Effects of Pineapple Peel on the Nutritional and Microbial Profiles of Napier Grass–Sugarcane Top Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Ensiling Materials and Mixed-Silage Preparation
2.2. Chemical Analysis
2.3. Microbial Populations and Bacterial Community Analysis
2.4. In Vitro Ruminal Fermentation Characteristics
2.5. Statistical Analysis
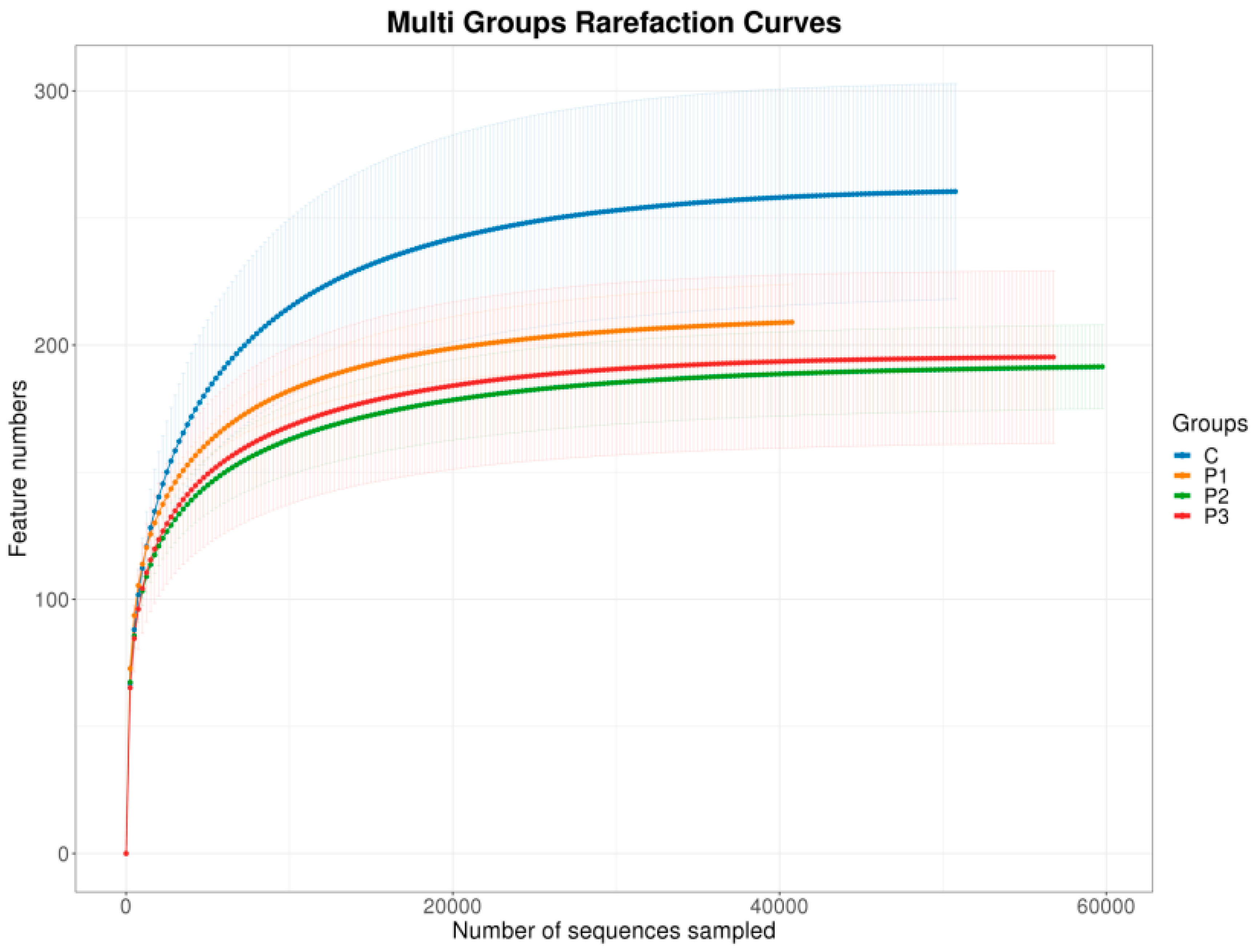
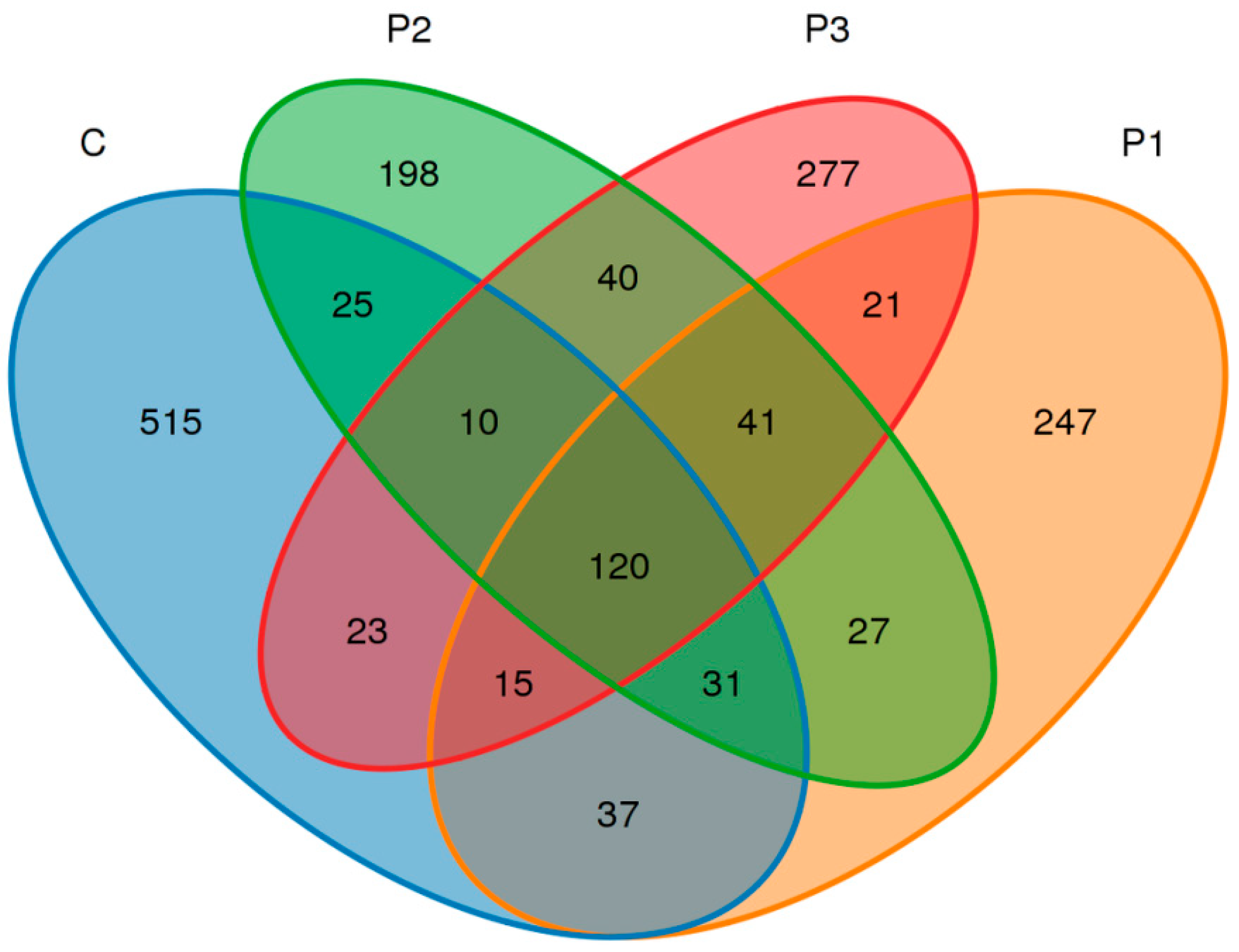
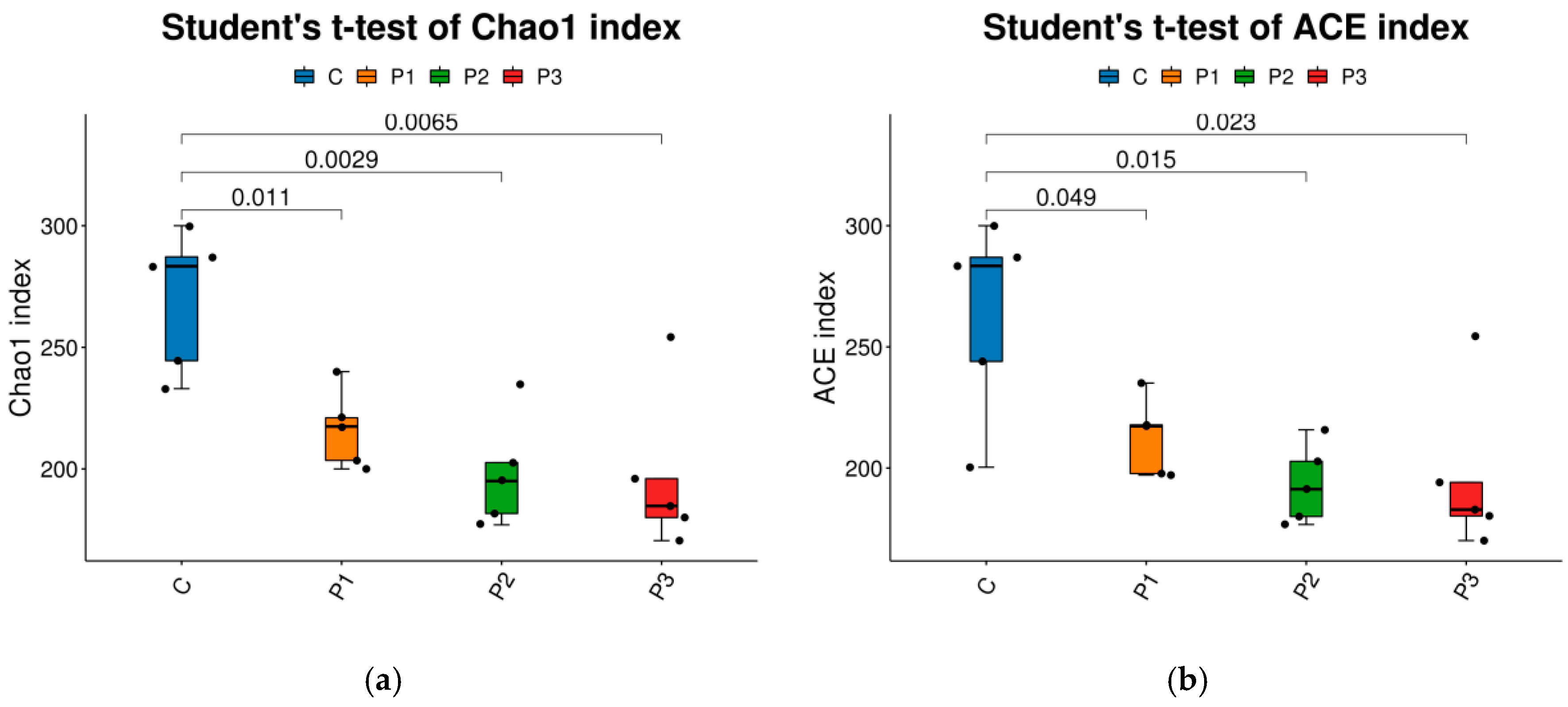
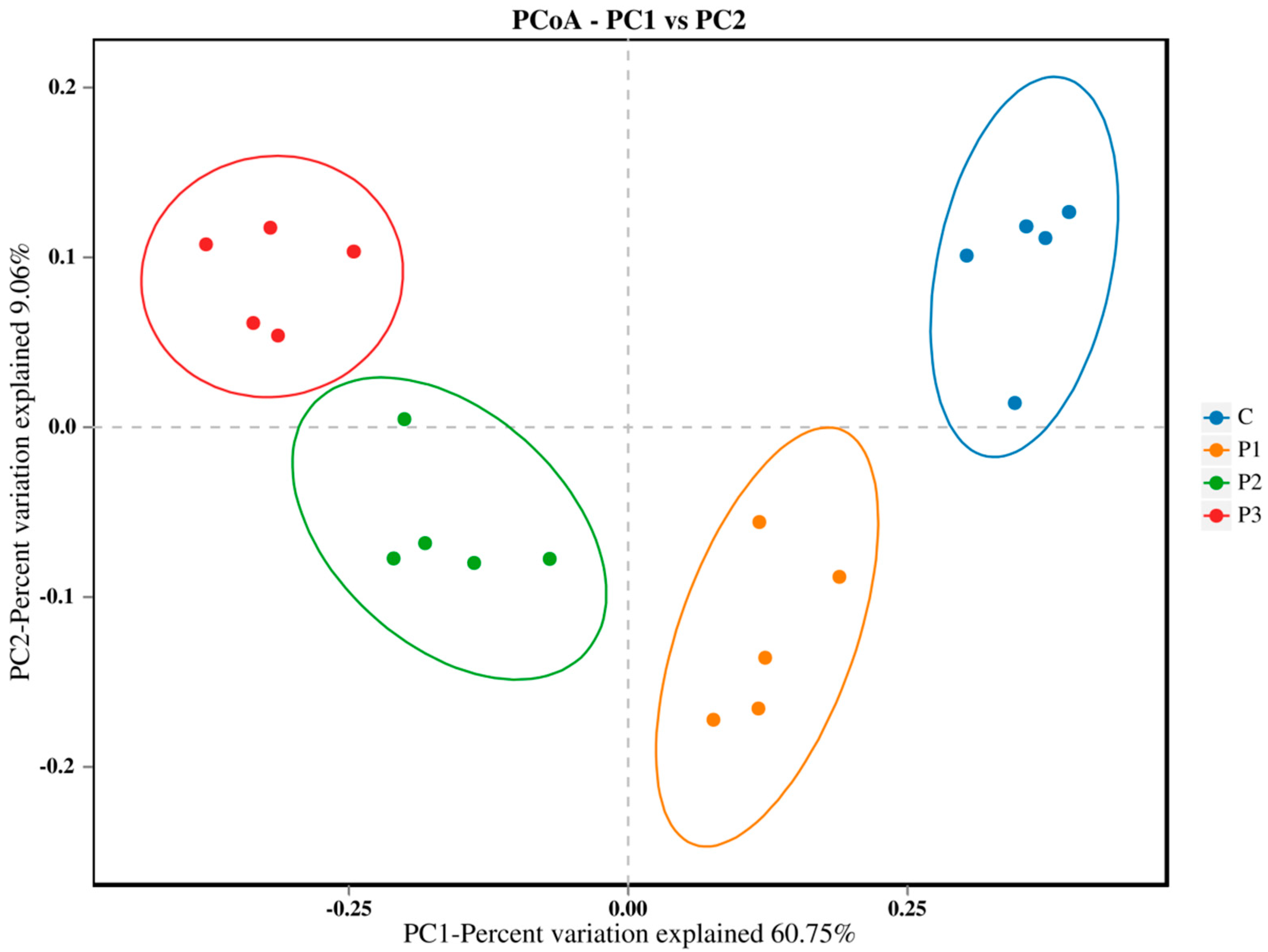
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADF | Acid detergent fiber |
| CP | Crude protein |
| DM | Dry matter |
| FM | Fresh matter |
| IVDMD | In vitro dry matter digestibility |
| LAB | Lactic acid bacteria |
| NDF | Neutral detergent fiber |
| NG | Napier grass |
| OM | Organic matter |
| ST | Sugarcane top |
| WSC | Water soluble carbohydrate |
| VFA | Volatile fatty acid |
References
- Dai, H.; Ou, S.; Liu, Z.; Huang, H. Pineapple peel carboxymethyl cellulose/polyvinyl alcohol/mesoporous silica SBA-15 hydrogel composites for papain immobilization. Carbohyd. Polym. 2017, 169, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Vandarkuzhali, S.A.A.; Natarajan, S.; Jeyabalan, S.; Sivaraman, G.; Singaravadivel, S.; Muthusubramanian, S.; Viswanathan, B. Pineapple Peel-Derived Carbon Dots: Applications as Sensor, Molecular Keypad Lock, and Memory Device. ACS Omega 2018, 3, 12584–12592. [Google Scholar] [CrossRef]
- Mehraj, M.; Das, S.; Feroz, F.; Waheed, W.A.; Dar, S.Q.; Kumar, S.; Wani, A.K.; Farid, A. Nutritional Composition and Therapeutic Potential of Pineapple Peel—A Comprehensive Review. Chem. Biodivers. 2024, 21, e202400315. [Google Scholar] [CrossRef] [PubMed]
- Selani, M.M.; Brazaca, S.G.; Dos, S.D.C.T.; Ratnayake, W.S.; Flores, R.A.; Bianchini, A. Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 2014, 163, 23–30. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Vasconcelos, S.T.; Thomaz, M.C.; Castelini, F.R.; Alvarenga, P.V.A.; Alves, O.J.; Ferreira, R.G.; Keith, O.R.; Milani, N.C.; Ruiz, S.U. Evaluation of pineapple byproduct at increasing levels in heavy finishing pigs feeding. Anim. Feed Sci. Technol. 2020, 269, 114664. [Google Scholar] [CrossRef]
- Chaosap, C.; Sahatsanon, K.; Sitthigripong, R.; Sawanon, S.; Setakul, J. The Effects of Using Pineapple Stem Starch as an Alternative Starch Source and Ageing Period on Meat Quality, Texture Profile, Ribonucleotide Content, and Fatty Acid Composition of Longissimus Thoracis of Fattening Dairy Steers. Foods 2021, 10, 2319. [Google Scholar] [CrossRef]
- Nazar, M.; Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Ali, K.N.; Shao, T. Effects of various epiphytic microbiota inoculation on the fermentation quality and microbial community dynamics during the ensiling of sterile Napier grass. J. Appl. Microbiol. 2021, 130, 1466–1480. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, H.; Li, L.; He, J.; Hu, Z.; Yang, X.; Xie, X. Effects of applying cellulase and starch on the fermentation characteristics and microbial communities of Napier grass (Pennisetum purpureum Schum.) silage. J. Anim. Sci. Technol. 2021, 63, 1301–1313. [Google Scholar] [CrossRef]
- Islam, M.R.; Garcia, S.C.; Sarker, N.R.; Islam, M.A.; Clark, C.E.F. Napier grass (Pennisetum purpureum Schum) management strategies for dairy and meat production in the tropics and subtropics: Yield and nutritive value. Front. Plant Sci. 2023, 14, 1269976. [Google Scholar] [CrossRef]
- Ferreira, D.J.; Lana, R.P.; Zanine, A.M.; Santos, E.M.; Veloso, C.M.; Ribeiro, G.A. Silage fermentation and chemical composition of elephant grass inoculated with rumen strains of Streptococcus bovis. Anim. Feed Sci. Technol. 2013, 183, 22–28. [Google Scholar] [CrossRef]
- Li, H.; Ran, Q.; Jia, Z.; Shuai, Y.; Zhou, Q.; Guan, H. Effect of different dry matter content on fermentation characteristics and nutritional quality of Napier grass silage with novel lactic acid bacteria strains. Lett. Appl. Microbiol. 2023, 76, ovad018. [Google Scholar] [CrossRef] [PubMed]
- Thorat, B.S.; Pawar, G.R.; Sushir, K.V.; Talekar, S.D.; Repale, J.M.; Kadlag, A.D. Sugarcane: A Climate Resilient Devine Crop. Int. J. Environ. Clim. Change 2024, 14, 555–577. [Google Scholar] [CrossRef]
- Yang, H.; Wang, T.; Yu, X.; Yang, Y.; Wang, C.; Yang, Q.; Wang, X. Enhanced sugar accumulation and regulated plant hormone signalling genes contribute to cold tolerance in hypoploid Saccharum spontaneum. BMC Genom. 2020, 21, 507. [Google Scholar] [CrossRef]
- Wang, J.; Roe, B.; Macmil, S.; Yu, Q.; Murray, J.E.; Tang, H.; Chen, C.; Najar, F.; Wiley, G.; Bowers, J.; et al. Microcollinearity between autopolyploid sugarcane and diploid sorghum genomes. BMC Genom. 2010, 11, 261. [Google Scholar] [CrossRef]
- NBS: China Statistical Yearbook, National Bureau of Statistics of China, 12th ed.; China Statistics Press: Beijing, China, 2024.
- Getahun, K.; Ashenafi, M.; Getnet, A.; Getachew, A. Nutritional and fermentative quality of sugarcane (Saccharum officinarum) top ensiled with or without urea and molasses. Afr. J. Agric. Res. 2018, 13, 1010–1017. [Google Scholar] [CrossRef]
- Roberto, C.H.V.; Villela, S.D.J.; Leonel, F.P.; Silva, L.D.; Bastos, P.H.F.; Martins, P.A. Performance and economic evaluation of feedlot cattle fed sugarcane tops. Livest. Sci. 2019, 230, 103827. [Google Scholar] [CrossRef]
- Wu, S.; Wang, C.; Chen, D.; Zhou, W.; Chen, X.; Wang, M.; Zhang, Q. Effects of pyroligneous acid as silage additive on fermentation quality and bacterial community structure of waste sugarcane tops. Chem. Biol. Technol. Agric. 2022, 9, 67. [Google Scholar] [CrossRef]
- Hundal, J.S.; Sharma, A.; Pal, R.; Grewal, R.S. Harnessing the In Vitro Nutritional Potential of Different Varieties of Sugarcane Tops Silages Enriched with Molasses and Bacterial Inoculants as an Unconventional Feed Resource. Sugar Tech 2021, 23, 923–932. [Google Scholar] [CrossRef]
- Nyakira, B.S.; Tuitoek, J.K.; Onjoro, P.A.; Ambula, M. K Determination of the Nutritive Value of Sugar Cane Tops, Mulberry Leaves (M. alba) and Calliandra (C. calothyrsus) as Feed Supplements for Goats in Kenya. J. Anim. Sci. Adv. 2015, 5, 1225–1233. [Google Scholar] [CrossRef]
- Sindhu, R.; Gnansounou, E.; Binod, P.; Pandey, A. Bioconversion of sugarcane crop residue for value added products—An overview. Renew Energy 2016, 98, 203–215. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Cao, Y.; Shi, W.; Xuan, Z.; Zhou, J.; Zhang, J.; Zhong, J. Effects of Lactobacillus hilgardii 60TS-2, with or without homofermentative Lactobacillus plantarum B90, on the aerobic stability, fermentation quality and microbial community dynamics in sugarcane top silage. Bioresour. Technol. 2020, 312, 123600. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Kumari, N.; Mishra, D.B.; Mani, V.; Tyagi, N. Dynamic Changes in Microbial Succession and Fermentation Profiles of Sugarcane Tops Silage Treated with Exogenous Enzymes and Lactic Acid Bacteria Following Various Duration of Ensiling. Sugar Tech 2023, 25, 592–602. [Google Scholar] [CrossRef]
- Chacon, S.A.R.G.; Araujo, T.L.A.C.; Pinedo, L.A.; Lima, D.M.; Assis, L.C.S.L.C.; Pereira, M.W.F.; Lima, P.O. Effect of pineapple peel addition on sorghum ensilage. S. Afr. J. Anim. Sci. 2024, 53, 485–492. [Google Scholar] [CrossRef]
- Kiggundu, M.; Kabi, F. Effect of wilting organic pineapple by-products and jack bean (Canavalia ensiformis) foliage inclusion on silage fermentation and its nutritive value. Anim. Feed Sci. Technol. 2019, 258, 114303. [Google Scholar] [CrossRef]
- Nadzirah, K.Z.; Zainal, S.; Noriham, A.; Normah, I.; Siti, A.M.; Nadya, H. Physico-chemical properties of pineapple variety N36 harvested and stored at different maturity stages. Int. Food Res. J. 2013, 20, 225–231. [Google Scholar]
- Wan, J.; Guo, J.; Miao, Z.; Guo, X. Reverse micellar extraction of bromelain from pineapple peel--Effect of surfactant structure. Food Chem. 2016, 197, 450–456. [Google Scholar] [CrossRef]
- Xie, H.; Xie, F.; Guo, Y.; Liang, X.; Peng, L.; Li, M.; Tang, Z.; Peng, K.; Yang, C. Fermentation quality, nutritive value and in vitro ruminal digestion of Napier grass, sugarcane top and their mixed silages prepared using lactic acid bacteria and formic acid. Grassl. Sci. 2023, 69, 23–32. [Google Scholar] [CrossRef]
- Romruen, O.; Kaewprachu, P.; Karbowiak, T.; Rawdkuen, S. Isolation and Characterization Cellulose Nanosphere from Different Agricultural By-Products. Polymers 2022, 14, 2534. [Google Scholar] [CrossRef]
- Firmino, S.S.; Lima, P.O.; Oliveira, P.V.C.; Souza, J.T.; Araújo, T.L.A.C.; Pereira, M.W.F.; Macedo, M.F.; Leite, H.M.S.; Veríssimo, V.M.S.; Pimentel, F.C.S.; et al. Effects of refused melon fruit in Canarana grass ensilage on intake, digestibility, serum biochemistry, performance, carcass characteristics and meat attributes of feedlot lambs. Trop. Anim. Health Prod. 2024, 56, 153. [Google Scholar] [CrossRef]
- Ash, A.J.; Elliott, R. Tropical crop and crop by-product additives can improve the quality of taro leaf (Colocasia esculenta) silage. J. Agric. Sci. 1991, 117, 233–239. [Google Scholar] [CrossRef]
- Maneerat, W.; Prasanpanich, S.; Tumwasorn, S.; Laudadio, V.; Tufarelli, V. Evaluating agro-industrial by-products as dietary roughage source on growth performance of fattening steers. Saudi J. Biol. Sci. 2015, 22, 580–584. [Google Scholar] [CrossRef]
- Xie, H.; Zeng, F.; Luo, X.; Li, Z.; Pan, Y.; Guo, Y.; Peng, L.; Liang, L.; Li, J.; Liang, Y.; et al. Silage Making of Napier Grass and Sugarcane Top at Different Proportions: Evolution of Natural Fermentation Characteristics, Chemical Composition, and Microbiological Profile. Fermentation 2024, 10, 525. [Google Scholar] [CrossRef]
- Xie, H.; Peng, L.; Li, M.; Guo, Y.; Liang, X.; Peng, K.; Yang, C. Effects of mixed sugarcane tops and napiergrass silages on fermentative quality, nutritional value, and milk yield in water buffaloes. Anim. Sci. J. 2023, 94, e13824. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, H.; Zeng, F.; Luo, X.; Peng, L.; Sun, X.; Wang, X.; Yang, C. An Assessment on the Fermentation Quality and Bacterial Community of Corn Straw Silage with Pineapple Residue. Fermentation 2024, 10, 242. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. 2018, 58, 486–511. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Guo, R.; Zou, C.; Chen, H.; Chen, D.; Yang, L.; Tan, H.; Wu, S.; Lv, Y.; Xiao, Z.; et al. Insight into the chemical composition, antioxidant capacity, meat quality, fatty acid profile, and volatile compounds of yellow-feathered chickens fed with fermented pineapple residue. Food Chem. 2024, 24, 101874. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Udén, P. In vitro studies on microbial efficiency from two cuts of ryegrass (Lolium perenne, cv. Aberdart) with different proportions of sugars and protein. Anim. Feed Sci. Technol. 2006, 126, 145–156. [Google Scholar] [CrossRef]
- Cai, Y. Analysis method for silage. In Japanese Society of Grassland Science, Field and Laboratory Methods for Grassland Science; Tosho Printing Co., Ltd.: Tokyo, Japan, 2004; pp. 279–282. [Google Scholar]
- Byrne, E.; McCormack, S. Determination of ammonium nitrogen in silage samples by an ammonia electrode. Commun. Soil Sci. Plant 2008, 9, 667–684. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, Y.; Hirakubo, T.; Fukui, H.; Matsuyama, H. Fermentation characteristics and microorganism composition of total mixed ration silage with local food by-products in different seasons. Anim. Sci. J. 2011, 82, 259–266. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on Ruminant Saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Sharifi, A.; Fazaeli, H.; Azarfar, A.; Jonker, A.; Kiani, A. Effect of transferring lignocellulose-degrading bacteria from termite to rumen fluid of sheep on in vitro gas production, fermentation parameters, microbial populations and enzyme activity. J. Integr. Agric. 2020, 19, 1323–1331. [Google Scholar] [CrossRef]
- Cherney, J.H.; Cherney, D.J.R. Assessing Silage Quality. In Silage Science and Technology; Wiley: Hoboken, NJ, USA, 2003; pp. 141–198. [Google Scholar]
- Mitiku, A.A.; Andeta, A.F.; Borremans, A.; Lievens, B.; Bossaert, S.; Crauwels, S.; Aernouts, B.; Kechero, Y.; Van, L. Silage making of maize stover and banana pseudostem under South Ethiopian conditions: Evolution of pH, dry matter and microbiological profile. Microb. Biotechnol. 2020, 13, 1477–1488. [Google Scholar] [CrossRef]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Wu, P.; Li, L.; Jiang, J.; Sun, Y.; Yuan, Z.; Feng, X.; Guo, Y. Effects of fermentative and non-fermentative additives on silage quality and anaerobic digestion performance of Pennisetum purpureum. Bioresour. Technol. 2020, 297, 122425. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, W.; Tian, H.; Wang, M.; Gao, C.; Guo, Y.; Sun, B. A preliminary study on the possibility of fermented pineapple peel residue partially replacing whole corn silage in feeding Chuanzhong black goats. Front. Microbiol. 2022, 13, 959857. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.M.; Juniper, D.T.; Bryant, M.J.; Beever, D.E. Apparent digestibility and nitrogen utilisation of diets based on maize and grass silage fed to beef steers. Anim. Feed Sci. Technol. 2005, 119, 55–68. [Google Scholar] [CrossRef]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of mixing Neolamarckia cadamba leaves on fermentation quality, microbial community of high moisture alfalfa and stylo silage. Microb. Biotechnol. 2019, 12, 869–878. [Google Scholar] [CrossRef]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 2019, 284, 240–247. [Google Scholar] [CrossRef]
- Singh, D.; Johnson, T.A.; Tyagi, N.; Malhotra, R.; Behare, P.V.; Kumar, S.; Tyagi, A.K. Synergistic Effect of LAB Strains (Lb. fermentum and Pediococcus acidilactisci) with Exogenous Fibrolytic Enzymes on Quality and Fermentation Characteristics of Sugarcane Tops Silage. Sugar Tech 2023, 25, 141–153. [Google Scholar] [CrossRef]
- Kung, L.J.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Bolsen, K.K.; Lin, C.J. History of Silage. In Silage Science and Technology; Wiley: Hoboken, NJ, USA, 2003; pp. 1–30. [Google Scholar]
- Bernardes, T.F. Advances in Silage Sealing. In Advances in Silage Production and Utilization; InTechOpen: London, UK, 2016; pp. 53–62. [Google Scholar]
- Daniel, J.L.P.; Bernardes, T.F.; Jobim, C.C.; Schmidt, P.; Nussio, L.G. Production and utilization of silages in tropical areas with focus on Brazil. Grass Forage Sci. 2019, 74, 188–200. [Google Scholar] [CrossRef]
- Moura, Z.A.; Sene, O.A.; Jesus, F.D.; Parente, H.N.; Oliveira, M.P.M.; Pinho, R.M.A.; Santos, E.M.; Nascimento, T.V.C.; Oliveira, L.A.G.V.; Perazzo, A.F. Fermentative profile, losses and chemical composition of silage soybean genotypes amended with sugarcane levels. Sci. Rep. 2020, 10, 21064. [Google Scholar]
- Cao, Y.; Zang, Y.; Jiang, Z.; Han, Y.; Hou, J.; Liu, H.; Zhong, R.; Fang, J.; Zhang, A.; Yoshida, N. Fermentation quality and nutritive value of fresh and fermented total mixed rations containing Chinese wildrye or corn stover. Grassl. Sci. 2016, 62, 213–223. [Google Scholar] [CrossRef]
- Broberg, A.; Jacobsson, K.; Strom, K.; Schnurer, J. Metabolite profiles of lactic acid bacteria in grass silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Huang, K.; Gao, Y.; Lin, Y.; Yuan, B.; Wang, X.; Xu, G.; Nussio, L.G.; Yang, F.; et al. Multi-omics analysis reveals the core microbiome and biomarker for nutrition degradation in alfalfa silage fermentation. mSystems 2024, 9, e00682-24. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S.S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.W.H.O.; Spoelstra, S.F. Microbiology of Ensiling. In Silage Science and Technology; Wiley: Hoboken, NJ, USA, 2003; pp. 31–93. [Google Scholar]
- Dubois, P.C.A.; Trynka, G.; Franke, L.; Hunt, K.A.; Romanos, J.; Curtotti, A.; Zhernakova, A.; Heap, G.A.R.; Ádány, R.; Aromaa, A.; et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010, 42, 295–302. [Google Scholar] [CrossRef]
- Savoie, P.; Jofriet, J.C. Silage Storage. In Silage Science and Technology; Wiley: Hoboken, NJ, USA, 2003; pp. 405–467. [Google Scholar]
- Paz-Arteaga, S.L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Cadena-Chamorro, E.; Serna-Cock, L.; Aguilar-González, M.A.; Ramírez-Guzmán, N.; Torres-León, C. Bioprocessing of pineapple waste for sustainable production of bioactive compounds using solid-state fermentation. Innov. Food Sci. Emerg. 2023, 85, 103313. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Zhang, Q.; Yang, Y.; Zou, C.; Li, L.; Liang, X.; Wei, S.; Lin, B. Ruminal fermentation and microbial community differently influenced by four typical subtropical forages in vitro. Anim. Nutr. 2018, 4, 100–108. [Google Scholar] [CrossRef]
- Li, M.; Zi, X.; Zhou, H.; Hou, G.; Cai, Y. Effects of sucrose, glucose, molasses and cellulase on fermentation quality and in vitro gas production of king grass silage. Anim. Feed Sci. Technol. 2014, 197, 206–212. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Liu, G.; Gao, R.; Bao, J.; Wang, L.; Wu, Z.; Yu, Z. Associative effects of ensiling mixtures of sweet sorghum and korshinsk pea shrub on fermentation quality, chemical composition, and in vitro rumen digestion characteristics. Anim. Sci. J. 2022, 93, e13700. [Google Scholar] [CrossRef]
- Dorta, E.; Sogi, D.S. Value added processing and utilization of pineapple by-products. In Handbook of Pineapple Technology; Wiley: Hoboken, NJ, USA, 2017; pp. 196–220. [Google Scholar]
- Gómez-Trinidad, M.; Sánchez-Santillán, P.; Ayala-Monter, M.A.; Saavedra-Jimenez, L.A.; Sollano-Mendieta, C.E.; López-Torres, B.J. In vitro gas production, in situ digestibility, intake, weight gain and ruminal characteristics of calves fed a diet containing 60% waste papaya silage. Trop. Anim. Health Prod. 2024, 56, 370. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.X.; Wei, L.Y.; Walker, N.D.; Liu, F.Z.; Chen, L.Y.; Beauchemin, K.A.; Yang, W.Z. Comparison of non-encapsulated and encapsulated active dried yeast on ruminal pH and fermentation, and site and extent of feed digestion in beef heifers fed high-grain diets. Anim. Feed Sci. Technol. 2017, 228, 13–22. [Google Scholar] [CrossRef]
- Akinfemi, A.; Adesanya, A.O.; Ay, V.E. Use of an in vitro gas production technique to evaluate some Nigerian feedstuffs. Am-Euras. J. Sci. Res. 2009, 4, 240–245. [Google Scholar]
- Melesse, A.; Steingass, H.; Boguhn, J.; Rodehutscord, M. In vitro fermentation characteristics and effective utilisable crude protein in leaves and green pods of Moringa stenopetala and Moringa oleifera cultivated at low and mid-altitudes. J. Anim. Physiol. Anim. Nutr. 2013, 97, 537–546. [Google Scholar] [CrossRef]
- Gultepe, E.E.; Uyarlar, C.; Cetingul, I.S.; Iqbal, A.; Ozcinar, U.; Bayram, I.; Bradford, B.J. Comparison of ruminal digestibility of Origanum onites L. leaves in dairy buffalo and cows. Trop. Anim. Health Prod. 2020, 52, 2063–2071. [Google Scholar] [CrossRef]


| Items | Content (% DM Basis) |
|---|---|
| Ingredient | |
| Distiller’s grains | 30.00 |
| Corn straw silage | 52.00 |
| Peanut straw | 7.00 |
| Marigold meal | 10.00 |
| NaCl | 0.35 |
| Premix 1 | 0.65 |
| Total | 100.00 |
| Nutrient levels 2 | |
| CP | 13.22 |
| NDF | 52.26 |
| ADF | 33.98 |
| Ash | 8.27 |
| Ca | 0.73 |
| p | 0.30 |
| GE/(MJ/kg) | 15.48 |
| Item | Fresh Forage | Silage † | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| NG | ST | PP | C | P1 | P2 | P3 | |||
| DM (%) | 16.71 | 26.21 | 11.91 | 21.17 a | 18.86 b | 17.32 c | 15.51 d | 0.077 | <0.0001 |
| CP (%DM) | 13.26 | 7.31 | 9.50 | 9.51 a | 9.01 b | 8.76 c | 8.63 c | 0.069 | <0.0001 |
| OM (%DM) | 91.22 | 90.99 | 92.31 | 87.19 c | 88.29 b | 88.50 b | 89.42 a | 0.104 | <0.0001 |
| WSC (%DM) | 2.17 | 13.89 | 12.78 | 5.05 | 5.21 | 5.30 | 5.47 | 0.142 | 0.2277 |
| NDF (%DM) | 67.81 | 71.72 | 33.85 | 67.94 a | 64.40 b | 63.27 c | 61.37 d | 0.354 | <0.0001 |
| ADF (%DM) | 38.02 | 40.26 | 19.29 | 38.50 a | 37.94 a | 35.92 b | 33.88 c | 0.316 | <0.0001 |
| Item | Silage † | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | P1 | P2 | P3 | |||
| pH | 4.23 | 4.16 | 4.18 | 4.21 | 0.023 | 0.2232 |
| Lactic acid (g/kg DM) | 40.36 | 43.88 | 39.81 | 39.06 | 1.667 | 0.2190 |
| Acetic acid (g/kg DM) | 14.37 b | 15.46 b | 17.30 a | 18.85 a | 0.569 | 0.0002 |
| Propionic acid (g/kg DM) | 5.74 c | 5.89 c | 7.40 b | 7.92 a | 0.155 | <0.0001 |
| Butyric acid (g/kg DM) | ND | ND | ND | ND | - | - |
| NH3-N (g/kg DM) | 0.28 b | 0.21 c | 0.31 b | 0.39 a | 0.018 | <0.0001 |
| LAB (lg cfu/g FM) | 8.46 a | 8.41 a | 7.98 b | 7.20 c | 0.101 | <0.0001 |
| Yeasts (lg cfu/g FM) | 6.49 c | 6.71 b | 6.88 a | 6.94 a | 0.052 | <0.0001 |
| Mold (lg cfu/g FM) | ND | ND | ND | ND | - | - |
| Item | Silage † | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | P1 | P2 | P3 | |||
| IVDMD (%) | 37.54 c | 39.96 bc | 42.13 ab | 42.99 a | 0.888 | 0.0023 |
| Gas production (mL/g DM) | ||||||
| Total Gas | 96.58 | 98.08 | 97.28 | 96.82 | 2.095 | 0.9592 |
| H2 | 0.19 | 0.20 | 0.21 | 0.22 | 0.023 | 0.7252 |
| Methane | 17.65 | 15.27 | 16.12 | 16.52 | 1.022 | 0.4490 |
| pH | 6.44 a | 6.39 b | 6.42 ab | 6.46 a | 0.014 | 0.0164 |
| VFA (mmol/L) | ||||||
| Total | 17.92 | 18.83 | 18.10 | 17.70 | 1.331 | 0.9367 |
| Acetate | 9.28 | 10.70 | 9.95 | 9.54 | 0.771 | 0.5961 |
| Propionate | 5.91 | 5.61 | 5.62 | 5.47 | 0.565 | 0.9543 |
| Isobutyrate | 0.08 | 0.07 | 0.06 | 0.07 | 0.021 | 0.9414 |
| Butyrate | 2.17 | 2.06 | 2.08 | 2.15 | 0.169 | 0.9622 |
| Isovalerate | 0.20 | 0.15 | 0.15 | 0.19 | 0.038 | 0.6855 |
| Valerate | 0.28 | 0.25 | 0.24 | 0.28 | 0.026 | 0.6988 |
| Acetate/propionate | 1.57 | 2.05 | 1.78 | 1.79 | 0.219 | 0.5053 |
| NH3-N (mg/L) | 51.87 | 51.51 | 48.61 | 48.47 | 1.140 | 0.0916 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Tang, Z.; Zeng, F.; Luo, X.; Xie, F.; Liang, L.; Li, J.; Liao, P.; Peng, L.; Li, Z.; et al. Effects of Pineapple Peel on the Nutritional and Microbial Profiles of Napier Grass–Sugarcane Top Silage. Microorganisms 2025, 13, 1314. https://doi.org/10.3390/microorganisms13061314
Xie H, Tang Z, Zeng F, Luo X, Xie F, Liang L, Li J, Liao P, Peng L, Li Z, et al. Effects of Pineapple Peel on the Nutritional and Microbial Profiles of Napier Grass–Sugarcane Top Silage. Microorganisms. 2025; 13(6):1314. https://doi.org/10.3390/microorganisms13061314
Chicago/Turabian StyleXie, Huade, Zhenhua Tang, Fanquan Zeng, Xianqing Luo, Fang Xie, Li Liang, Jingzhen Li, Pinfeng Liao, Lijuan Peng, Zhipei Li, and et al. 2025. "Effects of Pineapple Peel on the Nutritional and Microbial Profiles of Napier Grass–Sugarcane Top Silage" Microorganisms 13, no. 6: 1314. https://doi.org/10.3390/microorganisms13061314
APA StyleXie, H., Tang, Z., Zeng, F., Luo, X., Xie, F., Liang, L., Li, J., Liao, P., Peng, L., Li, Z., Bai, H., Guo, X., & Yang, C. (2025). Effects of Pineapple Peel on the Nutritional and Microbial Profiles of Napier Grass–Sugarcane Top Silage. Microorganisms, 13(6), 1314. https://doi.org/10.3390/microorganisms13061314






