.-M.; Ali, M.; Hsia, S.-M. The Vaginal Microbiome: Associations with Vaginal pH, Menopause and Metabolic Parameters. Microorganisms 2025, 13, 1317. https://doi.org/10.3390/microorganisms13061317
Chen Y-C, Chiang Y-F, Huang K-C, Wang K-L, Huang Y-J, Shieh T-M, Ali M, Hsia S-M. The Vaginal Microbiome: Associations with Vaginal pH, Menopause and Metabolic Parameters. Microorganisms. 2025; 13(6):1317. https://doi.org/10.3390/microorganisms13061317
Chicago/Turabian StyleChen, Yi-Chun, Yi-Fen Chiang, Ko-Chieh Huang, Kai-Lee Wang, Yun-Ju Huang, Tzong-Ming Shieh, Mohamed Ali, and Shih-Min Hsia. 2025. "The Vaginal Microbiome: Associations with Vaginal pH, Menopause and Metabolic Parameters" Microorganisms 13, no. 6: 1317. https://doi.org/10.3390/microorganisms13061317
APA StyleChen, Y.-C., Chiang, Y.-F., Huang, K.-C., Wang, K.-L., Huang, Y.-J., Shieh, T.-M., Ali, M., & Hsia, S.-M. (2025). The Vaginal Microbiome: Associations with Vaginal pH, Menopause and Metabolic Parameters. Microorganisms, 13(6), 1317. https://doi.org/10.3390/microorganisms13061317










