The Community Structure of Aerobic Anoxygenic Photosynthetic Bacteria in Biocrusts on Tropical Coral Islands and Their Application in Ecological Restoration, South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Area
2.2. Sampling and Storage
2.3. Measurement of the Soil Physicochemical Properties
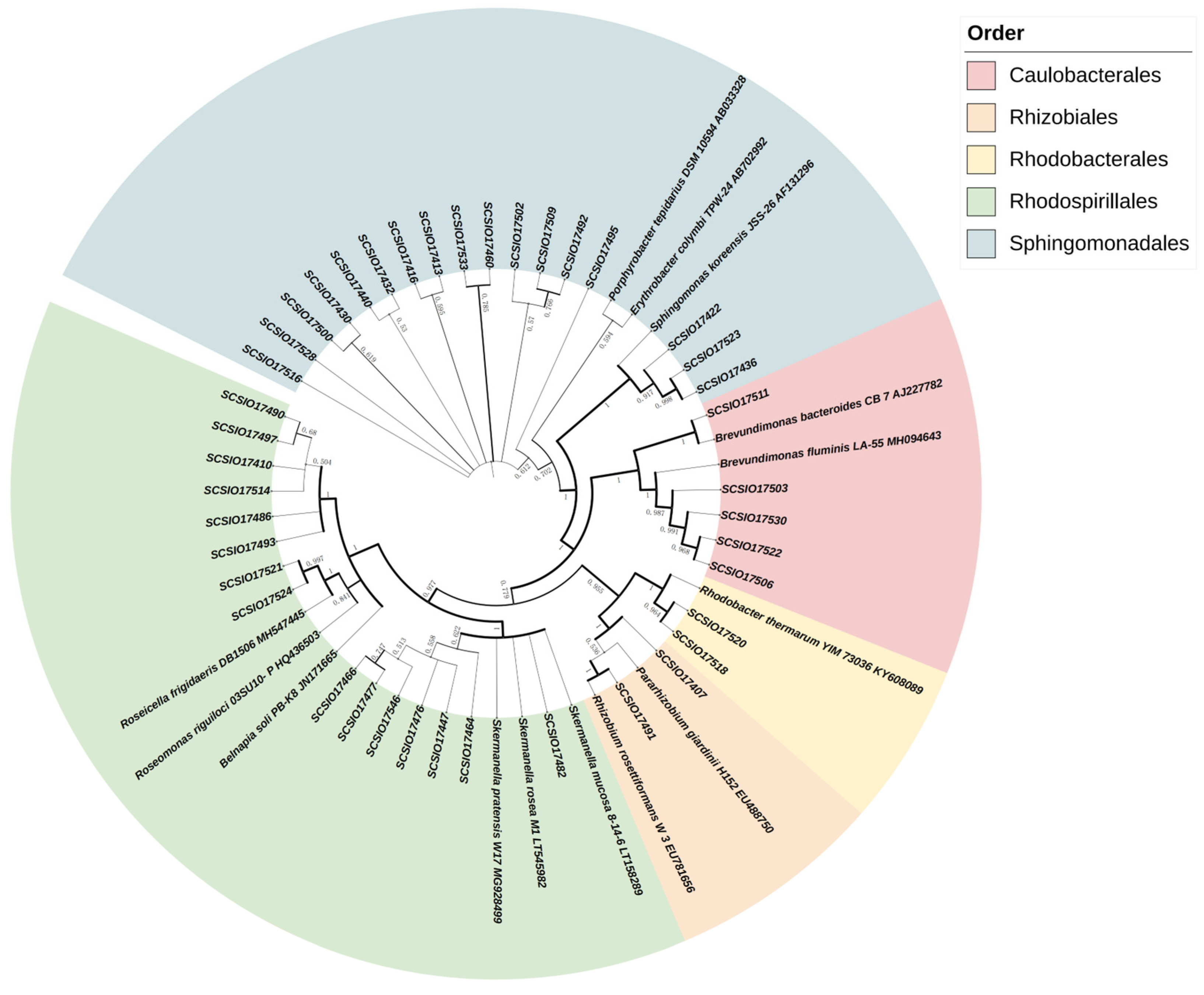
2.4. Isolation, Purification, Identification, and Functional Screening of AAPB
2.5. DNA Extraction, Amplification, and Sequencing
2.6. Real-Time PCR
2.7. Statistical Analyses
3. Results
3.1. Physicochemical Soil Properties
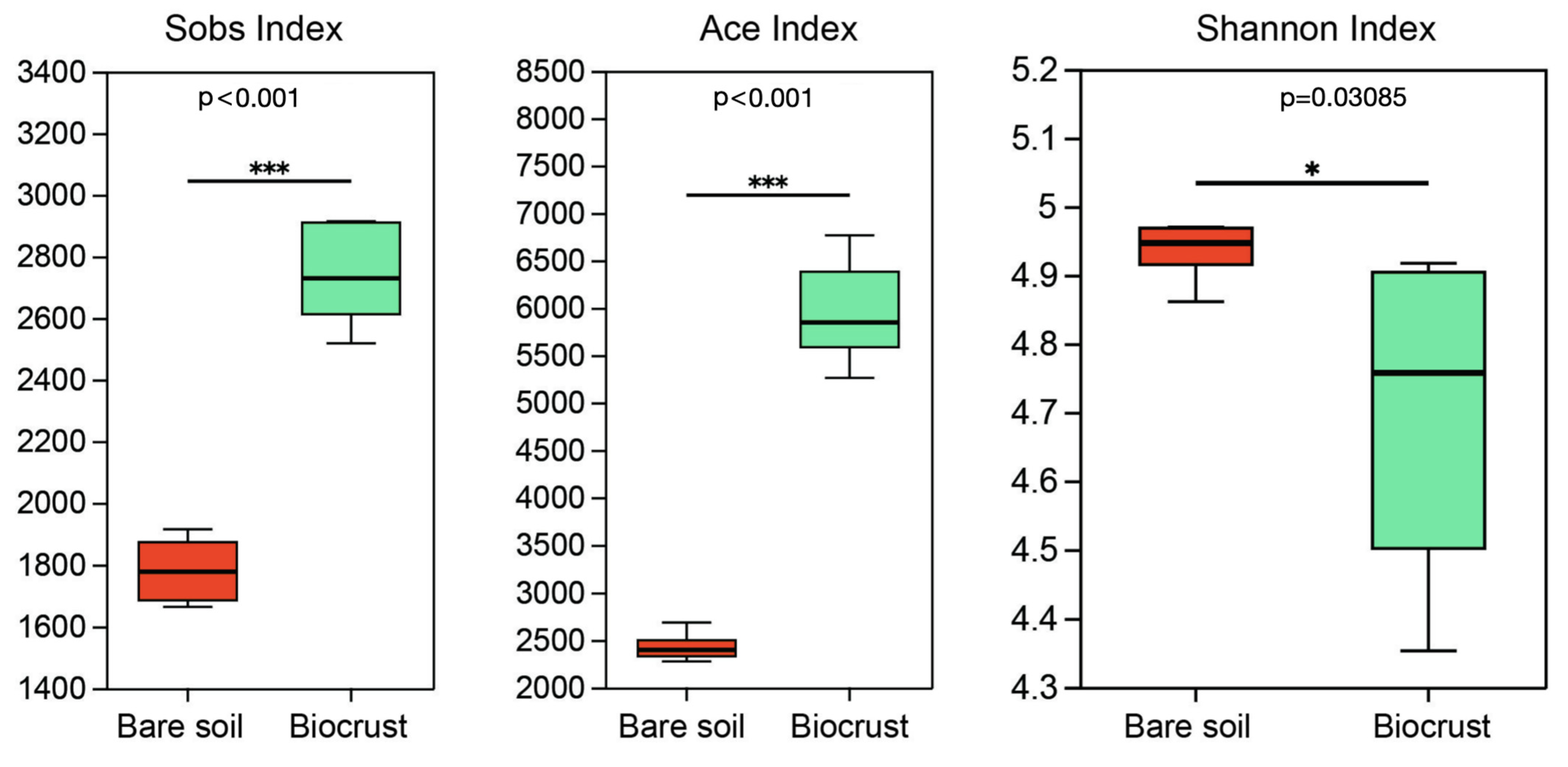
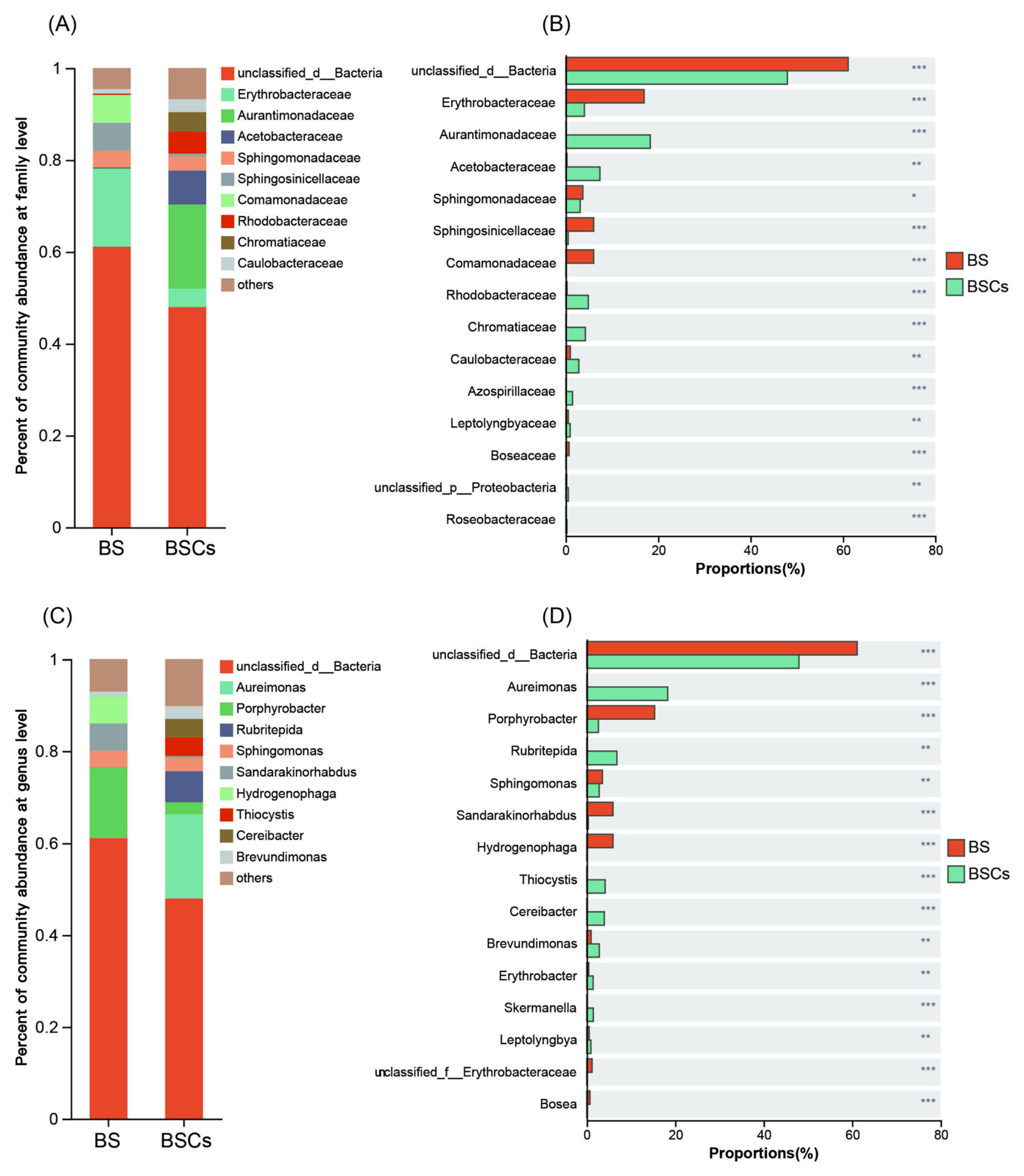
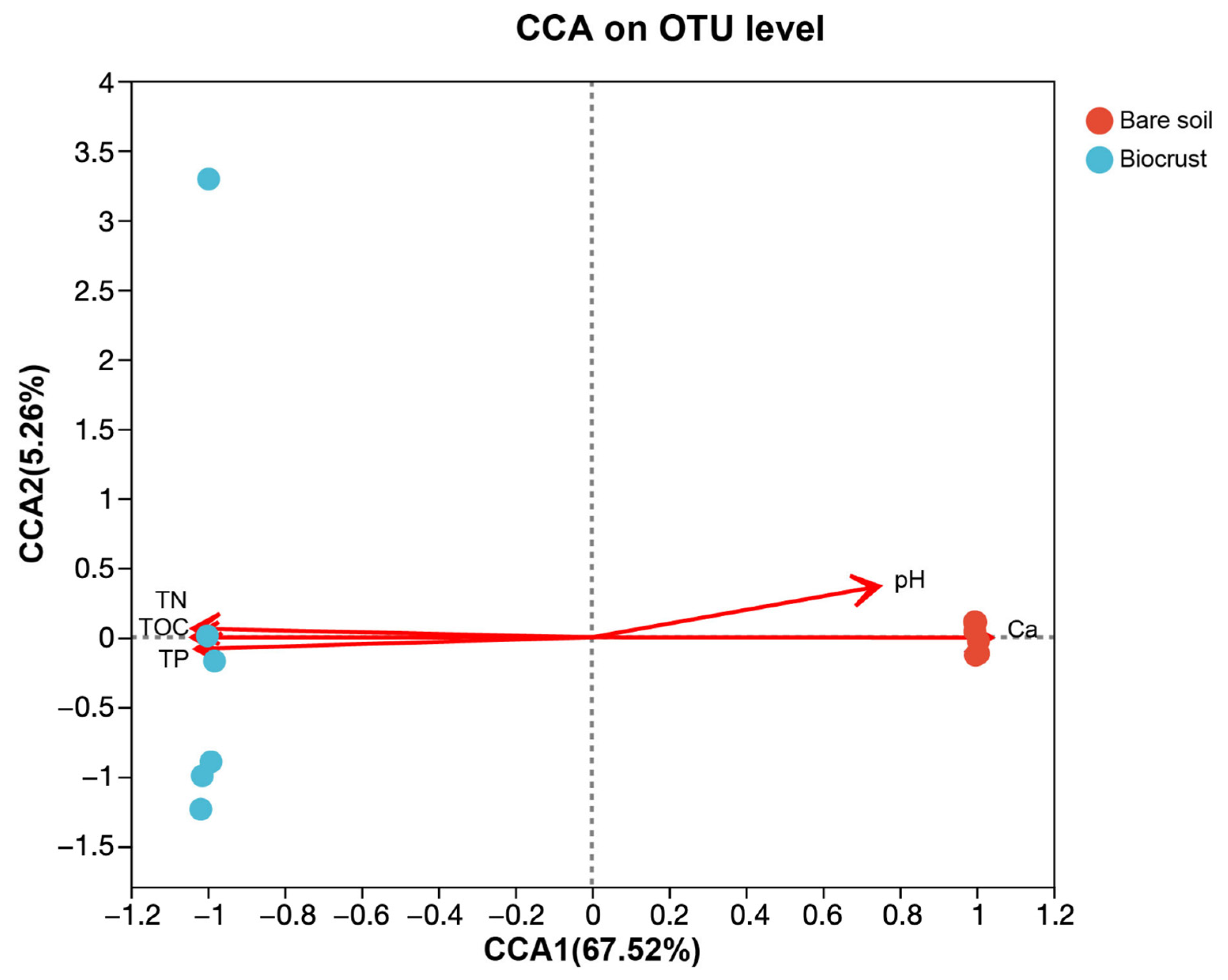
3.2. Culture-Independent Analysis of AAPB Community Diversity and Composition
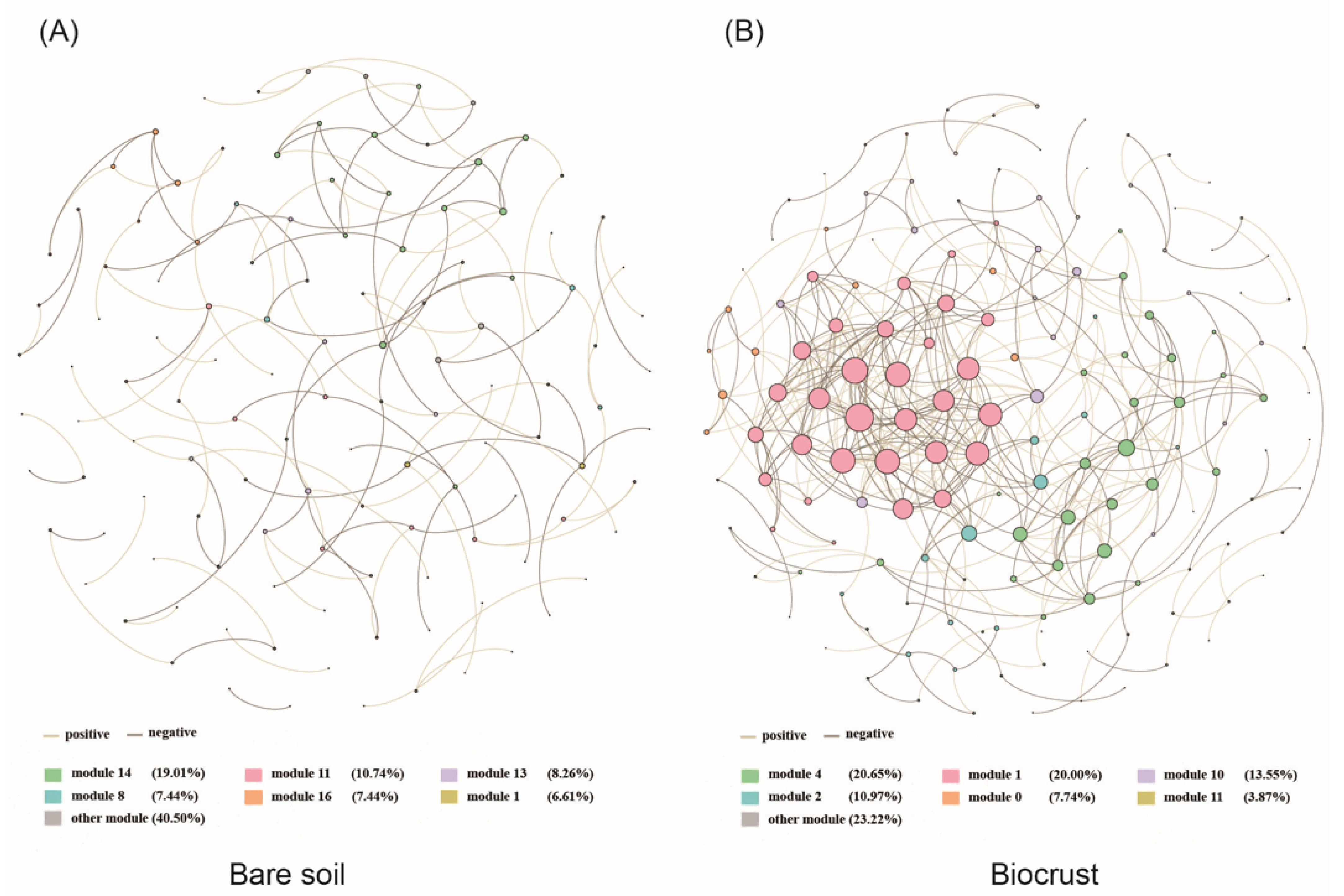
3.3. The Co-Occurrence Network of AAPB
3.4. Culture-Dependent Isolation and Taxonomic Diversity of AAPB Strains
3.5. Exopolysaccharide Content and Sand-Fixing Ability of AAPB Strains
4. Discussion
4.1. AAPB Community and Its Influencing Factors with Regard to Biocrust Formation
4.2. Sand-Fixing Characteristics and Anti-Erosion Ability of AAPB Strains
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, T.M.; Cassey, P.; Duncan, R.P.; Evans, K.L.; Gaston, K.J. Avian extinction and mammalian introductions on oceanic islands. Science 2004, 305, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Li, Y.; Chen, Z.; Peng, C.; Huang, G.; Niu, H.; Zhang, H. Assessment of habitat change on bird diversity and bird–habitat network of a Coral Island, South China Sea. BMC Ecol. Evol. 2021, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Tamm, A.; Wu, D.; Caesar, J.; Grube, M.; Weber, B. Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. ISME J. 2018, 12, 1032–1046. [Google Scholar] [CrossRef]
- Belnap, J.; Weber, B.; Büdel, B. Biological Soil Crusts as an Organizing Principle in Drylands. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–13. [Google Scholar]
- Weber, B.; Belnap, J.; Büdel, B. Synthesis on Biological Soil Crust Research. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 527–534. [Google Scholar]
- Rodriguez-Caballero, E.; Belnap, J.; Büdel, B.; Crutzen, P.J.; Andreae, M.O.; Pöschl, U.; Weber, B. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 2018, 11, 185–189. [Google Scholar] [CrossRef]
- Couradeau, E.; Karaoz, U.; Lim, H.C.; Nunes da Rocha, U.; Northen, T.; Brodie, E.; Garcia-Pichel, F. Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nat. Commun. 2016, 7, 10373. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Reed, S.; Travers, S.K.; Bowker, M.A.; Maestre, F.T.; Ding, J.; Havrilla, C.; Rodriguez-Caballero, E.; Barger, N.; Weber, B.; et al. The pervasive and multifaceted influence of biocrusts on water in the world’s drylands. Glob. Change Biol. 2020, 26, 6003–6014. [Google Scholar] [CrossRef]
- Benko, Z.R. Biological Soil Crusts: Structure, Function, and Management. Community Ecol. 2003, 4, 118–119. [Google Scholar]
- Chamizo, S.; Cantón, Y.; Rodríguez-Caballero, E.; Domingo, F. Biocrusts positively affect the soil water balance in semiarid ecosystems. Ecohydrology 2016, 9, 1208–1221. [Google Scholar] [CrossRef]
- Belnap, J.; Walker, B.J.; Munson, S.M.; Gill, R.A. Controls on sediment production in two U.S. deserts. Aeolian Res. 2014, 14, 15–24. [Google Scholar] [CrossRef]
- Tang, K.; Jia, L.; Yuan, B.; Yang, S.; Li, H.; Meng, J.; Zeng, Y.; Feng, F. Aerobic anoxygenic phototrophic bacteria promote the development of biological soil crusts. Front. Microbiol. 2018, 9, 2715. [Google Scholar] [CrossRef]
- Yang, H.; Hu, C. Soil Chemistry and Nutrients Influence the Distribution of Aerobic Anoxygenic Phototrophic Bacteria and Eukaryotic Phototrophic Microorganisms of Physical Soil Crusts at Different Elevations on the Tibetan Plateau. Microb. Ecol. 2022, 83, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C. Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Yurkov, V.; Hughes, E. Aerobic Anoxygenic Phototrophs: Four Decades of Mystery. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Hallenbeck, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 193–214. [Google Scholar]
- Koblížek, M. Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol. Rev. 2015, 39, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yu, Y.; Chen, L. Response of surface soil hydrology to the micro-pattern of bio-crust in a dry-land Loess environment, China. PLoS ONE 2015, 10, e0133565. [Google Scholar] [CrossRef]
- Csotonyi, J.T.; Swiderski, J.; Stackebrandt, E.; Yurkov, V. A New Extreme Environment for Aerobic Anoxygenic Phototrophs: Biological Soil Crusts. In Recent Advances in Phototrophic Prokaryotes; Hallenbeck, P.C., Ed.; Springer New York: New York, NY, USA, 2010; pp. 3–14. [Google Scholar]
- Tang, K.; Yuan, B.; Jia, L.; Pan, X.; Feng, F.; Jin, K. Spatial and temporal distribution of aerobic anoxygenic phototrophic bacteria: Key functional groups in biological soil crusts. Environ. Microbiol. 2021, 23, 3554–3567. [Google Scholar] [CrossRef]
- Cottrell, M.T.; Ras, J.; Kirchman, D.L. Bacteriochlorophyll and community structure of aerobic anoxygenic phototrophic bacteria in a particle-rich estuary. ISME J. 2010, 4, 945–954. [Google Scholar] [CrossRef]
- Yurkov, V.; Csotonyi, J.T. New light on aerobic anoxygenic phototrophs. In The Purple Phototrophic Bacteria; Springer: Berlin/Heidelberg, Germany, 2009; pp. 31–55. [Google Scholar]
- Bill, N.; Tomasch, J.; Riemer, A.; Müller, K.; Kleist, S.; Schmidt-Hohagen, K.; Wagner-Döbler, I.; Schomburg, D. Fixation of CO2 using the ethylmalonyl-CoA pathway in the photoheterotrophic marine bacterium Dinoroseobacter shibae. Environ. Microbiol. 2017, 19, 2645–2660. [Google Scholar] [CrossRef]
- Tahon, G.; Tytgat, B.; Stragier, P.; Willems, A. Analysis of cbbL, nifH, and pufLM in soils from the Sør Rondane Mountains, Antarctica, reveals a large diversity of autotrophic and phototrophic bacteria. Microb. Ecol. 2016, 71, 131–149. [Google Scholar] [CrossRef]
- Godinho, A.L.; Bhosle, S. Sand aggregation by exopolysaccharide-producing Microbacterium arborescens––AGSB. Curr. Microbiol. 2009, 58, 616–621. [Google Scholar] [CrossRef]
- Bowker, M.A.; Belnap, J.; Miller, M.E. Spatial Modeling of Biological Soil Crusts to Support Rangeland Assessment and Monitoring. Rangel. Ecol. Manag. 2006, 59, 519–529. [Google Scholar] [CrossRef]
- Kuske, C.R.; Yeager, C.M.; Johnson, S.; Ticknor, L.O.; Belnap, J. Response and resilience of soil biocrust bacterial communities to chronic physical disturbance in arid shrublands. ISME J. 2012, 6, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rossi, F.; Deng, S.; Liu, Y.; Wang, G.; Adessi, A.; De Philippis, R. Macromolecular and chemical features of the excreted extracellular polysaccharides in induced biological soil crusts of different ages. Soil Biol. Biochem. 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Meng, D.; Wu, J.; Chen, K.; Li, H.; Jin, W.; Shu, S.; Zhang, J. Effects of extracellular polymeric substances and microbial community on the anti-scouribility of sewer sediment. Sci. Total Environ. 2019, 687, 494–504. [Google Scholar] [CrossRef]
- Stomp, M.; Huisman, J.; Stal, L.J.; Matthijs, H. Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME J. 2007, 1, 271–282. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Chen, T.; Zhang, H.; Zhao, M.; Yan, H. Two centuries-long records of skeletal calcification in massive Porites colonies from Meiji Reef in the southern South China Sea and its responses to atmospheric CO2 and seawater temperature. Sci. China. Earth Sci. 2012, 55, 1. [Google Scholar] [CrossRef]
- Gan, J.; Li, H.; Curchitser, E.N.; Haidvogel, D.B. Modeling South China Sea circulation: Response to seasonal forcing regimes. J. Geophys. Res. Ocean. 2006, 111. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, S. A Comprehensive Network Integrating Signature Microbes and Crucial Soil Properties During Early Biological Soil Crust Formation on Tropical Reef Islands. Front. Microbiol. 2022, 13, 831710. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Zhang, S.; Huang, H.; Yang, J.; Tian, X.-P.; Long, L.-J. Highly Heterogeneous Bacterial Communities Associated with the South China Sea Reef Corals Porites lutea, Galaxea fascicularis and Acropora millepora. PLoS ONE 2013, 8, e71301. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Cruz, L.; Sotomayor-Ramírez, D.; Pérez-Alegría, L. Enzyme activities as affected by soil properties and land use in a tropical watershed. Appl. Soil Ecol. 2007, 35, 35–45. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, Z.; Wu, Y.; Liu, S.; Cui, L.; Zhang, J.; Huang, X. Origins of sediment organic matter and their contributions at three contrasting wetlands in a coastal semi-enclosed ecosystem. Mar. Pollut. Bull. 2019, 139, 32–39. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocesswengaowenor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Revelle, W.R. psych: Procedures for Personality and Psychological Research. 2017. Available online: https://www.scholars.northwestern.edu/en/publications/psych-procedures-for-personality-and-psychological-research/ (accessed on 25 March 2025).
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. Proc. Int. AAAI Conf. Web Soc. Media 2009, 3, 361–362. [Google Scholar] [CrossRef]
- Xue, R.; Wang, C.; Zhao, L.; Sun, B.; Wang, B. Agricultural intensification weakens the soil health index and stability of microbial networks. Agric. Ecosyst. Environ. 2022, 339, 108118. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Béjà, O.; Suzuki, M.T.; Heidelberg, J.F.; Nelson, W.C.; Preston, C.M.; Hamada, T.; Eisen, J.A.; Fraser, C.M.; DeLong, E.F. Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 2002, 415, 630–633. [Google Scholar] [CrossRef]
- Díaz-Cárdenas, C.; Bernal, L.F.; Caro-Quintero, A.; López, G.; David Alzate, J.; Gonzalez, L.N.; Restrepo, S.; Shapiro, N.; Woyke, T.; Kyrpides, N.C. Draft genome and description of Consotaella salsifontis gen. nov. sp. nov., a halophilic, free-living, nitrogen-fixing alpha proteobacterium isolated from an ancient terrestrial saline spring. Int. J. Syst. Evol. Microbiol. 2017, 67, 3744–3751. [Google Scholar] [CrossRef]
- Saravanan, V.; Madhaiyan, M.; Osborne, J.; Thangaraju, M.; Sa, T. Ecological occurrence of Gluconacetobacter diazotrophicus and nitrogen-fixing Acetobacteraceae members: Their possible role in plant growth promotion. Microb. Ecol. 2008, 55, 130–140. [Google Scholar] [CrossRef]
- Kiledal, E.A.; Keffer, J.L.; Maresca, J.A. Bacterial communities in concrete reflect its composite nature and change with weathering. Msystems 2021, 6, e01153-20. [Google Scholar] [CrossRef]
- Cania, B.; Vestergaard, G.; Kublik, S.; Köhne, J.M.; Fischer, T.; Albert, A.; Winkler, B.; Schloter, M.; Schulz, S. Biological soil crusts from different soil substrates harbor distinct bacterial groups with the potential to produce exopolysaccharides and lipopolysaccharides. Microb. Ecol. 2020, 79, 326–341. [Google Scholar] [CrossRef]
- Becker, R.; Ulrich, K.; Behrendt, U.; Schneck, V.; Ulrich, A. Genomic Characterization of Aureimonas altamirensis C2P003—A Specific Member of the Microbiome of Fraxinus excelsior Trees Tolerant to Ash Dieback. Plants 2022, 11, 3487. [Google Scholar] [CrossRef]
- Chamizo, S.; Cantón, Y.; Miralles, I.; Domingo, F. Biological soil crust development affects physicochemical characteristics of soil surface in semiarid ecosystems. Soil Biol. Biochem. 2012, 49, 96–105. [Google Scholar] [CrossRef]
- Schulz, K.; Mikhailyuk, T.; Dreßler, M.; Leinweber, P.; Karsten, U. Biological Soil Crusts from Coastal Dunes at the Baltic Sea: Cyanobacterial and Algal Biodiversity and Related Soil Properties. Microb. Ecol. 2016, 71, 178–193. [Google Scholar] [CrossRef]
- Concostrina-Zubiri, L.; Huber-Sannwald, E.; Martínez, I.; Flores, J.F.; Escudero, A. Biological soil crusts greatly contribute to small-scale soil heterogeneity along a grazing gradient. Soil Biol. Biochem. 2013, 64, 28–36. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhang, Y. Responses of microbial activities and soil physical-chemical properties to the successional process of biological soil crusts in the Gurbantunggut Desert, Xinjiang. J. Arid. Land 2015, 7, 101–109. [Google Scholar] [CrossRef]
- You, Y.; Wang, J.; Huang, X.; Tang, Z.; Liu, S.; Sun, O.J. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 2014, 4, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kong, W.; Wu, N.; Zhang, Y. Bacterial diversity and community along the succession of biological soil crusts in the Gurbantunggut Desert, Northern China. J. Basic Microbiol. 2016, 56, 670–679. [Google Scholar] [CrossRef]
- Blay, E.S.; Schwabedissen, S.G.; Magnuson, T.S.; Aho, K.A.; Sheridan, P.P.; Lohse, K.A. Variation in Biological Soil Crust Bacterial Abundance and Diversity as a Function of Climate in Cold Steppe Ecosystems in the Intermountain West, USA. Microb. Ecol. 2017, 74, 691–700. [Google Scholar] [CrossRef]
- Borchhardt, N.; Baum, C.; Thiem, D.; Köpcke, T.; Karsten, U.; Leinweber, P.; Hrynkiewicz, K. Soil microbial phosphorus turnover and identity of algae and fungi in biological soil crusts along a transect in a glacier foreland. Eur. J. Soil Biol. 2019, 91, 9–17. [Google Scholar] [CrossRef]
- Pushkareva, E.; Pessi, I.S.; Wilmotte, A.; Elster, J. Cyanobacterial community composition in Arctic soil crusts at different stages of development. FEMS Microbiol. Ecol. 2015, 91, fiv143. [Google Scholar] [CrossRef]
- Naqqash, T.; Imran, A.; Hameed, S.; Shahid, M.; Majeed, A.; Iqbal, J.; Hanif, M.K.; Ejaz, S.; Malik, K.A. First report of diazotrophic Brevundimonas spp. as growth enhancer and root colonizer of potato. Sci. Rep. 2020, 10, 12893. [Google Scholar] [CrossRef]
- Barger, N.N.; Herrick, J.E.; Van Zee, J.; Belnap, J. Impacts of biological soil crust disturbance and composition on C and N loss from water erosion. Biogeochemistry 2006, 77, 247–263. [Google Scholar] [CrossRef]
- Mager, D.M.; Thomas, A.D. Extracellular polysaccharides from cyanobacterial soil crusts: A review of their role in dryland soil processes. J. Arid. Environ. 2011, 75, 91–97. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Zeng, X.; Wei, X.; Sun, C.; Niu, J.; Yan, W.; Du, J.; Sun, Y.; Cheng, H. Mangrove restoration promotes the anti-scouribility of the sediments by modifying inherent microbial community and extracellular polymeric substance. Sci. Total Environ. 2022, 811, 152369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, Y.; Dong, Y.; Lapen, D.R.; Liu, J.; Chen, W. Subsoiling and conversion to conservation tillage enriched nitrogen cycling bacterial communities in sandy soils under long-term maize monoculture. Soil Tillage Res. 2022, 215, 105197. [Google Scholar] [CrossRef]
- Koblížek, M.; Béjà, O.; Bidigare, R.R.; Christensen, S.; Benitez-Nelson, B.; Vetriani, C.; Kolber, M.K.; Falkowski, P.G.; Kolber, Z.S. Isolation and characterization of Erythrobacter sp. strains from the upper ocean. Arch. Microbiol. 2003, 180, 327–338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Mai, Z.; Li, J.; Wang, L.; Zhang, S. The Community Structure of Aerobic Anoxygenic Photosynthetic Bacteria in Biocrusts on Tropical Coral Islands and Their Application in Ecological Restoration, South China Sea. Microorganisms 2025, 13, 1265. https://doi.org/10.3390/microorganisms13061265
Wen J, Mai Z, Li J, Wang L, Zhang S. The Community Structure of Aerobic Anoxygenic Photosynthetic Bacteria in Biocrusts on Tropical Coral Islands and Their Application in Ecological Restoration, South China Sea. Microorganisms. 2025; 13(6):1265. https://doi.org/10.3390/microorganisms13061265
Chicago/Turabian StyleWen, Jing, Zhimao Mai, Jie Li, Lin Wang, and Si Zhang. 2025. "The Community Structure of Aerobic Anoxygenic Photosynthetic Bacteria in Biocrusts on Tropical Coral Islands and Their Application in Ecological Restoration, South China Sea" Microorganisms 13, no. 6: 1265. https://doi.org/10.3390/microorganisms13061265
APA StyleWen, J., Mai, Z., Li, J., Wang, L., & Zhang, S. (2025). The Community Structure of Aerobic Anoxygenic Photosynthetic Bacteria in Biocrusts on Tropical Coral Islands and Their Application in Ecological Restoration, South China Sea. Microorganisms, 13(6), 1265. https://doi.org/10.3390/microorganisms13061265




