Abstract
Daqu, a crucial fermentation starter for Chinese Baijiu, develops distinct microbial and physicochemical profiles depending on fermentation temperature, which significantly influence enzymatic activity and flavor formation. While high-temperature (HT-Daqu, 65 °C) and medium-temperature (MT-Daqu, 60 °C) variants are known to produce different liquor aromas, systematic comparisons of their microbial and physicochemical dynamics remain limited. This study integrated physicochemical assays (moisture, starch, acidity, enzymatic activity) with 16S rRNA and ITS (Internal Transcribed Spacer) sequencing to analyze HT-Daqu (HQ1–HQ3) and MT-Daqu (MQ1–MQ3) from Sichuan breweries. Results revealed that HT-Daqu exhibited significantly lower moisture (p < 0.05) and starch content (p < 0.05) but higher acidity (p < 0.05) compared to MT-Daqu. Enzymatic activities were generally reduced in HT-Daqu, except for neutral protease. Microbial profiling revealed distinct microbial dynamics between HT-Daqu and MT-Daqu: HT-Daqu harbored thermophilic Bacillus (40–60% relative abundance) with reduced fungal diversity, while MT-Daqu prioritized fungal consortia—Aspergillus dominated MQ1 (78%) and Saccharomyces transiently peaked in MQ2 (35%)—which correlated with enhanced saccharification enzyme activities and esterification potential. Alpha-diversity indices confirmed higher bacterial diversity in HT-Daqu and greater fungal richness in MT-Daqu. Correlation networks highlighted temperature-driven linkages, such as Bacillus positively associating with acidity. These findings elucidate the trade-offs between microbial stress adaptation and metabolic efficiency under different thermal regimes, providing actionable insights for optimizing Daqu production through targeted microbial management and temperature control to enhance liquor quality.
1. Introduction
Chinese Baijiu, a traditional distilled spirit and one of the world’s oldest fermented beverages, is renowned for its complex flavor profiles and cultural significance. Its production relies on a starch-rich substrate (e.g., sorghum, wheat) that undergoes solid-state fermentation, a process driven by Daqu—a microbial-rich starter culture critical for saccharification, alcohol synthesis, and flavor development [1]. Daqu is crafted by fermenting crushed grains under open conditions, enabling natural inoculation of environmental microorganisms, including bacteria, molds, yeasts, and actinomycetes [2]. These microbes produce hydrolytic enzymes (e.g., amylases, proteases) and metabolic precursors that collectively shape the aroma, texture, and quality of Baijiu [1].
Daqu is categorized by its peak fermentation temperature: high-temperature Daqu (HT-Daqu; 60–65 °C), medium-temperature Daqu (MT-Daqu; 50–60 °C), and low-temperature Daqu (LT-Daqu; <50 °C) [2]. Each type supports distinct microbial consortia and physicochemical properties, which directly correlate with the flavor characteristics of the final product [3]. For instance, HT-Daqu, pivotal for sauce-aroma Baijiu (e.g., Moutai), harbors thermotolerant Bacillus and Aspergillus species that generate pyrazines and phenolic compounds under prolonged high-temperature fermentation [3]. MT-Daqu, used for strong-aroma liquors, exhibits balanced microbial diversity dominated by Saccharopolyspora and Lactobacillus, contributing to ester-rich profiles [3]. LT-Daqu, employed in light-aroma varieties, favors Rhizopus and Pichia species, which enhance fruity and floral notes through cooler, shorter fermentation cycles [3]. These temperature-driven differences in microbial composition also influence enzymatic activities, such as alcohol acyltransferases (AATs), which catalyze ester synthesis and are highly temperature-dependent [4].
Key physicochemical parameters—including moisture, starch content, acidity, and enzyme activities (e.g., saccharification, liquefaction)—are highly sensitive to thermal conditions. HT-Daqu typically exhibits reduced starch content and lower enzymatic activity (except neutral protease) compared to MT-Daqu, likely due to heat-induced inactivation [5]. Conversely, MT-Daqu fosters fungal dominance (e.g., Aspergillus and Saccharomyces), enhancing saccharification and ester synthesis [6]. These differences highlight temperature-regulated trade-offs between microbial stress adaptation and metabolic efficiency. However, controversies persist regarding the trade-offs between microbial diversity and functional enzyme activity under different thermal regimes, necessitating systematic comparisons [7].
Microbial community structure further differentiates Daqu functionality. HT-Daqu selects for thermophilic bacteria like Bacillus, which contribute to protein degradation and aroma precursor synthesis under extreme heat [8]. In contrast, MT-Daqu supports synergistic fungal–bacterial consortia (e.g., Saccharomycopsis and Lactobacillus) that optimize starch hydrolysis [9]. Recent advances in high-throughput sequencing confirm that HT-Daqu harbors greater bacterial diversity but reduced fungal richness compared to MT-Daqu [10]. This temperature-driven microbial succession underscores the need for comparative analyses to guide Daqu process optimization. Nevertheless, the mechanistic links between temperature-induced microbial shifts and key physicochemical parameters, such as neutral protease activity or reducing sugar content, remain underexplored [11].
Despite advances in understanding Daqu’s microbiome, critical knowledge gaps persist. Comparative studies on temperature-dependent microbial assembly patterns between HT- and MT-Daqu remain sparse, particularly regarding functional shifts in enzyme kinetics and metabolic networks. We hypothesize that Daqu-production temperature governs enzymatic profiles and microbial diversity, with HT-Daqu favoring bacterial dominance over fungal activity. By integrating conventional physicochemical assays (moisture, starch, acidity, reducing sugars) and enzyme activity measurements (saccharification, liquefaction, neutral protease) with high-throughput sequencing, we systematically evaluated microbial and functional divergences. The findings elucidate temperature-driven trade-offs in microbial functionality and offer actionable strategies to optimize fermentation for enhanced liquor quality.
2. Materials and Methods
2.1. Sample Collection
HT-Daqu (HQ1–HQ3 denote high-temperature Daqu replicates; peak fermentation temperature: 65 °C) and MT-Daqu (MQ1–MQ3 represent medium-temperature Daqu replicates; peak fermentation temperature: 60 °C) were obtained from three independent brewing enterprises in Yibin City and Luzhou City, respectively. The Daqu samples were produced using a standardized protocol: locally sourced soft wheat was first crushed, mixed with water (approximately 40–45% moisture content), and pressed into brick-shaped blocks (dimensions: 25 × 15 × 6 cm). These blocks underwent spontaneous solid-state fermentation under controlled temperature and humidity conditions. For HT-Daqu, the fermentation process included a high-temperature stage (peaking at 65 °C for 5–7 days) to promote thermophilic microbial succession (e.g., Bacillus, Thermoascus) and enzyme activation, while MT-Daqu maintained moderate thermal conditions (peaking at 60 °C for 3–5 days) to preserve fungal diversity (e.g., Aspergillus, Saccharomyces). Post-fermentation, the Daqu bricks were air-dried naturally for 15–20 days and stored in ventilated rooms at ambient temperature (20–25 °C) for 3–4 months to ensure microbial stabilization and flavor maturation. Prior to analysis, samples were collected from the outer, middle, and inner layers of the Daqu bricks to account for spatial heterogeneity, homogenized under sterile conditions, and stored at −80 °C for microbial profiling and 4 °C for enzymatic assays. At the time of sampling, the Daqu exhibited key physicochemical characteristics: moisture content (10.5–12.8%), acidity (pH 5.2–5.8), liquefying activity (1.2–1.6 U/g), and esterifying capacity (120–150 mg/100 g), which correlate with their enzymatic and microbial functionality during Baijiu fermentation.
Eighteen samples (9 per group) were collected in August 2024 at the end of the microbial incubation phase (45 days for HT-Daqu; 30 days for MT-Daqu) (Supplementary Figure S1). To ensure sample homogeneity, each Daqu block was homogenized via a three-point sampling protocol (core, crust, and edge regions). Each homogenized sample (600 g) was divided into two aliquots: 300 g stored at −20 °C for physicochemical characterization, and 300 g snap-frozen in liquid nitrogen and preserved at −80 °C for microbial DNA extraction (16S rRNA gene and ITS sequencing). Biological triplicates (three independent fermentation batches per group) were included to enhance experimental robustness.
2.2. Physicochemical Properties and Enzyme Activity Analysis
The physicochemical properties of Daqu, including moisture, acidity, starch content, liquefying enzyme activity (measured via α-amylase-mediated hydrolysis of starch into dextrins at 65 °C), saccharifying enzyme activity (assessed via glucoamylase-driven conversion of dextrins to glucose at 60 °C), fermentation capacity (quantified by CO2 production during 72-h anaerobic fermentation of Daqu slurry, normalized to glucose equivalents), and esterification capacity, were measured according to the Brewing Daqu Quality Control Standards (QB/T 4257-2011) [12] (Supplementary Figure S1). Protease activities (acidic and neutral) were evaluated using the Technical Specifications for Strong-Flavor Daqu Analysis (DB 34/T 3085-2018) [13]. Cellulase activity was determined via the spectrophotometric protocol outlined in the Feed Additive Testing Guidelines (NY/T 912-2020) [14], while hemicellulase activity was analyzed following the Xylanase Assay Method for Feed Additives (GB/T 23874-2009) [15]. Reducing sugar content was quantified through the National Food Safety Standard (GB 5009.7-2016, Method 2) [16], with values reported as glucose equivalent concentrations.
2.3. Sample DNA Extraction and Microbial Community Structure Analysis
Total genomic DNA was extracted from 0.3 g of wheat-based Daqu samples using the PowerSoil® DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions, with triplicate extractions per sample pooled to minimize variability. DNA concentration and purity were evaluated by spectrophotometry (NanoDrop, Wilmington, DE, USA) and agarose gel electrophoresis. The V3-V4 regions of bacterial 16S rRNA genes were amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) [17], while fungal ITS regions were amplified with primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [18]. Purified amplicons were sequenced on the Illumina MiSeq platform (2 × 250 bp paired-end) at Biomarker Technologies Co., Ltd. (Beijing, China), with raw data processed through QIIME2 (v2021.11) for quality filtering, denoising, and feature table generation.
2.4. Bioinformatics Analysis
Raw sequencing data were analyzed using CCS 6.0.0 (PacBio, Menlo Park, CA, USA) to produce high-accuracy circular consensus reads. Quality filtering involved adapter removal through the fastx-toolkit_v0.0.14-6 and elimination of chimeric sequences with VSEARCH v2.14.1’s reference-based uchime method. Sequence deduplication was executed via the fastx_uniques function in USEARCH v11.0.667, followed by Amplicon Sequence Variant (ASV) derivation through the unoise3 pipeline (minimum read abundance: minsize = 8). Taxon-specific filtration was implemented using in-house scripts to retain fungal sequences from ITS regions and bacterial sequences from 16S datasets (Supplementary Figure S1). Operational taxonomic units (OTUs) were defined through 97% similarity clustering with USEARCH (v9.2.612), followed by taxonomic classification via NCBI NT database alignment using BLASTn v2.2.28 (e-value cutoff: 1 × 10−5, minimum similarity: 97%). α-diversity assessments were performed in R v4.3.0 using the vegan package [19], with calculations based on normalized ASV abundance matrices.
2.5. Alpha Diversity Analysis
To systematically characterize microbial community profiles, three α-diversity metrics (Chao1, Shannon, Simpson) were utilized. The Chao1 index quantifies taxonomic abundance, whereas Shannon and Simpson indices incorporate both population quantity and distribution uniformity—essential parameters for evaluating metabolic consistency and functional adaptability within Daqu fermentation systems. Elevated Shannon indices coupled with reduced Simpson coefficients correlate with enhanced biodiversity, suggesting an equilibrated community structure. All statistical processing was performed within the QIIME2 computational framework.
2.6. Statistical Analysis
The data were analyzed using SPSS 26.0 (IBM Corp., Armonk, NY, USA). Differences among groups were evaluated by one-way analysis of variance (ANOVA), followed by Tukey’s HSD post hoc test, with significance defined at p < 0.05. Spearman correlation analysis, conducted using SAS v8 (SAS Institute Inc., Cary, NC, USA), was employed to examine the relationships between dominant Daqu microbial taxa and physicochemical parameters. The results were visualized using Cytoscape v3.7.2 (Cytoscape Consortium, San Diego, CA, USA).
3. Results
3.1. Daqu-Making Temperature Modulates Physicochemical Properties
All Daqu samples exhibited water content below 13% (w/w) (Figure 1A), conforming to standard fermentation parameters. HT-Daqu (HQ1–3) demonstrated significantly lower moisture (8.88–10.75%) than MT- counterparts (MQ1–3: 11.68–12.51%) (p < 0.05) yet displayed elevated acidity (1.70–2.16 mmol/10 g vs. 1.10–1.22 mmol/10 g; Figure 1A; Supplementary Table S1). Intra-group variations revealed extremes in water content (MQ1: 12.51%; HQ1: 8.88%) and acidity (HQ2: 2.16 mmol/10 g; MQ3: 1.09 mmol/10 g).
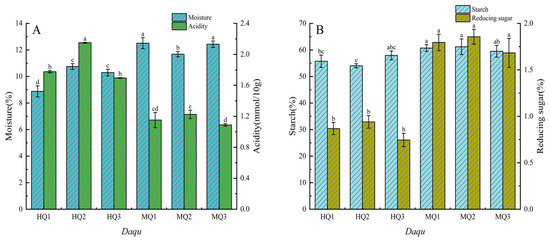
Figure 1.
Changes in physicochemical indexes in different medium-high temperature Daqu. (A) Acidity, moisture. (B) Starch, reducing sugar. Data are presented as mean ± SD (n = 3). Bars with different letters indicate significant difference (p < 0.05).
HT-Daqu (HQ1–3) exhibited significantly lower reducing sugar (0.75–0.94%) and starch content (54.04–57.93%) compared to MT-Daqu (MQ1–3: 1.68–1.86% reducing sugar, 59.50–61.20% starch) (p < 0.05, Figure 1B; Supplementary Table S1). Notable intra-group variations were observed, with HQ3 (0.75%) and MQ2 (1.86%) representing the respective extremes of reducing sugar content, while starch content ranged from 54.04% (HQ2) to 61.20% (MQ2).
3.2. Temperature-Driven Regulation of Enzymatic Activities
Significant temperature-dependent variations in enzymatic profiles were observed between MT and HT Daqu. MT-Daqu exhibited 1.6-fold higher neutral protease activity than HT-Daqu (p < 0.05; Figure 2A; Supplementary Table S2), whereas acidic protease activity was markedly reduced in MT-Daqu. Saccharification enzyme activity in MT-Daqu (MQ2) surpassed that of HT-Daqu (HQ1) by 38% (p < 0.05), though comparable liquefaction activity was maintained across all groups (Figure 2B). Cellulase and hemicellulase activities peaked in MT-Daqu (MQ1), showing 2.3- and 1.9-fold increases relative to HT-Daqu (p < 0.01; Figure 2C; Supplementary Table S2). Notably, MT-Daqu demonstrated superior esterase activity and fermentation capacity, with MQ1 outperforming all HT-Daqu samples by 27% (p < 0.05; Figure 2D).
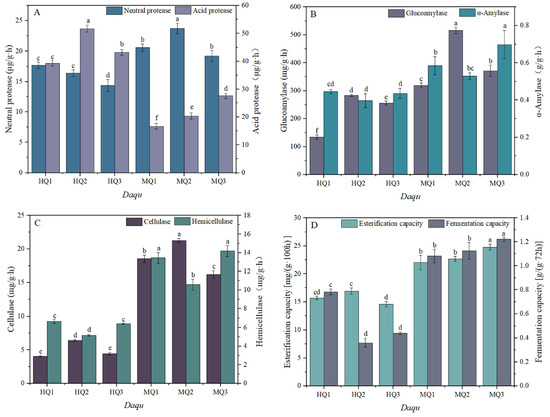
Figure 2.
Changes in enzyme activity in medium-high temperature Daqu. (A) Neutral protease activity, acid protease. (B) Glucoamylase, α-amylase. (C) Cellulase, hemicellulase. (D) Fermentation capacity, esterification capacity. Data are presented as mean ± SD (n = 3). Bars with different letters indicate significant difference (p < 0.05).
3.3. Daqu-Making Temperature Shapes α-Diversity
Alpha-diversity metrics of bacterial communities revealed systematic differences between HT-Daqu (HQ1–3) and MT-Daqu (MQ1–3). HT-Daqu exhibited significantly higher ASV counts (HQ1: 961 vs. MQ3: 77; p < 0.01; Table 1), while MT-Daqu MQ1 showed the highest Chao1 index (642.03 ± 0.021) (Table 1), surpassing all HT counterparts (e.g., HQ1: 396.36 ± 2.810). The Shannon index was significantly elevated in HT-Daqu (HQ1: 7.83 ± 0.073 vs. MQ3: 1.76 ± 0.042), whereas the Simpson index displayed an inverse pattern (HQ1: 0.039 ± 0.001 vs. MQ1: 0.980 ± 0.002, Table 1). No significant difference was observed in the Shannon index between HQ2 (4.81 ± 0.009) and MQ1 (4.35 ± 0.008) (p > 0.05). All samples achieved sequencing coverage > 99.9% (Table 1), with HT-Daqu demonstrating greater intra-group variability in Chao1 indices (e.g., HQ1: ±2.810) compared to MT groups (e.g., MQ1: ±0.021).

Table 1.
The impact of peak temperature on the α-diversity of bacterial communities in medium-high Daqu.
Alpha diversity analysis of fungal communities in HT (HQ1–3) and MT-Daqu (MQ1–3) revealed significant ecological divergence (Table 2). MT-Daqu exhibited markedly higher species richness, with ASV counts (635–2037) and Chao1 indices (209.79–952.11) substantially exceeding those of HT groups (ASVs: 331–368; Chao1: 47.83–122.24; Table 2). The maximum Chao1 value was recorded in MQ2 (952.11 ± 0.004), contrasting sharply with the minimum in HQ2 (47.83 ± 5.137), demonstrating statistically significant intergroup variation (p < 0.05). Species diversity patterns further distinguished the groups. MT- Daqu showed significantly higher Shannon indices (MQ1: 8.44 ± 0.022; MQ2: 7.63 ± 0.010) compared to HT counterparts (1.53–2.01). Conversely, Simpson indices revealed inverse trends (Table 2), with HT groups displaying lower dominance values (0.37–0.63) than MT samples (0.64–0.99). Sequencing coverage exceeded 99.9% for all samples (Table 2), ensuring robust community characterization through sufficient sequencing depth. These metrics collectively highlight temperature-dependent restructuring of fungal community complexity in Daqu ecosystems.

Table 2.
The impact of peak temperature on the α-diversity of fungal communities in medium-high temperature Daqu.
3.4. Genus-Level Abundance Dynamics Driven by Qu-Making Temperature
Bacterial profiling identified 856 genera across HT- and MT-Daqu. HT variants were dominated by Bacillus (40–60% relative abundance), Kroppenstedtia, and Caldibacillus (Figure 3). In contrast, MT-Daqu exhibited predominance of mesophilic genera including Virgibacillus (particularly in MQ1), Saccharopolyspora, and Desmospora (Figure 3). Minor taxa (unclassified genera) constituted <20% of communities in both groups.
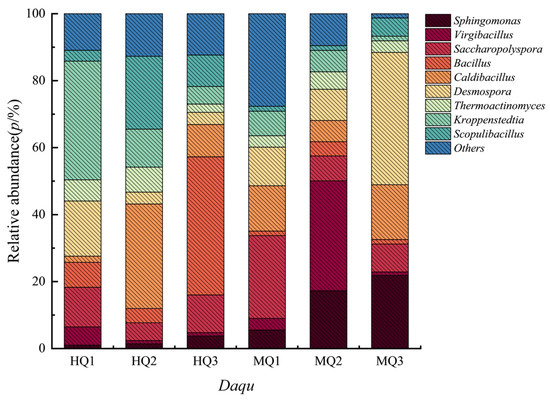
Figure 3.
The bacterial taxonomic abundance in HT- and MT-Daqu.
Fungal community analysis identified 932 genera across HT- and MT-Daqu. HT- Daqu exhibited dominance of thermotolerant taxa: Paecilomyces (52% relative abundance), Talaromyces (28%), and Rasamsonia (33%) (Figure 4). In contrast, MT-Daqu was characterized by mesophilic genera Aspergillus (78%), Saccharomyces (35%), and Wickerhamomyces (31%), demonstrating temperature-driven fungal consortia stratification (Figure 4).
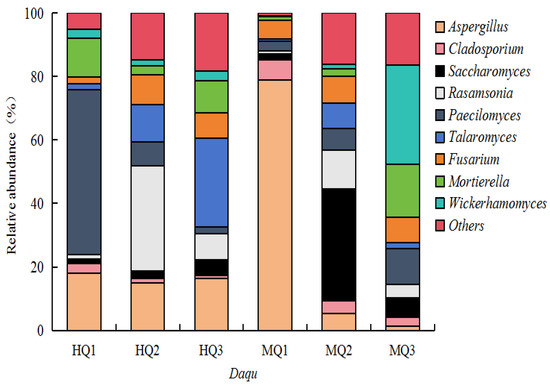
Figure 4.
The fungal taxonomic abundance in HT- and MT-Daqu.
3.5. Microbial-Physicochemical Correlations in HT- and MT-Daqu
The study revealed significant correlations between microbial taxa and physicochemical properties in HT- and MT-Daqu. Bacterial genera exhibited distinct functional roles: Sphingomonas showed highly significant positive associations with acid protease and glucoamylase activities (p < 0.01) (Figure 5), alongside significant correlations with esterase activity and fermentation capacity (p < 0.05), but displayed a strong negative correlation with acidity (p < 0.01). Bacillus was positively linked to acidity but negatively correlated with moisture content (p < 0.01) and reducing sugar/esterase activity (p < 0.05). Desmospora demonstrated significant positive correlations with amylase and hemicellulase activities (p < 0.01), while Scopulibacillus was negatively associated with starch content and neutral protease activity (p < 0.05) (Figure 5). Kroppenstedtia exhibited dual correlations—positive with acidity and negative with acid protease, glucoamylase, and fermentation capacity.
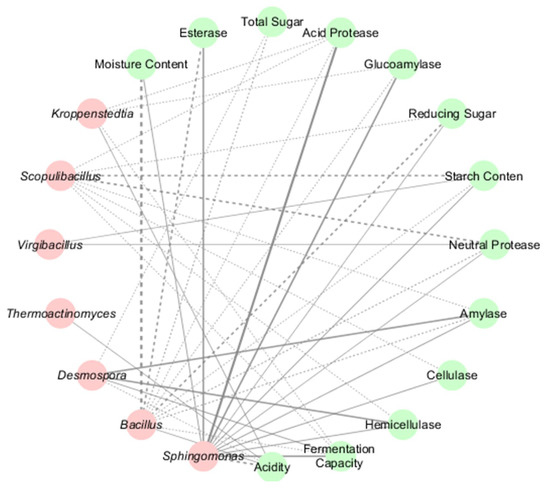
Figure 5.
Bacterial–Physicochemical Network in HT- and MT-Daqu. Red spheres represent bacterial genera, while green spheres denote physicochemical indicators. Positive and negative associations are denoted by solid and dashed lines, respectively. Line color intensity corresponds to correlation strength (darker hues indicate higher absolute values), while bold lines signify statistically significant relationships (p < 0.05).
Fungal genera displayed contrasting behaviors: Aspergillus negatively correlated with acid protease, glucoamylase, and esterase activities, diverging from its typical starch-degrading roles. Cladosporium and Saccharomyces showed significant positive associations with neutral protease (p < 0.05) and acid protease (p < 0.05) (Figure 6), respectively, while Talaromyces strongly negatively correlated with total sugar (p < 0.01). These findings highlight the temperature-dependent microbial–physicochemical interplay, emphasizing the multifunctionality of understudied taxa like Sphingomonas and the context-dependent roles of ubiquitous genera such as Aspergillus. The results provide actionable insights for optimizing fermentation parameters and microbial management in Daqu production.
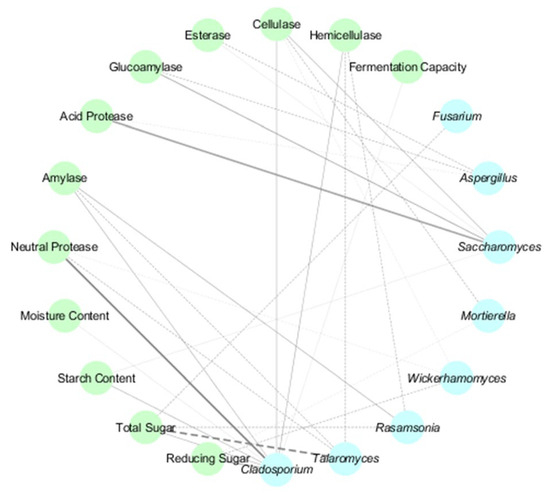
Figure 6.
Fungal–Physicochemical Network in HT- and MT-Daqu. Blue spheres represent fungal genera, while green spheres denote physicochemical indicators. Positive and negative associations are denoted by solid and dashed lines, respectively. Line color intensity corresponds to correlation strength (darker hues indicate higher absolute values), while bold lines signify statistically significant relationships (p < 0.05).
4. Discussion
The findings of this study reveal critical temperature-dependent differences in Daqu’s physicochemical properties, offering both alignment and divergence from prior research. Consistent with Xing et al. [20], intra-group variations in moisture and acidity were observed, but our data uniquely demonstrated that high-temperature Daqu (HQ) exhibited significantly lower moisture (8.88–10.75%) and higher acidity (1.70–2.15 mmol/10 g) compared to medium-temperature Daqu (MQ: 11.68–12.51% moisture, 1.10–1.22 mmol/10 g acidity). This contrasts with Chen et al. [21], who reported higher acidity in medium-temperature Daqu dominated by Weissella and Lactobacillus. The elevated acidity in HQ may instead arise from thermotolerant acid-producing microbes (e.g., Thermoascus or Bacillus [22]), which thrive under high-temperature conditions and accelerate organic acid synthesis. Similarly, HQ’s lower reducing sugar (0.75–0.94%) and starch (54.04–57.93%) compared to MQ (1.68–1.86% reducing sugar; 59.50–61.20% starch) align with Liu et al. [23], who linked starch depletion to microbial activity. High temperatures likely enhance amylolytic enzyme activity (e.g., from Rhizomucor or Aspergillus [21]), accelerating starch hydrolysis. However, the rapid microbial consumption of reducing sugars in HQ for energy or secondary metabolites—less pronounced in MQ due to moderate enzymatic kinetics—explains its lower residual sugar levels.
The observed temperature-driven variations in enzymatic activities align with prior findings on microbial community dynamics and functional shifts in Daqu. Similar to Chen et al. [21], MT-Daqu exhibited higher saccharification and esterification capacities than HT-Daqu. While prior studies attribute such functional advantages to thermotolerant fungi (e.g., Aspergillus) and bacteria (e.g., Lactobacillus) under moderate temperatures [3,11], our data reveal a distinct pattern: although Aspergillus dominated fungal communities in the initial MT-Daqu batch (MQ1: 78%), its abundance sharply declined in subsequent batches (MQ2–3: <35%). Furthermore, Lactobacillus was undetected across all samples, whereas thermotolerant taxa such as Bacillus (22–36%) and Virgibacillus (18–24%) were enriched, suggesting temperature-driven selection of these genera likely underpins MT-Daqu’s metabolic superiority through enhanced carbohydrate metabolism and ester precursor synthesis.
The elevated neutral protease activity in MT-Daqu contrasts with HT-Daqu’s reduced acidic protease activity, possibly reflecting microbial adaptations to temperature-regulated pH and substrate availability [3]. Notably, the 2.3-fold higher cellulase activity in MT-Daqu parallels findings by Huang et al. [11], who linked enhanced lignocellulose degradation to Bacillus-dominated communities under moderate thermal stress. However, the comparable liquefaction activity across all groups diverges from Feng et al. [3], where HT-Daqu showed reduced amylase activity, suggesting strain-specific thermostability or compensatory metabolic pathways in this study. The superior esterase activity in MT-Daqu may stem from thermotolerant taxa such as Virgibacillus and Bacillus, which thrive under moderate thermal conditions (45–55 °C) and exhibit enzymatic synergy with fungi (e.g., Aspergillus) to drive ester precursor synthesis [24,25]. These results underscore temperature as a pivotal regulator of microbial consortia and their enzymatic repertoires, with MT-Daqu’s balanced microbial network enabling multifunctional enzymatic advantages.
The study revealed temperature-driven divergence in microbial α-diversity between HT and MT-Daqu. HT-Daqu exhibited higher bacterial ASV counts (HQ1: 961 vs. MQ3: 77) and Shannon indices (7.83 vs. 1.76), aligning with the dominance of thermotolerant Bacillus and Thermoactinomyces [11]. However, MT-Daqu unexpectedly surpassed HT-Daqu in bacterial Chao1 richness (MQ1: 642.03 vs. HQ1: 396.36), contrasting with Fu et al. [26], likely due to transient dominance of mesophilic taxa (Lactobacillus, Weissella) during early fermentation [23]. Fungal diversity diverged sharply: MT-Daqu showed superior richness (ASVs: 2037 vs. 331–368) and Shannon indices (8.44 vs. 1.53–2.01), consistent with moderate-temperature support for Aspergillus and Thermoascus activity [27]. In contrast, HT-Daqu’s lower fungal Simpson indices (0.37–0.63 vs. 0.64–0.99) deviated from Pang et al. [22], potentially reflecting regional humidity variations favoring extremophiles (Thermoascus) and over generalists (Candida) [6,28]. Thermal gradients in HT-Daqu likely drove bacterial niche partitioning between thermophiles and mesophiles [29], while MT-Daqu’s fungal versatility stemmed from Aspergillus-mediated polysaccharide degradation [3]. The elevated Chao1 in MT-Daqu may arise from rare taxa (Pediococcus) proliferating during cooling phases under reduced competition. These findings underscore temperature as a pivotal regulator of microbial assembly, highlighting the need for strain-level functional analyses to resolve thermal adaptation trade-offs [30], thereby guiding fermentation optimization for balanced diversity and functional stability.
The study revealed distinct microbial stratification between HT and MT-Daqu. Temperature-driven microbial consortia stratification in MT-Daqu involves Virgibacillus and Saccharopolyspora alongside Aspergillus, forming a multi-taxa functional network rather than single-genus dominance. This contrasts with studies reporting Weissella and Lactobacillus in other regional Daqu systems [31], suggesting temperature-driven microbial selection varies significantly across production lineages. However, the prominence of Kroppenstedtia in HT-Daqu contrasts with Deng et al. [32], potentially reflecting regional fermentation practices favoring its heat-resistant enzymes [33]. Fungal communities exhibited temperature-driven divergence: Paecilomyces (52%) and Talaromyces (28%) dominated HT-Daqu, aligning with thermotolerant taxa in Fu et al. [26] but differing from studies emphasizing Thermoascus [32,33], possibly due to Paecilomyces’ superior amylolytic thermostability [25]. Unexpectedly low Thermoascus abundance in HT-Daqu diverged from Deng et al. [33], possibly reflecting transient temperature drops favoring Paecilomyces over sustained bio-heat (>50 °C) that enriches Thermoascus [6]. Thermophilic adaptations explain these patterns: Bacillus and Kroppenstedtia produce heat-stable proteases/amylases [11], while Paecilomyces outcompetes via sporulation efficiency [25]. Our findings on Bacillus and Virgibacillus dominance in MT-Daqu align with Zheng et al.’s categorization of Daqu types based on thermal regimes [34]. While Zheng et al. emphasized the prevalence of Aspergillus and Rhizopus in HT-Daqu [34], our data reveal a contrasting pattern in MT-Daqu, where Bacillus (22–36%) and Virgibacillus (18–24%) dominate after initial fungal decline. This divergence likely reflects temperature-driven microbial selection: MT-Daqu’s moderate thermal conditions (45–55 °C) favor thermotolerant Bacillus strains over mesophilic fungi like Aspergillus, corroborating recent studies on temperature-dependent microbial succession.
Our investigation of medium-high temperature Daqu microbiota-physicochemistry interplay demonstrates partial alignment with previous findings while revealing novel distinctions. Consistent with thermophilic biomarker identification [35], genus-specific enzymatic associations were observed, notably Sphingomonas showing strong positive correlations with acid protease (r = 0.72, p < 0.01) and glucoamylase (r = 0.68, p < 0.01), paralleling Saccharomycopsis’ saccharification functions [36]. However, our data diverge from aroma-type Daqu studies [36,37], identifying Sphingomonas as a multifunctional taxon governing both starch hydrolysis and esterification pathways, potentially attributable to regional microbial biogeography [38]. Bacillus demonstrated significant acid tolerance (r = 0.54, p < 0.05), contrasting with Lactobacillus-dominated pH modulation in premium Daqu [37], suggesting metabolic niche specialization under thermal stress. Fungal dynamics revealed unexpected functional shifts: Aspergillus exhibited negative enzymatic correlations despite its established starch-degrading role [35], possibly due to thermophilic bacterial competition (e.g., Thermoascus), while Cladosporium maintained protease synergy as reported in barley-based systems [39]. Methodologically, we expanded microbial networks to encompass previously overlooked genera (e.g., Desmospora), contrasting with regional bacterial disparity studies [38]. The absence of fungal-esterification linkages contrasts with succession models [37], highlighting temperature–regime specificity. These findings advance Daqu microbial ecology by: (1) resolving functional redundancy/antagonism among ubiquitous taxa (e.g., Kroppenstedtia’s dual acid–enzyme correlations vs. Thermoactinomyces [40]); (2) identifying context-dependent enzymatic drivers (Sphingomonas vs. Pantoea/Furfurilactobacillus [36]; and (3) establishing temperature-mediated microbial trade-offs.
This study has certain limitations that warrant future investigation. While microbial diversity and enzymatic activities were characterized, the direct metabolic roles of key taxa (e.g., Bacillus, Aspergillus) in flavor formation remain unvalidated due to the absence of functional assays or multi-omics approaches. Additionally, enzymatic profiles were not correlated with volatile flavor compounds, leaving mechanistic connections between microbial activity and liquor aroma unresolved. Future research should prioritize integrating metagenomics, metatranscriptomics, and metabolomics to resolve functional contributions of microbial taxa to enzymatic and flavor pathways. Linking enzyme activities to volatile organic compounds via GC-MS would clarify direct flavor contribution mechanisms. Furthermore, employing advanced sequencing techniques (e.g., PacBio) or strain isolation could characterize thermophilic Bacillus subspecies and other biotechnologically relevant strains, enabling targeted microbial management to optimize Daqu functionality and liquor quality.
5. Conclusions
This study elucidates the pivotal role of fermentation temperature in governing microbial consortia assembly and functional divergence in Daqu, a key microbial starter for Chinese Baijiu. Comparative analysis of HT and MT-Daqu revealed temperature-driven ecological trade-offs: HT-Daqu exhibited bacterial dominance (Bacillus spp.) with enhanced thermotolerant adaptations, while MT-Daqu fostered fungal-rich communities (Aspergillus, Saccharomyces) demonstrating superior enzymatic capacities in carbohydrate metabolism and flavor precursor synthesis. These microbial divergences were mechanistically linked to temperature-modulated physicochemical parameters, including moisture, acidity, and starch utilization efficiency.
The findings from this study hold significant practical implications for the agri-food sector, particularly in optimizing traditional fermentation practices and enhancing product quality. The enzymatic superiority of MT-Daqu in fungal-driven ester synthesis (e.g., ethyl acetate, ethyl hexanoate) positions it as a strategic tool for Baijiu producers aiming to amplify fruity and floral flavor profiles, while HT-Daqu’s Bacillus-mediated proteolysis can be leveraged to elevate savory pyrazines (e.g., tetramethylpyrazine), aligning with market demands for Maotai-flavor Baijiu. By modulating fermentation temperatures (60 °C vs. 65 °C), producers can selectively enrich microbial consortia—prioritizing MT-Daqu for carbohydrate-active enzymes (amylases, hemicellulases) or HT-Daqu for thermostable proteases—thereby tailoring Daqu production to regional flavor preferences. Collectively, these insights bridge microbial ecology with biotechnology, providing a framework to harness microbial diversity for reproducible, high-quality fermentations while preserving the artisanal heritage of Baijiu production.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13061312/s1, Figure S1: Experimental design of the study. Table S1: Changes in physicochemical indexes (starch, reducing sugar, acidity, moisture) in different medium-high temperature Daqu. Table S2: Changes in enzyme activity (glucoamylase, α-amylase, fermentation capacity, esterification capacity, neutral protease activity, acid protease, cellulase, hemicellulase) in different medium-high temperature Daqu. Table S3: Alpha diversity of bacterial communities in medium-high temperature Daqu. Table S4: Alpha diversity of fungal communities in medium-high temperature Daqu.
Author Contributions
Conceptualization, H.Y. and K.L.; methodology, L.D.; software, J.Z.; validation, H.W. and Q.J.; formal analysis, T.X.; investigation, Q.J. and C.Z.; resources, J.Z.; data curation, C.Z.; writing—original draft preparation, H.Y.; writing—review and editing, K.L.; visualization, G.N.; supervision, K.L.; project administration, L.L.; funding acquisition, H.W. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the open foundation of key laboratory of Wuliangye-flavor liquor solid-state fermentation, China national light industry (2024JJ011), The solid-state fermentation resource utilization key laboratory of Sichuan province (2024GTJJ02), Sichuan higher education engineering research center for agri-food standardization and inspection (24NSYJZX07), Sichuan province engineering technology research center of oil cinnamon (24YZY05). The APC was funded by 2024GTJJ02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request for non-commercial research purposes.
Acknowledgments
The authors acknowledge the use of generative AI tools for language editing and refinement during the preparation of this manuscript. The scientific content and conclusions remain the sole responsibility of the authors.
Conflicts of Interest
The involvement of Yibin Wuliangye Co., Ltd. was strictly limited to providing financial support in the form of salary payments for the researchers Jia Zheng and Hong Wang, along with research funding allocation. It should be explicitly stated that the company exercised no influence whatso-ever on the research methodology, data interpretation, or final outcomes of this academic investi-gation. There are no patents, products in development, or marketed products associated with this research to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare no conflicts of interest.
References
- Wang, M.Y.; Yang, J.G.; Zhao, Q.S.; Zhang, K.Z.; Su, C. Research Progress on Flavor Compounds and Microorganisms of Maotai Flavor Baijiu. J. Food Sci. 2019, 84, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zheng, J.; Xie, J.; Zhou, R.; Jin, Y.; Zhao, D.; Huang, J.; Wu, C. Effects of Environmental Factors on the Microbial Community Changes during Medium-High Temperature Daqu Manufacturing. Food Res. Int. 2022, 153, 110955. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.T.; Lu, Z.M.; Shi, W.; Xiao, C.; Zhang, X.J.; Chai, L.J.; Wang, S.T.; Shen, C.H.; Shi, J.S.; Xu, Z.H. Effects of Different Culture Temperatures on Microbial Community Structure, Enzyme Activity, and Volatile Compounds in Daqu. Chin. J. Appl. Environ. Biol. 2021, 27, 760–767. [Google Scholar] [CrossRef]
- Huang, P.; Wang, Z.; Zheng, J.; Jin, Y.; Huang, J.; Zhao, D.; Zhou, R.; Wu, C. Research Progress in Microbial Community Structure in Nongxiangxing Daqu. Microbiol. China 2023, 50, 3170–3186. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, H.; Wu, Z.; Zhang, W. Microbial Diversity in Jiuqu and Its Fermentation Features: Saccharification, Alcohol Fermentation and Flavors Generation. Appl. Microbiol. Biotechnol. 2023, 107, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Lu, Z.M.; Zhang, X.J.; Wang, S.T.; Ao, L.; Shen, C.H.; Shi, J.S.; Xu, Z.H. Bio-Heat Is a Key Environmental Driver Shaping the Microbial Community of Medium-Temperature Daqu. Appl. Environ. Microbiol. 2017, 83, e01550-17. [Google Scholar] [CrossRef]
- Bo, T.; Lv, N.; Yang, K.; Zhang, J.; Zhang, J.; Bai, B.; Tian, X.; Fan, S. Research Progress on Microbial Communities and Functions during Daqu Fermentation for Baijiu Production. Food Sci. 2023, 44, 385–393. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Li, H.; Liu, W.; Zhang, Z.; Han, S.; Hou, J.; Pan, C. Combined Microbiome and Metabolomics Analysis of Taorong-Type Baijiu High-Temperature Daqu and Medium-Temperature Daqu. PeerJ 2024, 12, e16621. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Li, H.; Zhang, H.; Shen, X.; Zhang, L.; Han, S.; Pan, C. Taorong-Type Baijiu Starter: Analysis of Fungal Community and Metabolic Characteristics of Middle-Temperature Daqu and High-Temperature Daqu. PLoS ONE 2022, 17, e0274881. [Google Scholar] [CrossRef]
- Wang, Y.; Gai, J.; Hou, Q.; Wang, J.; Li, L.; Yu, Q.; Chang, X.; Dong, X.; Chen, M.; Li, P.; et al. Ultra-High-Depth Macrogenomic Sequencing Revealed Differences in Microbial Composition and Function Between High Temperature and Medium-High Temperature Daqu. World J. Microbiol. Biotechnol. 2023, 39, 337. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Mu, Y.; Zhu, Z.; Wu, Y.; Qi, Q.; Mu, Y.; Su, W. Exploring the Heterogeneity of Community and Function and Correspondence of “Species-Enzymes” among Three Types of Daqu with Different Fermentation Peak-Temperature via High-Throughput Sequencing and Metagenomics. Food Res. Int. 2024, 176, 113805. [Google Scholar] [CrossRef] [PubMed]
- QB/T 4257-2011; General Analysis Methods for Brewing Daqu. Standardization Administration of China: Beijing, China, 2011.
- DB 34/T 3085-2018; Operating Procedures for Testing Strong-Flavor Daqu. Anhui Provincial Bureau of Quality and Technical Supervision: Hefei, China, 2018.
- NY/T 912-2020; Determination of Cellulase Activity in Feed Additives—Spectrophotometric Method. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020.
- GB/T 23874-2009; Determination of Xylanase Activity in Feed Additives—Spectrophotometric Method. Standardization Administration of China: Beijing, China, 2009.
- GB 5009.7-2016; National Food Safety Standard—Determination of Reducing Sugars in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- Dueholm, M.S.; Andersen, K.S.; McIlroy, S.J.; Kristensen, J.M.; Yashiro, E.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. Generation of Comprehensive Ecosystem-Specific Reference Databases with Species-Level Resolution by High-Throughput Full-Length 16S rRNA Gene Sequencing and Automated Taxonomy Assignment (AutoTax). mBio 2020, 11, e01557-20. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural Intensification Reduces Microbial Network Complexity and the Abundance of Keystone Taxa in Roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan v2.6: Community Ecology Package. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 September 2019).
- Xing, G.; Ao, Z.; Wang, S.; Deng, B.; Wang, X.; Dong, Z. Analysis of the Change in Physiochemical Indexes during the Production Process of Daqu of Different Temperature. Liquor-Making Sci. Technol. 2014, 6, 4. [Google Scholar] [CrossRef]
- Chen, K.; Wu, X.; Chen, Y.; Liu, T.; Wan, Y.; Liu, C.; Wu, C.; Fu, G. Effect of Peak Temperature on the Succession of Physicochemical Index and Microbial Community of Special-Flavor Daqu. Food Ferment. Ind. 2020, 46, 52–58. [Google Scholar] [CrossRef]
- Pang, Z.; Li, W.; Hao, J.; Xu, Y.; Du, B.; Zhang, C.; Wang, K.; Zhu, H.; Wang, H.; Zhang, G.; et al. Correlational Analysis of the Physicochemical Indexes, Volatile Flavor Components, and Microbial Communities of High-Temperature Daqu in the Northern Region of China. Foods 2023, 12, 326. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Y.; Wang, X.; Zhao, J.; Yuan, Y.; Zhuo, L.; Liang, J.; Zhou, W. Study on Physicochemical Indexes and Microbial Succession Law during Fermentation of Medium/High-Temperature Daqu. Liquor-Making Sci. Technol. 2024, 8, 25–29. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Li, X.D.; Xie, S.K.; Yu, P.B.; Chen, J.X. Interactions between Lactic Acid Bacteria and Yeasts in Light-Aroma Baijiu. Microbiol. Bull. 2017, 44, 1767–1776. [Google Scholar] [CrossRef]
- Nie, X.; Jia, X.; Zhu, K.; Ling, Z.; Chen, H.; Xie, J.; Ao, Z.; Song, C.; Shen, C.; Chai, L.; et al. Dynamic Changes and Potential Correlations between Microbial Diversity and Volatile Flavor Compounds in Chinese Medium-Temperature Daqu during Manufacturing. Molecules 2024, 29, 4851. [Google Scholar] [CrossRef]
- Fu, G.; Deng, M.; Chen, K.; Chen, Y.; Cai, W.; Wu, C.; Liu, C.; Wu, S.; Wan, Y. Peak-Temperature Effects of Starter Culture (Daqu) on Microbial Community Succession and Volatile Substances in Solid-State Fermentation (Jiupei) during Traditional Chinese Special-Flavour Baijiu Production. LWT 2021, 152, 112132. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, Y.; Qu, D.; Zhao, H.; Tian, L.; Zhou, J.; Liu, J.; Guo, Z. Microbial Communities, Functional, and Flavor Differences among Three Different-Colored High-Temperature Daqu: A Comprehensive Metagenomic, Physicochemical, and Electronic Sensory Analysis. Food Res. Int. 2024, 184, 114257. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Mao, X.; Liu, D.; Ning, X.Q.; Shen, Y.; Chen, B.; Luo, H.B. Comparative Analysis of Physicochemical Properties and Microbial Composition in High-Temperature Daqu with Different Colors. Front. Microbiol. 2020, 11, 588117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wu, X.; Xu, Y.; Zhang, Y.; Wang, Z.; Shen, L.; Yang, W.; Sun, J.; Liu, Y. Microbial Composition and Dynamic Succession during the Daqu Production Process of Northern Jiang-Flavored Liquor in China. aBiotech 2021, 11, 224. [Google Scholar] [CrossRef]
- Han, P.J.; Song, L.; Wen, Z.; Zhu, H.Y.; Wei, Y.H.; Wang, J.W.; Bai, M.; Luo, L.J.; Wang, J.W.; Chen, S.X.; et al. Species-Level Understanding of the Bacterial Community in Daqu Based on Full-Length 16S rRNA Gene Sequences. Food Microbiol. 2024, 123, 104566. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.H.; Zheng, Y.L.; Zhao, T.; Mao, H.; Fang, S.L.; Chen, M.B.; Liu, S.L. Changes in the Microbial Community Structure during the Digitally Managed Fermentation of Medium-Temperature Daqu. Food Sci. Technol. 2022, 42, e87122. [Google Scholar] [CrossRef]
- Deng, C.; Gao, R.J.; Zhao, Y.W.; Miao, L.H.; Wang, M.K.; Liu, P.L.; Fan, P.W. Relationship between Sensory Indexes, Physicochemical Indexes, Microbial Community, and Volatile Compounds in High-Temperature Daqu. Food Ferment. Ind. 2022, 48, 78–85. [Google Scholar] [CrossRef]
- Deng, J.; Zheng, J.; Huang, D.; Huang, Z.; Ye, G.; Luo, H. Characterization of Physicochemical Properties, Volatile Compounds and Microbial Community Structure in Four Types of Daqu. LWT 2023, 184, 115064. [Google Scholar] [CrossRef]
- Zheng, X.-W.; Tabrizi, M.R.; Nout, M.J.R.; Han, B.-Z. Daqu—A Traditional Chinese Liquor Fermentation Starter. J. Inst. Brew. 2011, 117, 82–90. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, M.S.; Dai, Q.L.; Li, S.; Shen, C.H.; Wang, S.T. Comparative Study on Physicochemical Properties and Fungal Community Composition of Medium and High-Temperature Daqu from the Same Region. Sci. Technol. Food Ind. 2023, 44, 150–159. [Google Scholar] [CrossRef]
- Mao, W.D.; Feng, W.C.; Sun, W.; Chen, H.; Liao, B.; Fang, S.L. Correlation Analysis of Microbial Communities and Physicochemical Indicators in Daqu of Different Flavor Types. China Brew. 2024, 43, 147–153. [Google Scholar]
- Xiang, G.X.; Chen, Y.Q.; Shen, Y.; Wang, X.; Zhang, Y.D.; Luo, H.B. Comparative Analysis of Microbial Community Structure and Physicochemical Properties of Different Grades of Strong-Flavor Daqu. Food Sci. 2022, 43, 184–191. [Google Scholar] [CrossRef]
- Wu, S.K.; Xie, J.; Cheng, T.Y.; Wei, C.H.; Huang, Z.G.; Deng, J. Correlation Between Quality Indicators and Bacterial Communities in Strong-Flavor Daqu from Different Regions. Food Res. Dev. 2019, 40, 7. [Google Scholar] [CrossRef]
- Gu, C.Y.; Ran, M.F.; Wei, Y.; Zhang, L.Q.; Wang, S.T.; Shen, C.H. Correlation Between Physicochemical Characteristics and Microbial Communities in Highland Barley Daqu with Different Ingredients. Food Ferment. Ind. 2021, 47, 126–133. [Google Scholar] [CrossRef]
- Tang, H.F.; Huang, J.; Zhou, R.Q.; Qin, H.; Zhang, S.Y.; Dong, Y. Effects of Spatial Heterogeneity on Microbial Communities and Quality of Medium-High Temperature Daqu. Food Ferment. Ind. 2023, 49, 237–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).