PCR-Based Legionella Risk Evaluation of Drinking Water Systems—An Empirical Field Evaluation
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling Procedure
2.2. Legionellae Detection Using Culture Methods
2.3. Legionellae Detection Using Molecular Methods
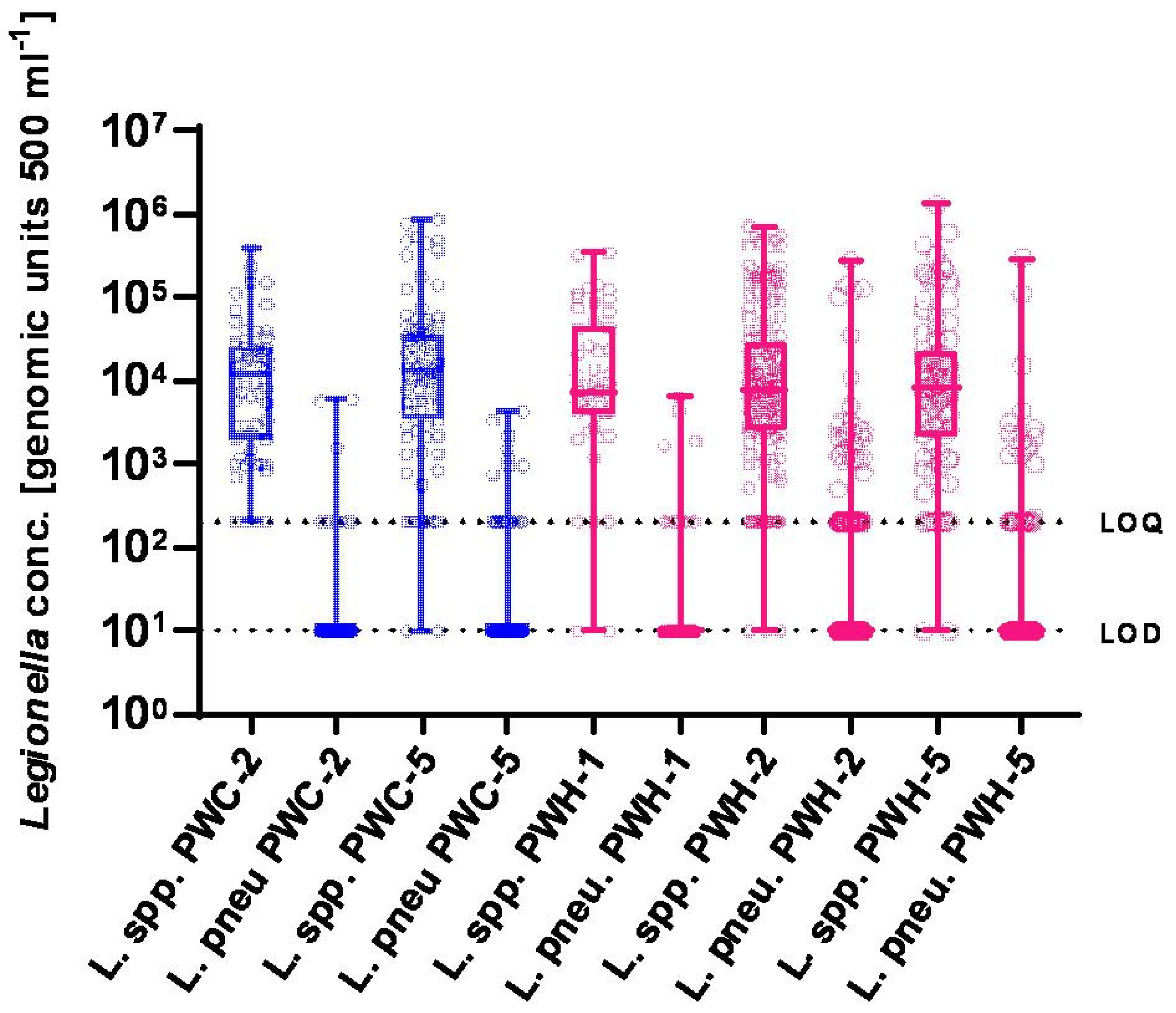
3. Results
3.1. Cultural Detection of Legionellae
3.2. Molecular Detection of Legionellae
3.3. Comparability of Results by Culture vs. DNA Detection
3.4. Comparison of Molecular Legionella Detection Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition, Incorprating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Alleron, L.; Khemiri, A.; Koubar, M.; Lacombe, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Frere, J. VBNC Legionella pneumophila cells are still able to produce virulence proteins. Water Res. 2013, 47, 6606–6617. [Google Scholar] [CrossRef]
- Kirschner, A.K.T. Determination of viable legionellae in engineered water systems: Do we find what we are looking for? Water Res. 2016, 93, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O., III. Common features of opportunistic premise plumbing pathogens. Int. J. Environ. Res. Public Health 2015, 12, 4533–4545. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.; Abdelhady, H.; Garduno, R.A. The many forms of a pleomorphic bacterial pathogen-the developmental network of Legionella pneumophila. Front. Microbiol. 2014, 5, 670. [Google Scholar] [CrossRef] [PubMed]
- van Heijnsbergen, E.; Schalk, J.A.; Euser, S.M.; Brandsema, P.S.; Den Boer, J.W.; de Roda Husman, A.M. Confirmed and potential sources of legionella reviewed. Environ. Sci. Technol. 2015, 49, 4797–4815. [Google Scholar] [CrossRef]
- Phin, N.; Parry-Ford, F.; Harrison, T.; Stagg, H.R.; Zhang, N.; Kumar, K.; Lortholary, O.; Zumla, A.; Abubakar, I. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect. Dis. 2014, 14, 1011–1021. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Legionnaires’ Disease: Annual Epidemiological Report for 2021; ECDC: Stockholm, Sweden, 2023; Volume 2023. [Google Scholar]
- Barskey, A.E.L.S.; Hannpel, E.; Smith, J.; Edens, C. Legionnaires’ Disease Surveillance Summary Report, United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/legionella/health-depts/surv-reporting/2018-19-surv-report-508.pdf (accessed on 18 May 2025).
- Bonita, B.; Udo, B. Epidemiologie der Legionärskrankheit in Deutschland—Entwicklungen in den Jahren 2010 bis 2020.—[Epidmiology of Legionnaires’ Disease in Germany—Trend from 2010 to 2020]. Epid. Bull. 2021, 42, 14. [Google Scholar] [CrossRef]
- Van Kenhove, E.; Dinne, K.; Janssens, A.; Laverge, J. Overview and comparison of Legionella regulations worldwide. Am. J. Infect. Control 2019. [Google Scholar] [CrossRef]
- ISO 11731:2017; Water Quality—Enumeration of Legionella. International Organization for Standardization: Geneva, Switzerland, 2017.
- German Federal Environment Agency. Systemische Untersuchungen von Trinkwasser-Installationen auf Legionellen nach Trinkwasserverordnung—Probennahme, Untersuchungsgang und Angabe des Ergebnisses (Systemic examinations of drinking water installations for Legionella according to the Drinking Water Ordinance—Sampling, examination process and indication of the result). Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 2019, 62, 1032–1037. [Google Scholar] [CrossRef]
- Dilger, T.; Melzl, H.; Gessner, A. Legionella contamination in warm water systems: A species-level survey. Int. J. Hyg. Environ. Health 2017, 221, 199–210. [Google Scholar] [CrossRef]
- Kruse, E.B.; Wehner, A.; Wisplinghoff, H. Prevalence and distribution of Legionella spp in potable water systems in Germany, risk factors associated with contamination, and effectiveness of thermal disinfection. Am. J. Infect. Control 2016, 44, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Völker, S.; Schreiber, C.; Kistemann, T. Drinking water quality in household supply infrastructure—A survey of the current situation in Germany. Int. J. Hyg. Environ. Health 2010, 213, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O. Living with Legionella and Other Waterborne Pathogens. Microorganisms 2020, 8, 2026. [Google Scholar] [CrossRef] [PubMed]
- Valcina, O.; Pule, D.; Malisevs, A.; Trofimova, J.; Makarova, S.; Konvisers, G.; Berzins, A.; Krumina, A. Co-Occurrence of Free-Living Amoeba and Legionella in Drinking Water Supply Systems. Medicina 2019, 55, 492. [Google Scholar] [CrossRef]
- Walker, J.T.; McDermott, P.J. Confirming the Presence of Legionella pneumophila in Your Water System: A Review of Current Legionella Testing Methods. J. AOAC Int. 2021, 104, 1135–1147. [Google Scholar] [CrossRef]
- Shih, H.Y.; Lin, Y.E. Caution on interpretation of Legionella results obtained using real-time PCR for environmental water samples. Appl. Environ. Microbiol. 2006, 72, 6859. [Google Scholar] [CrossRef]
- Duda, S.; Baron, J.L.; Wagener, M.M.; Vidic, R.D.; Stout, J.E. Lack of correlation between Legionella colonization and microbial population quantification using heterotrophic plate count and adenosine triphosphate bioluminescence measurement. Environ. Monit. Assess. 2015, 187, 393. [Google Scholar] [CrossRef]
- Whiley, H.; Taylor, M. Legionella detection by culture and qPCR: Comparing apples and oranges. Crit. Rev. Microbiol. 2014, 42, 65–74. [Google Scholar] [CrossRef]
- Zacharias, N.; Wasser, F.; Freier, L.; Spies, K.; Koch, C.; Pleischl, S.; Mutters, N.T.; Kistemann, T. Legionella in drinking water: The detection method matters. J. Water Health 2023, 21, 884–894. [Google Scholar] [CrossRef]
- Collins, S.; Stevenson, D.; Walker, J.; Bennett, A. Evaluation of Legionella real-time PCR against traditional culture for routine and public health testing of water samples. J. Appl. Microbiol. 2017, 122, 1692–1703. [Google Scholar] [CrossRef]
- Toplitsch, D.; Platzer, S.; Zehner, R.; Maitz, S.; Mascher, F.; Kittinger, C. Comparison of Updated Methods for Legionella Detection in Environmental Water Samples. Int. J. Environ. Res. Public Health 2021, 18, 5436. [Google Scholar] [CrossRef] [PubMed]
- Almonacid Garrido, M.C.; Villanueva-Suarez, M.J.; Montes Martin, M.J.; Garcia-Alonso, A.; Tenorio Sanz, M.D. Prevalence and distribution of Legionella in municipal drinking water supply systems in Madrid (Spain) and risk factors associated. Sci. Total Environ. 2024, 954, 176655. [Google Scholar] [CrossRef] [PubMed]
- Lizana, X.; Lopez, A.; Benito, S.; Agusti, G.; Rios, M.; Pique, N.; Marques, A.M.; Codony, F. Viability qPCR, a new tool for Legionella risk management. Int. J. Hyg. Environ. Health 2017, 220, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- ISO 19458-2006; Water Quality—Sampling for Microbiological Analysis. International Organization for Standardization: Geneva, Switzerland, 2006.
- Helbig, J.H.; Bernander, S.; Castellani Pastoris, M.; Etienne, J.; Gaia, V.; Lauwers, S.; Lindsay, D.; Luck, P.C.; Marques, T.; Mentula, S.; et al. Pan-European study on culture-proven Legionnaires’ disease: Distribution of Legionella pneumophila serogroups and monoclonal subgroups. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 710–716. [Google Scholar] [CrossRef]
- ISO/TS 12869:2019; Water Quality—Detection and Quantification of Legionella spp. and/or Legionella Pneumophila by Concentration and Genic Amplification by Quantitative Polymerase Chain Reaction (qPCR). International Organization for Standardization: Geneva, Switzerland, 2019.
- Ji, P.; Rhoads, W.J.; Edwards, M.A.; Pruden, A. Impact of water heater temperature setting and water use frequency on the building plumbing microbiome. ISME J. 2017, 11, 1318–1330. [Google Scholar] [CrossRef]
- Miyamoto, H.; Jitsurong, S.; Shiota, R.; Maruta, K.; Yoshida, S.; Yabuuchi, E. Molecular determination of infection source of a sporadic Legionella pneumonia case associated with a hot spring bath. Microbiol. Immunol. 1997, 41, 197–202. [Google Scholar] [CrossRef]
- Ratcliff, R.M.; Lanser, J.A.; Manning, P.A.; Heuzenroeder, M.W. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J. Clin. Microbiol. 1998, 36, 1560–1567. [Google Scholar] [CrossRef]
- Benitez, A.J.; Winchell, J.M. Clinical application of a multiplex real-time PCR assay for simultaneous detection of Legionella species, Legionella pneumophila, and Legionella pneumophila serogroup 1. J. Clin. Microbiol. 2013, 51, 348–351. [Google Scholar] [CrossRef]
- Lesnik, R.; Brettar, I.; Höfle, M.G. Legionella species diversity and dynamics from surface reservoir to tap water: From cold adaptation to thermophily. ISME J. 2016, 10, 1064–1080. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Diaz-Flores, A.; Montero, J.C.; Castro, F.J.; Alejandres, E.M.; Bayon, C.; Solis, I.; Fernandez-Lafuente, R.; Rodriguez, G. Comparing methods of determining Legionella spp. in complex water matrices. BMC Microbiol. 2015, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- van der Lugt, W.; Euser, S.M.; Bruin, J.P.; den Boer, J.W.; Yzerman, E.P.F. Wide-scale study of 206 buildings in the Netherlands from 2011 to 2015 to determine the effect of drinking water management plans on the presence of Legionella spp. Water Res. 2019, 161, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.V.; Lai, S.; Exner, M.; Lenz, J.; Gaia, V.; Casati, S.; Hartemann, P.; Lück, C.; Pangon, B.; Ricci, M.L.; et al. An international trial of quantitative PCR for monitoring Legionella in artificial water systems. J. Appl. Microbiol. 2011, 110, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Stevenson, D.; Bennett, A.; Walker, J. Occurrence of Legionella in UK household showers. Int. J. Hyg. Environ. Health 2017, 220 Pt B, 401–406. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Struewing, I.; Mistry, J.H.; Wahman, D.G.; Pressman, J.; Lu, J.R. Legionella and other opportunistic pathogens in full-scale chloraminated municipal drinking water distribution systems. Water Res. 2021, 205, 117571. [Google Scholar] [CrossRef]
- Heining, L.; Welp, L.; Hugo, A.; Elsner, M.; Seidel, M. Immunomagnetic separation coupled with flow cytometry for the analysis of Legionella pneumophila in aerosols. Anal. Bioanal. Chem. 2023, 415, 5139–5149. [Google Scholar] [CrossRef]
- Redwitz, J.; Streich, P.; Zamfir, M.; Walser-Reichenbach, S.M.; Seidel, M.; Herr, C.E.W.; Heinze, S.; Quartucci, C. Verification and application of qPCR and viability-qPCR for Legionella monitoring in evaporative cooling systems complementing the conventional culture method. Sci. Total Environ. 2024, 953, 176011. [Google Scholar] [CrossRef]
- Spica, V.R.; Borella, P.; Bruno, A.; Carboni, C.; Exner, M.; Hartemann, P.; Gianfranceschi, G.; Lagana, P.; Mansi, A.; Montagna, M.T.; et al. Legionnaires’ Disease Surveillance and Public Health Policies in Italy: A Mathematical Model for Assessing Prevention Strategies. Water 2024, 16, 2167. [Google Scholar] [CrossRef]
- Joly, P.; Falconnet, P.A.; Andr, J.; Weill, N.; Reyrolle, M.; Vandenesch, F.; Maurin, M.; Etienne, J.; Jarraud, S. Quantitative real-time Legionella PCR for environmental water samples: Data interpretation. Appl. Environ. Microbiol. 2006, 72, 2801–2808. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Frost, C.; Marre, R. Detection of legionellae in hospital water samples by quantitative real-time LightCycler PCR. Appl. Environ. Microbiol. 2001, 67, 3985–3993. [Google Scholar] [CrossRef]
- Lück, C.; Igel, L.; Helbig, J.H.; Kuhlisch, E.; Jatzwauk, L. Comparison of commercially available media for the recovery of Legionella species. Int. J. Hyg. Environ. Health 2004, 207, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Ta, A.C.; Stout, J.E.; Yu, V.L.; Wagener, M.M. Comparison of culture methods for monitoring Legionella species in hospital potable water systems and recommendations for standardization of such methods. J. Clin. Microbiol. 1995, 33, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, E.; Rhoads, W.J.; Julian, T.R.; Hammes, F. Quantification of Legionella pneumophila in building potable water systems: A meta-analysis comparing qPCR and culture-based detection methods. PLoS Water 2025, 4, e0000291. [Google Scholar] [CrossRef]
- Lautenschlager, K.; Boon, N.; Wang, Y.; Egli, T.; Hammes, F. Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Water Res. 2010, 44, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Völker, S.; Schreiber, C.; Kistemann, T. Modelling characteristics to predict Legionella contamination risk—Surveillance of drinking water plumbing systems and identification of risk areas. Int. J. Hyg. Environ. Health 2016, 219, 101–109. [Google Scholar] [CrossRef]
- Bédard, E.; Fey, S.; Charron, D.; Lalancette, C.; Cantin, P.; Dolcé, P. Temperature diagnostic to identify high risk areas and optimize Legionella pneumophila surveillance in hot water distribution systems. Water Res. 2015, 71, 244–256. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Legionnaires’ Disease: Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2021; Volume 2021. [Google Scholar]
- Dietersdorfer, E.; Kirschner, A.; Schrammel, B.; Ohradanova-Repic, A.; Stockinger, H.; Sommer, R.; Walochnik, J.; Cervero-Arago, S. Starved viable but non-culturable (VBNC) Legionella strains can infect and replicate in amoebae and human macrophages. Water Res. 2018, 141, 428–438. [Google Scholar] [CrossRef]
- Ditommaso, S.; Ricciardi, E.; Giacomuzzi, M.; Arauco Rivera, S.R.; Zotti, C.M. Legionella in water samples: How can you interpret the results obtained by quantitative PCR? Mol. Cell. Probes 2015, 29, 7–12. [Google Scholar] [CrossRef]
- Scaturro, M.; Fontana, S.; Dell’eva, I.; Helfer, F.; Marchio, M.; Stefanetti, M.V.; Cavallaro, M.; Miglietta, M.; Montagna, M.T.; De Giglio, O.; et al. A multicenter study of viable PCR using propidium monoazide to detect Legionella in water samples. Diagn. Microbiol. Infect. Dis. 2016, 85, 283–288. [Google Scholar] [CrossRef]
- Codony, F.; Dinh-Thanh, M.; Agusti, G. Key Factors for Removing Bias in Viability PCR-Based Methods: A Review. Curr. Microbiol. 2020, 77, 682–687. [Google Scholar] [CrossRef]
- Völker, S.; Kistemann, T. Field testing hot water temperature reduction as an energy-saving measure—Does the Legionella presence change in a clinic’s plumbing system? Environ. Technol. 2015, 36, 2138–2147. [Google Scholar] [CrossRef] [PubMed]

| Sampling Point | Analyzed Samples—DP (% L. spp. Positive) | Analyzed Samples—Filter (% L. spp. Positive) |
|---|---|---|
| PWC (before heater) | 96 (3) | 96 (2) |
| PWH 2nd L (after heater) | 85 (1) | 85 (4) |
| PWH 2nd L (recirculation) | 79 (1) | 79 (4) |
| PWH 1st L (periphery) | 76 (6) | 76 (9) |
| PWH 2nd L (periphery) | 229 (12) | 229 (26) |
| PWH 5th L (periphery) | 171 (6) | 171 (16) |
| PWC 5th L (periphery) | 172 (5) | 173 (17) |
| Total | 908 (34) | 909 (77) |
| Sampling Points | qPCR [<LoD] | qPCR [<LoQ] | qPCR [>LoQ] | ||||
|---|---|---|---|---|---|---|---|
| L. spp. [%] | L. pneumophila [%] | L. spp. [%] | L. pneumophila [%] | L. spp. [%] | L. pneumophila [%] | Total L. spp./L. p. | |
| PWC (before heater) | 0 [0.00] | 85 [88.54] | 4 [6.56] | 7 [7.29] | 51 [92.72] | 3 [3.16] | 55/95 |
| PWH 2nd L (after heater) | 0 [0.00] | 70 [82.35] | 6 [12.77] | 7 [8.24] | 41 [87.23] | 8 [9.41] | 47/85 |
| PWH 2nd L (recirculation) | 0 [0.00] | 64 [81.01] | 7 [15.91] | 9 [11.39] | 37 [84.09] | 6 [7.59] | 44/79 |
| PWH 1st L (periphery) | 2 [3.92] | 48 [81.35] | 3 [5.88] | 7 [11.86] | 46 [90.19] | 4 [6.78] | 51/59 |
| PWH 2nd L (periphery) | 3 [2.97] | 138[77.09] | 11 [10.89] | 24 [13.41] | 87 [86.14] | 17 [9.49] | 101/179 |
| PWH 5th L (periphery) | 2 [2.08] | 134 [79.29] | 9 [9.38] | 18 [10.65] | 85 [88.51] | 17 [9.49] | 96/169 |
| PWC 5th L (periphery) | 2 [2.00] | 148 [86.05] | 9 [9.09] | 16 [9.30] | 88 [88.89] | 8 [4.65] | 99/172 |
| Total | 9 [1.82] | 688 [82.10] | 49 [9.91] | 88 [10.50] | 436 [88.26] | 63 [7.52] | 494/838 |
| qPCR | Culture Positive [%] | Culture Negative [%] | Total | |||
| Legionella pneumophila | Positive | a | 32 [3.81] | c | 119 [14.18] | 150 |
| Negative * | b | 14 [1.67] | d | 681 [81.17] | 688 | |
| Legionella spp. | Positive | a | 56 [11.42] | c | 434 [88.57] | 490 |
| Negative * | b | 0 [0] | d | 4 [0.82] | 4 | |
| PPV (95% CI) | NPV (95% CI) | |
|---|---|---|
| Legionella pneumophila | 21.19 (17.27–25.72) | 97.99 (96.91–98.69) |
| Legionella spp. | 11.43 (11.29–11.57) | 100 (93.62–100.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petzold, M.; Zacharias, N.; Uhle, S.; Kieper, L.; Mutters, N.T.; Kistemann, T.; Schreiber, C. PCR-Based Legionella Risk Evaluation of Drinking Water Systems—An Empirical Field Evaluation. Microorganisms 2025, 13, 1311. https://doi.org/10.3390/microorganisms13061311
Petzold M, Zacharias N, Uhle S, Kieper L, Mutters NT, Kistemann T, Schreiber C. PCR-Based Legionella Risk Evaluation of Drinking Water Systems—An Empirical Field Evaluation. Microorganisms. 2025; 13(6):1311. https://doi.org/10.3390/microorganisms13061311
Chicago/Turabian StylePetzold, Markus, Nicole Zacharias, Sarah Uhle, Laurine Kieper, Nico Tom Mutters, Thomas Kistemann, and Christiane Schreiber. 2025. "PCR-Based Legionella Risk Evaluation of Drinking Water Systems—An Empirical Field Evaluation" Microorganisms 13, no. 6: 1311. https://doi.org/10.3390/microorganisms13061311
APA StylePetzold, M., Zacharias, N., Uhle, S., Kieper, L., Mutters, N. T., Kistemann, T., & Schreiber, C. (2025). PCR-Based Legionella Risk Evaluation of Drinking Water Systems—An Empirical Field Evaluation. Microorganisms, 13(6), 1311. https://doi.org/10.3390/microorganisms13061311






