Evolving Landscape of Paediatric Pneumococcal Meningitis in Argentina (2013–2023)
Abstract
1. Introduction
2. Materials and Methods
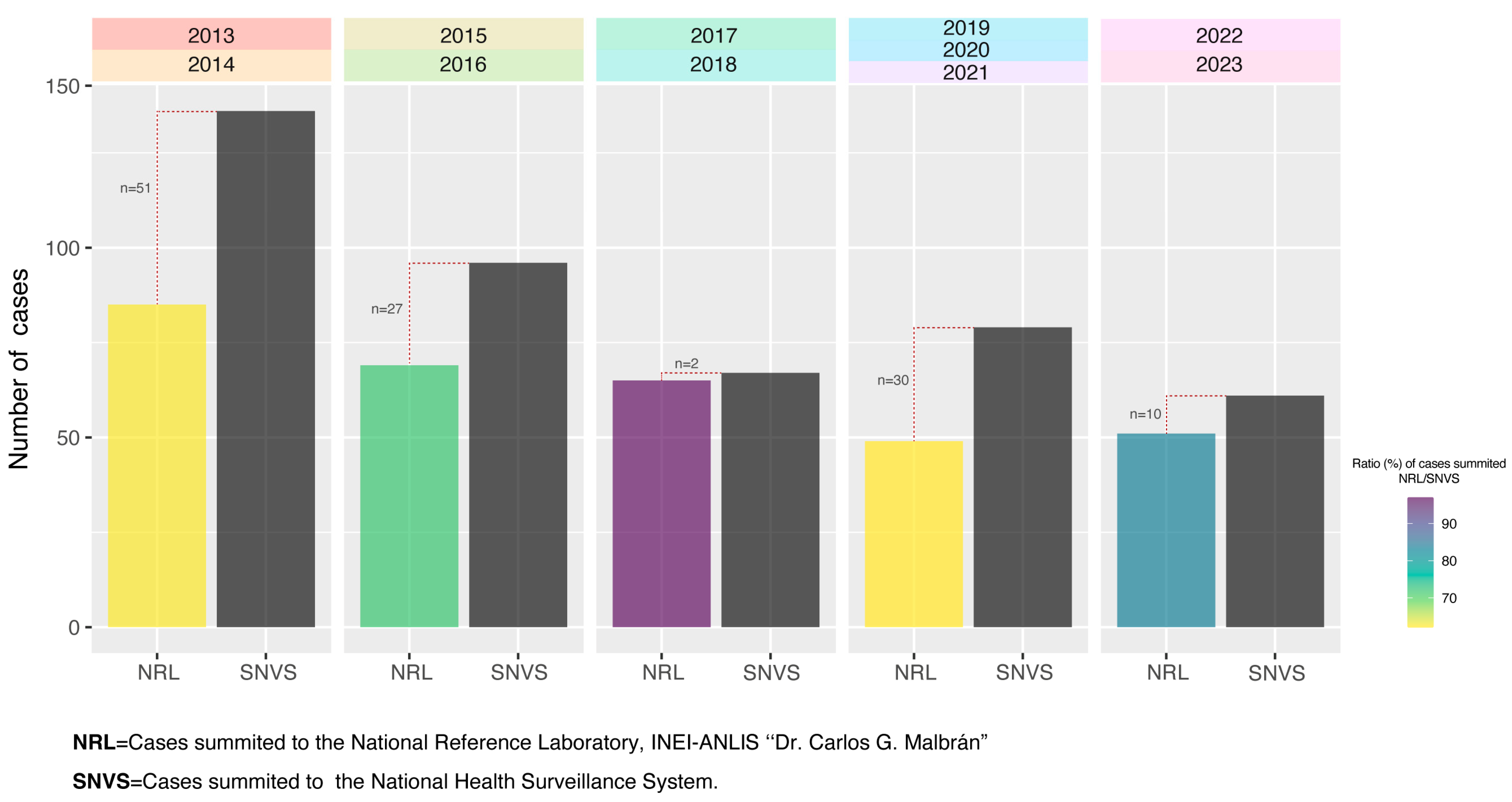
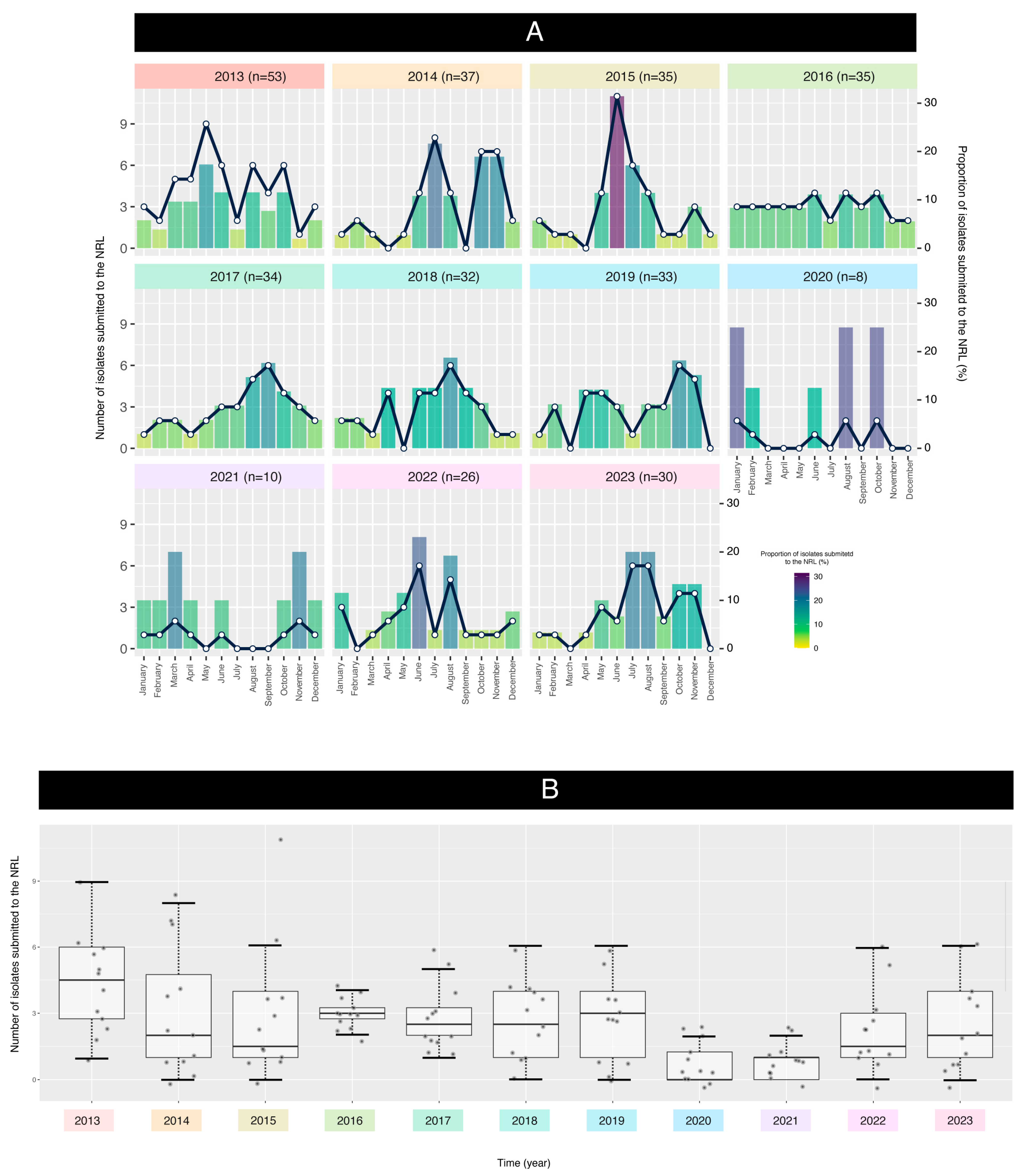
2.1. Collection of Bacterial Isolates
2.2. Isolate Analysis
2.2.1. Serotype Detection Methodology
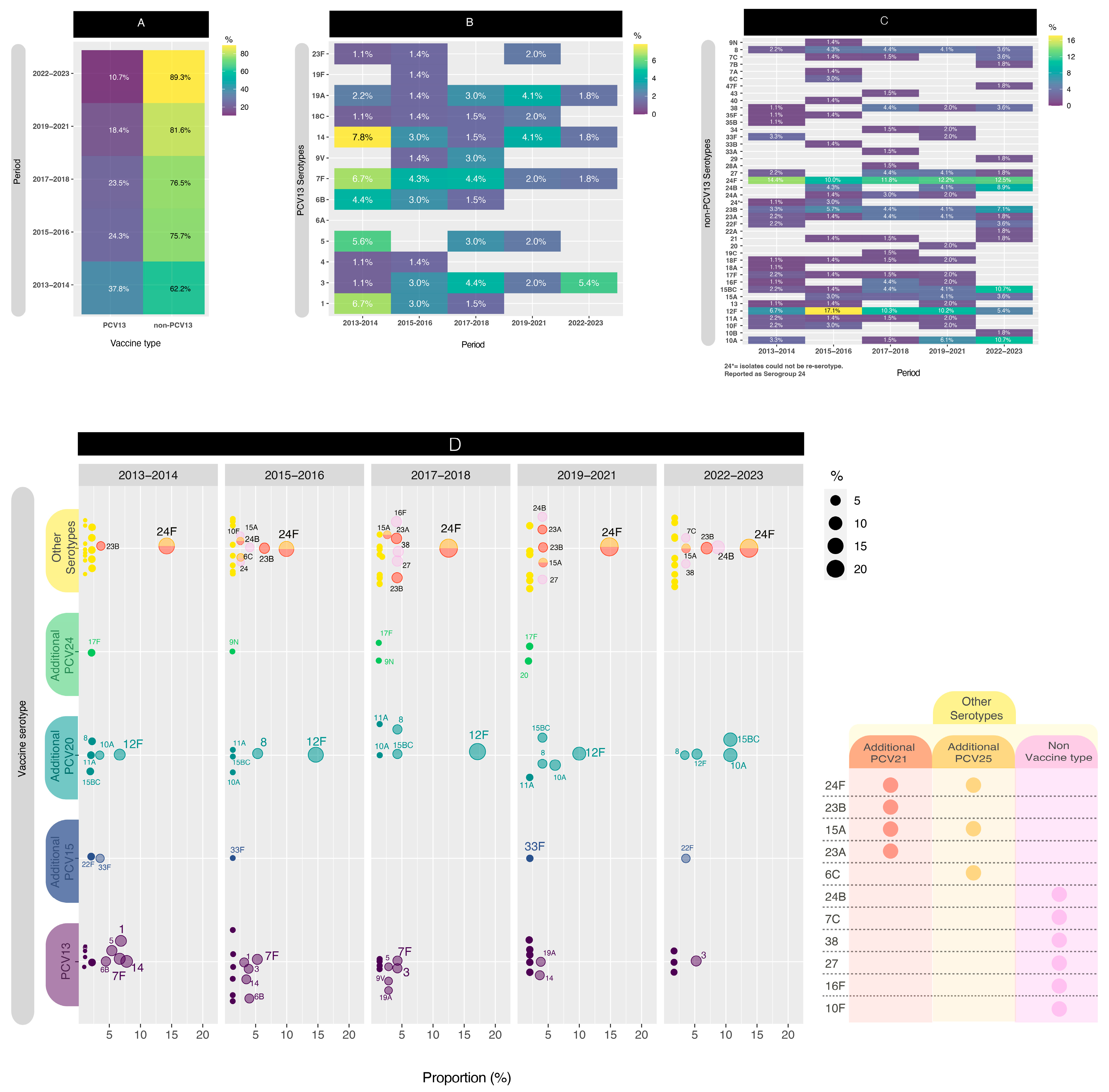
2.2.2. Establishing Serotype Categories
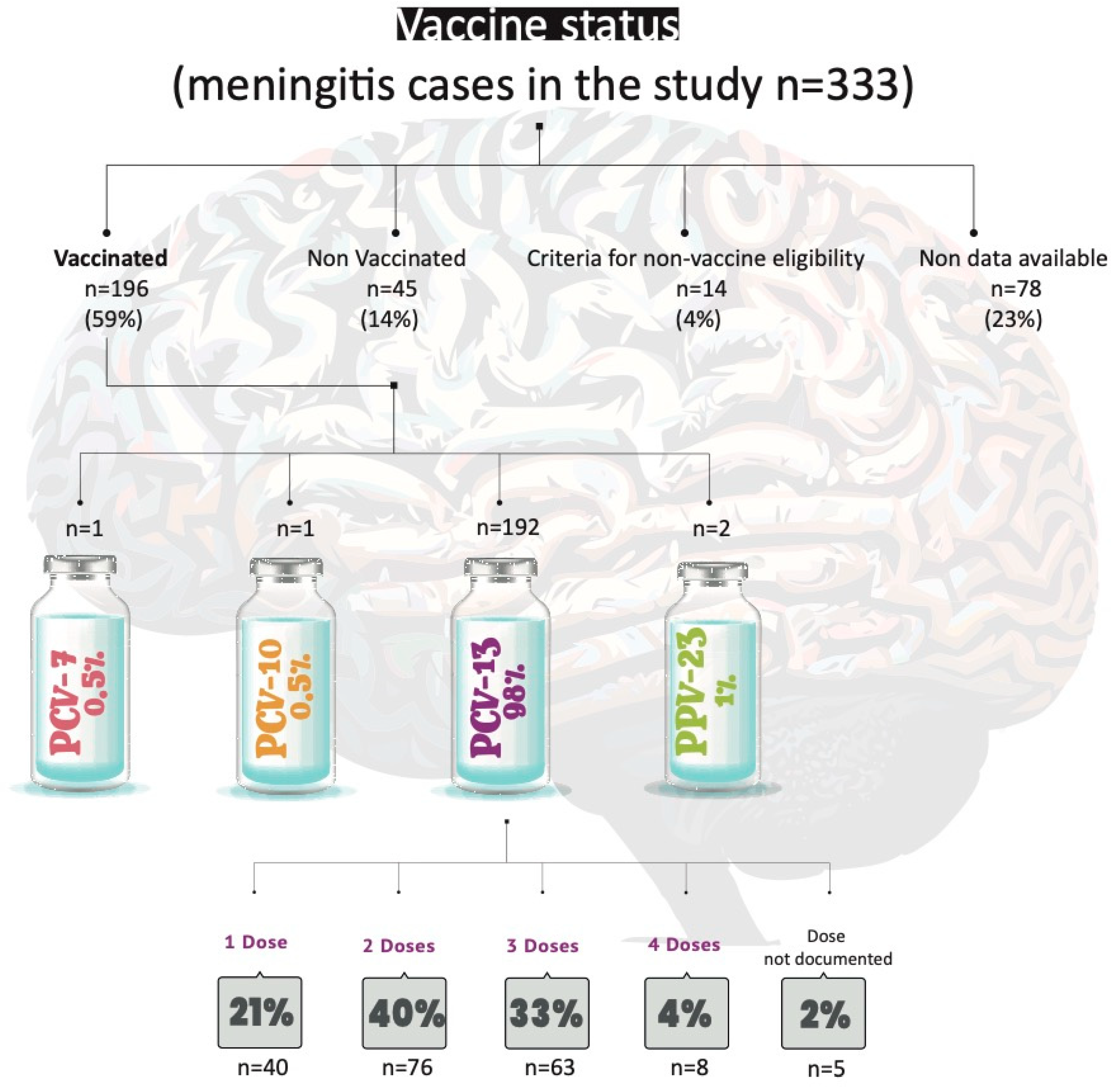
2.2.3. Vaccination Status of the Cases
2.2.4. Antimicrobial Susceptibility
2.3. Statistical Analysis
2.4. Theoretical Vaccination Coverage
3. Results
3.1. Serotype Distribution
3.2. Assessment of Vaccination Status in Cases
3.3. Antimicrobial Resistance Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brouwer, M.C.; van de Beek, D. Epidemiology of community-acquired bacterial meningitis. Curr. Opin. Infect. Dis. 2018, 31, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Schiess, N.; Groce, N.E.; Dua, T. The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review. Microorganisms 2021, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.C.A.; Costa, N.S.; Oliveira, L.M.A. World Meningitis Day and the World Health Organization0 s roadmap to defeat bacterial meningitis in the COVID-19 pandemic era. Int. J. Infect. Dis. 2021, 107, 219–220. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Meningitis Collaborators. Global, regional, and national burden of meningitis, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 1061–1082. [Google Scholar] [CrossRef]
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Thigpen, M.C.; Whitney, C.G.; Messonnier, N.E.; Zell, E.R.; Lynfield, R.; Hadler, J.L.; Harrison, L.H.; Farley, M.M.; Reingold, A.; Bennett, N.M.; et al. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 2011, 364, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://regionsanitaria1.ar/datosepidemio/normativas%20y%20tutoriales%20generales/vigilancia-clinica-de-enos.pdf#:~:text=Nacional%20de%20Vigilancia%20de%20la%20Salud%20(SNVS)%2C,en%20lo%20que%20se%20denomina%20Hoja%20C2 (accessed on 3 February 2025).
- Rodgers, G.L.; Whitney, C.G.; Klugman, K.P. Triumph of Pneumococcal Conjugate Vaccines: Overcoming a Common Foe. J. Infect. Dis. 2021, 224 (Suppl. S2), S352–S359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feemster, K.; Hausdorff, W.P.; Banniettis, N.; Platt, H.; Velentgas, P.; Esteves-Jaramillo, A.; Burton, R.L.; Nahm, M.H.; Buchwald, U.K. Implications of Cross-Reactivity and Cross-Protection for Pneumococcal Vaccine Development. Vaccines 2024, 12, 974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akkoyunlu, M. State of pneumococcal vaccine immunity. Hum. Vaccines Immunother. 2024, 20, 2336358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romanin, V.; Chiavetta, L.; Salvay, M.C.; Chiolo, M.J.; Regueira, M.; Barrios, A.; Califano, G.; García, S.; Gentile, A. Vacuna anti-Haemophilus influenzae de tipo b (Hib) en el Calendario Nacional de Argentina: Portación nasofaríngea de Hib tras 8 años de su introducción. Arch. Argent. Pediatr. 2007, 105, 498–505. [Google Scholar] [CrossRef]
- World Health Organization. Global Vaccine Action Plan: Defeating Meningitis by 2030 Meningitis Prevention and Control; World Health Organization Seventy-Third World Health Assembly: Geneva, Switzerland, 2020; pp. 1–4. [Google Scholar]
- Available online: https://www.argentina.gob.ar/sites/default/files/2024/04/ben_657_se_23.pdf (accessed on 3 February 2025).
- Lund, E. Omni-serum Rasmussen P. A diagnostic Pneumococcus serum, reacting with the 82 known types of Pneumococcus. Acta Pathol. Microbiol. Scand. 1966, 68, 458–460. [Google Scholar] [CrossRef]
- Satzke, C.; Turner, P.; Virolainen-Julkunen, A.; Adrian, P.V.; Antonio, M.; Hare, K.M.; Henao-Restrepo, A.M.; Leach, A.J.; Klugman, K.P.; Porter, B.D.; et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the WorldHealth Organization Pneumococcal Carriage Working Group. Vaccine 2013, 32, 165–179. [Google Scholar] [CrossRef]
- Carvalho, M.D.G.; Pimenta, F.C.; Jackson, D.; Roundtree, A.; Ahmad, Y.; Millar, E.V.; O’Brien, K.L.; Whitney, C.G.; Cohen, A.L.; Beall, B.W. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J. Clin. Microbiol. 2010, 48, 1611–1618. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme Corp. A Phase 3, Multicenter, Randomized, Double-Blind, Active Comparator-Controlled Study to Evaluate the Safety, Tolerability, and Immunogenicity of V114 in Healthy Adults 50 Years of Age or Older (PNEU-AGE). 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03950622 (accessed on 20 December 2020).
- Pfizer. A Phase 3, Randomized, Double-Blind Trial to Evaluate the Safety and Immunogenicity of a 20-Valent Pneumococcal Conjugate Vaccine in Pneumococcal Vaccine-Naive Adults 18 Years of Age and Older. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03760146 (accessed on 20 December 2020).
- Skinner, J.; Kaufhold, R.; McGuinness, D. Immunogenicity of PCV24, a next Generation Pneumococcal Conjugate Vaccine. In Proceedings of the 11th International Symposium on Pneumococci and Pneumococcal Diseases, Melbourne, Australia, 15 April 2018. [Google Scholar]
- Affinivax. Affinivax Announces the Presentation of Phase 1 Clinical Data for Its MAPSTM Vaccine for Streptococcus Pneumoniae at IDWeek 2020. Available online: https://affinivax.com/press-release-october-21-2020/ (accessed on 10 December 2020).
- van Selm, S.; vac Cann, L.M.; Kolkman, M.A.B.; van der Zeijst, B.A.M.; van Putten, J.P.M. Genetic Basis for the Structural Difference between Streptococcus Pneumoniae Serotype 15B and 15C Capsular Polysaccharides. Infect. Immun. 2003, 71, 6192–6198. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically M07-A9, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 3 February 2025).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- De Oliveira, L.H.; Shioda, K.; Valenzuela, M.T.; Janusz, C.B.; Rearte, A.; Sbarra, A.N.; Warren, J.L.; Toscano, C.M.; Weinberger, D.M. Declines in Pneumonia Mortality Following the Introduction of Pneumococcal Conjugate Vaccines in Latin American and Caribbean Countries. Clin. Infect. Dis. 2021, 73, 306–313. [Google Scholar] [CrossRef]
- Luca, D.L.; Kwong, J.C.; Chu, A.; Sander, B.; O’Reilly, R.; McGeer, A.J.; Bloom, D.E. Impact of Pneumococcal Vaccination on Pneumonia Hospitalizations and Related Costs in Ontario: A Population-Based Ecological Study. Clin. Infect. Dis. 2018, 66, 541–547. [Google Scholar] [CrossRef]
- Severiche-Bueno, D.F.; Severiche-Bueno, D.F.; Bastidas, A.; Caceres, E.L.; Silva, E.; Lozada, J.; Gomez, S.; Vargas, H.; Viasus, D.; Reyes, L.F. Burden of invasive pneumococcal disease (IPD) over a 10-year period in Bogotá, Colombia. Int. J. Infect. Dis. 2021, 105, 32–39. [Google Scholar] [CrossRef]
- Van Deursen, A.M.M.; Schurink-Van’t Klooster, T.M.; Man, W.H.; van de Kassteele, J.; van Gageldonk-Lafeber, A.B.; BruijningVerhagen, P.; de Melker, H.E.; Sanders, E.A.M.; Knol, M.J. Impact of infant pneumococcal conjugate vaccination on community acquired pneumonia hospitalization in all ages in the Netherlands. Vaccine 2017, 35, 7107–7113. [Google Scholar] [CrossRef]
- Pikis, A.; Kavaliotis, J.; Tsikoulas, J.; Andrianopoulos, P.; Venzon, D.; Manios, S. Long-term sequelae of pneumococcal meningitis in children. Clin. Pediatr. 1996, 35, 72–78. [Google Scholar] [CrossRef]
- Ruvinsky, R.O.; Regueira, M.; Fossati, M.S.; Gagetti, P.; Pace, J.; Rodríguez, M.; Gabastou, J.M.; Corso, A. Surveillance of invasive in Streptococcus pneumoniae in Argentina 1994–2007: Changes in serotype distribution, serotype coverage of pneumococcal conjugate vaccines and antibiotic resistance. J. Pediatr. Infect. Dis. 2010, 5, 263–269. [Google Scholar] [CrossRef]
- Garcia Quesada, M.; Yang, Y.; Bennett, J.C.; Hayford, K.; Zeger, S.L.; Feikin, D.R.; Peterson, M.E.; Cohen, A.L.; Almeida, S.C.G.; Ampofo, K.; et al. Serotype Distribution of Remaining Pneumococcal Meningitis in the Mature PCV10/13 Period: Findings from the PSERENADE Project. Microorganisms 2021, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.argentina.gob.ar/salud/inmunoprevenibles/coberturas-de-vacunacion (accessed on 3 February 2025).
- Available online: https://www.argentina.gob.ar/sites/default/files/2019/05/2024_08_26-cnv_2023-cierre-agosto-2024.pdf (accessed on 3 February 2025).
- Lodi, L.; Ricci, S.; Nieddu, F.; Moriondo, M.; Lippi, F.; Canessa, C.; Mangone, G.; Cortimiglia, M.; Casini, A.; Lucenteforte, E.; et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Severe Invasive Disease Caused by Serotype 3 Streptococcus pneumoniae in Italian Children. Vaccines 2019, 7, 128. [Google Scholar] [CrossRef]
- De Wals, P.; Gessner, B.D.; Isturiz, R.; Laferriere, C.; McLaughlin, J.M.; Pelton, S.; Schmitt, H.-J.; Suaya, J.A.; Jodar, L. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Invasive Disease Caused by Serotype 3 in Children: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Infect. Dis. 2019, 68, 2135–2143, Corrigendum to: Clin. Infect. Dis. 2021, 72, 1684–1685. [Google Scholar] [CrossRef]
- Available online: https://www.argentina.gob.ar/sites/default/files/2018/02/lineamiento_tecnico_vcn20_2024.pdf (accessed on 3 February 2025).


| Year | No. of Isolates (n) | Penicillin | Cefotaxime | Meropenem | Amoxicillin | Ceftaroline | Ceftobiprole | ||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||||||||||
| 2013–2014 | 90 | 28 | 31.1 | 3 | 3.3 | 5 | 5.6 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| 2015–2016 | 61 | 23 | 37.7 | 2 | 3.3 | 3 | 4.9 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| 2017–2018 | 66 | 22 | 33.3 | 3 | 4.5 | 3 | 4.5 | 1 | 1.5 | 0 | 0 | 0 | 0 | ||||||||
| 2019–2021 | 41 | 16 | 39.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| 2022–2023 | 53 | 26 | 49.1 | 1 | 1.9 | 1 | 1.9 | 1 | 1.9 | 0 | 0 | 0 | 0 | ||||||||
| Total 2013–2023 | 311 | 115 | 37.0 | 9 | 2.9 | 12 | 3.9 | 2 | 0.6 | 0 | 0 | 0 | 0 | ||||||||
| Year | No. of Isolates (n) | Vancomycin | Rifampicin | Chloramphenicol | Cotrimoxazole | Erythromycin | Clindamycin | Tetracycline | Doxycycline | Levofloxacin | MDR | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| 2013–2014 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 36.7 | 17 | 18.9 | 16 | 17.8 | 20 | 22.2 | 20 | 22.2 | 0 | 0 | 14 | 15.6 |
| 2015–2016 | 61 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 54.1 | 20 | 32.8 | 15 | 24.6 | 25 | 41.0 | 25 | 41.0 | 0 | 0 | 18 | 29.5 |
| 2017–2018 | 66 | 0 | 0 | 0 | 0 | 0 | 0 | 35 | 53.0 | 18 | 27.3 | 13 | 19.7 | 18 | 27.3 | 18 | 27.3 | 0 | 0 | 15 | 22.7 |
| 2019–2021 | 41 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 51.2 | 13 | 31.7 | 10 | 24.4 | 15 | 36.6 | 15 | 36.6 | 0 | 0 | 12 | 29.3 |
| 2022–2023 | 53 | 0 | 0 | 0 | 0 | 0 | 0 | 23 | 43.4 | 19 | 35.8 | 14 | 26.4 | 22 | 41.5 | 22 | 41.5 | 0 | 0 | 19 | 35.8 |
| Total 2013–2023 | 311 | 0 | 0 | 0 | 0 | 0 | 0 | 145 | 46.6 | 87 | 28.0 | 68 | 21.9 | 100 | 32.2 | 100 | 32.2 | 0 | 0 | 78 | 25.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zintgraff, J.; Gagetti, P.; Sanchez Eluchans, N.; Marchetti, P.; Moscoloni, M.A.; Argentina Spn Working Group; Lara, C.S.; Corso, A. Evolving Landscape of Paediatric Pneumococcal Meningitis in Argentina (2013–2023). Microorganisms 2025, 13, 1301. https://doi.org/10.3390/microorganisms13061301
Zintgraff J, Gagetti P, Sanchez Eluchans N, Marchetti P, Moscoloni MA, Argentina Spn Working Group, Lara CS, Corso A. Evolving Landscape of Paediatric Pneumococcal Meningitis in Argentina (2013–2023). Microorganisms. 2025; 13(6):1301. https://doi.org/10.3390/microorganisms13061301
Chicago/Turabian StyleZintgraff, Jonathan, Paula Gagetti, Nahuel Sanchez Eluchans, Paulina Marchetti, María Alicia Moscoloni, Argentina Spn Working Group, Claudia Sara Lara, and Alejandra Corso. 2025. "Evolving Landscape of Paediatric Pneumococcal Meningitis in Argentina (2013–2023)" Microorganisms 13, no. 6: 1301. https://doi.org/10.3390/microorganisms13061301
APA StyleZintgraff, J., Gagetti, P., Sanchez Eluchans, N., Marchetti, P., Moscoloni, M. A., Argentina Spn Working Group, Lara, C. S., & Corso, A. (2025). Evolving Landscape of Paediatric Pneumococcal Meningitis in Argentina (2013–2023). Microorganisms, 13(6), 1301. https://doi.org/10.3390/microorganisms13061301







