Effect of Maternal Probiotic and Piglet Dietary Tryptophan Level on Performance and Piglet Intestinal Health Parameters Pre-Weaning
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Management
2.1.1. Sow Management
2.1.2. Piglet Management
2.2. Data and Sample Collection
2.2.1. Data and Sample Collection—Sow
Sow Backfat and Feed Intake
Sow Fecal Sampling
2.2.2. Data and Sample Collection—Piglet
Performance and Creep Intake
Piglet Tissue and Digesta Sampling
2.3. Analysis
2.3.1. Microbial Analysis
Microbial DNA Extraction
Illumina Sequencing and Bioinformatic Analysis of Sow Feces and Offspring Colonic Digesta
Absolute Quantification of Escherichia coli in the Offspring Ileal Mucosa
2.3.2. Volatile Fatty Acid Analysis
2.3.3. Gene Expression Analysis
2.3.4. Morphological Analysis
2.3.5. Statistical Analysis
3. Results
3.1. Sow Reproductive and Offspring Performance
3.2. Stomach pH at Weaning
3.3. Duodenal Morphology
3.4. Small Intestine Gene Expression
3.4.1. Duodenum
3.4.2. Ileum
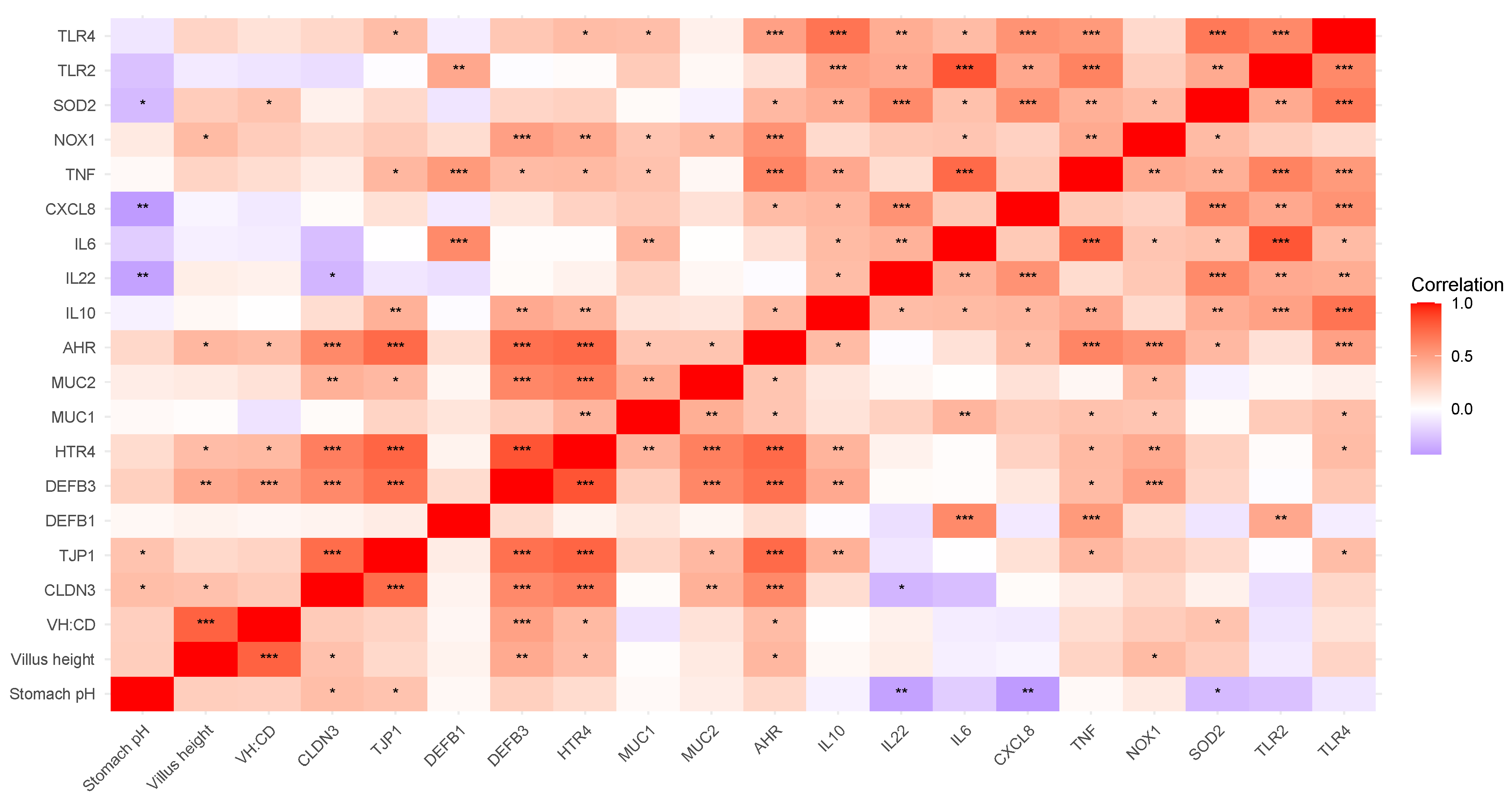
3.4.3. Correlation of Duodenal Gene Expression and Physiological Parameters
Nutrient Transporters
Immune Response and Barrier Defense
3.5. Volatile Fatty Acids
3.5.1. Cecal Volatile Fatty Acids
3.5.2. Colonic Volatile Fatty Acids
3.6. Microbial Composition
3.6.1. 16S rRNA Microbial Analysis of Sow Feces
Bacterial Richness and Diversity of Sow Feces
Differential Microbial Abundance Analysis of Sow Feces
- Phylum Level—Sow Feces
- Family Level—Sow Feces
- Genus Level—Sow Feces
3.6.2. Offspring Microbial Analysis
Absolute Quantification of Escherichia coli in Offspring Ileal Mucosa Associated Microbiota
16S rRNA Microbial Analysis of Offspring Colonic Digesta
- Bacterial Richness and Diversity—Offspring Colonic Digesta
- Differential Microbial Abundance Analysis—Offspring Colonic Digesta
- Phylum Level—Offspring Colonic Digesta
- Family Level—Offspring Colonic Digesta
- Genus Level—Offspring Colonic Digesta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Déru, V.; Bouquet, A.; Zemb, O.; Blanchet, B.; De Almeida, M.L.; Cauquil, L.; Carillier-Jacquin, C.; Gilbert, H. Genetic relationships between efficiency traits and gut microbiota traits in growing pigs being fed with a conventional or a high-fiber diet. J. Anim. Sci. 2022, 100, skac183. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef]
- Argüello, H.; Estellé, J.; Leonard, F.C.; Crispie, F.; Cotter, P.D.; O’sullivan, O.; Lynch, H.; Walia, K.; Duffy, G.; Lawlor, P.G.; et al. Influence of the Intestinal Microbiota on Colonization Resistance to Salmonella and the Shedding Pattern of Naturally Exposed Pigs. mSystems 2019, 4, e00021-19. [Google Scholar] [CrossRef]
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, Á. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porc. Health Manag. 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Van Breda, L.K.; Dhungyel, O.P.; Ginn, A.N.; Iredell, J.R.; Ward, M.P. Pre- and post-weaning scours in southeastern Australia: A survey of 22 commercial pig herds and characterisation of Escherichia coli isolates. PLoS ONE 2017, 12, e0172528. [Google Scholar] [CrossRef]
- Jacobson, M. On the Infectious Causes of Neonatal Piglet Diarrhoea—A Review. Vet. Sci. 2022, 9, 422. [Google Scholar] [CrossRef]
- Thompson, C.L.; Wang, B.; Holmes, A.J. The immediate environment during postnatal development has long-term impact on gut community structure in pigs. ISME J. 2008, 2, 739–748. [Google Scholar] [CrossRef]
- Schmidt, B.; Mulder, I.E.; Musk, C.C.; Aminov, R.I.; Lewis, M.; Stokes, C.R.; Bailey, M.; Prosser, J.I.; Gill, B.P.; Pluske, J.R.; et al. Establishment of normal gut microbiota is compromised under excessive hygiene conditions. PLoS ONE 2011, 6, e28284. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, C.A.; Lewis, M.C.; Berger, B.; Cloarec, O.; Heinzmann, S.S.; Charton, F.; Krause, L.; Levin, N.S.; Duncker, S.; Mercenier, A.; et al. Neonatal environment exerts a sustained influence on the development of the intestinal microbiota and metabolic phenotype. ISME J. 2016, 10, 145–157. [Google Scholar] [CrossRef]
- Sprockett, D.; Fukami, T.; Relman, D.A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 197–205. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.H.J.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef] [PubMed]
- Mulder, I.E.; Schmidt, B.; Lewis, M.; Delday, M.; Stokes, C.R.; Bailey, M.; Aminov, R.I.; Gill, B.P.; Pluske, J.R.; Mayer, C.-D.; et al. Restricting Microbial Exposure in Early Life Negates the Immune Benefits Associated with Gut Colonization in Environments of High Microbial Diversity. PLoS ONE 2011, 6, e28279. [Google Scholar] [CrossRef]
- Arnal, M.-E.; Zhang, J.; Messori, S.; Bosi, P.; Smidt, H.; Lallès, J.-P. Early changes in microbial colonization selectively modulate intestinal enzymes, but not inducible heat shock proteins in young adult Swine. PLoS ONE 2014, 9, e87967. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Wu, X.; Pan, Y.; Wang, L.; Cui, C.; Guo, Y.; Zhu, L.; Peng, J.; Wei, H. Early-Life Intervention Using Fecal Microbiota Combined with Probiotics Promotes Gut Microbiota Maturation, Regulates Immune System Development, and Alleviates Weaning Stress in Piglets. Int. J. Mol. Sci. 2020, 21, 503. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Ren, E.; Su, Y.; Zhu, W. Co-occurrence of early gut colonization in neonatal piglets with microbiota in the maternal and surrounding delivery environments. Anaerobe 2018, 49, 30–40. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Zhang, G.; Hou, C.; Li, N.; Yu, H.; Shang, L.; Zhang, X.; Trevisi, P.; Yang, F.; et al. Maternal Breast Milk and Fecal Microbes Guide the Spatiotemporal Development of Mucosa-Associated Microbiota and Barrier Function in the Neonatal Gut. BMC Biol. 2019, 17, 106. [Google Scholar] [CrossRef]
- Lim, J.-A.; Cha, J.; Choi, S.; Kim, J.-H.; Kim, D. Early Colonization of the Intestinal Microbiome of Neonatal Piglets Is Influenced by the Maternal Microbiome. Animals 2023, 13, 3378. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Ma, L. A Review Focusing on Microbial Vertical Transmission during Sow Pregnancy. Veter- Sci. 2023, 10, 123. [Google Scholar] [CrossRef]
- Adams, S.; Knapp, J.P.; Neujahr, A.; Burkey, T.; Miller, P.S.; Fernando, S.C. 276 Investigating the Colonization History of Early-Life Microbiome of Piglets. J. Anim. Sci. 2023, 101, 165–166. [Google Scholar] [CrossRef]
- Quan, J.; Xu, C.; Ruan, D.; Ye, Y.; Qiu, Y.; Wu, J.; Zhou, S.; Luan, M.; Zhao, X.; Chen, Y.; et al. Composition, function, and timing: Exploring the early-life gut microbiota in piglets for probiotic interventions. J. Anim. Sci. Biotechnol. 2023, 14, 143. [Google Scholar] [CrossRef]
- Kiernan, D.P.; O’Doherty, J.V.; Sweeney, T. The effect of maternal probiotic or synbiotic supplementation on sow and offspring microbiome, health, and performance. Animals 2023, 13, 2996. [Google Scholar] [CrossRef] [PubMed]
- Kritas, S.K.; Marubashi, T.; Filioussis, G.; Petridou, E.; Christodoulopoulos, G.; Burriel, A.R.; Tzivara, A.; Theodoridis, A.; Pískoriková, M. Reproductive performance of sows was improved by administration of a sporing bacillary probiotic (Bacillus subtilis C-3102). J. Anim. Sci. 2015, 93, 405–413. [Google Scholar] [CrossRef]
- Han, L.; Azad, A.K.; Huang, P.; Wang, W.; Zhang, W.; Blachier, F.; Kong, X. Maternal Supplementation with Different Probiotic Mixture from Late Pregnancy to Day 21 Postpartum: Consequences for Litter Size, Plasma and Colostrum Parameters, and Fecal Microbiota and Metabolites in Sows. Front. Vet. Sci. 2022, 9, 726276. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Zhang, D.; Wang, J.; Zhang, W.; Wang, Y.; Ji, H. Effects of dietary supplementation with Pediococcus acidilactici ZPA017 on reproductive performance, fecal microbial flora and serum indices in sows during late gestation and lactation. Asian-Australas. J. Anim. Sci. 2020, 33, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Piazuelo, D.; Gardiner, G.E.; Ranjitkar, S.; Bouwhuis, M.A.; Ham, R.; Phelan, J.P.; Marsh, A.; Lawlor, P.G. Maternal supplementation with Bacillus altitudinis spores improves porcine offspring growth performance and carcass weight. Br. J. Nutr. 2022, 127, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.A.; Davis, E.; Spencer, J.D.; Moser, R.; Rehberger, T. The effect of a Bacillus-based direct-fed microbial supplemented to sows on the gastrointestinal microbiota of their neonatal piglets. J. Anim. Sci. 2013, 91, 3390–3399. [Google Scholar] [CrossRef]
- Larsen, N.; Thorsen, L.; Kpikpi, E.N.; Stuer-Lauridsen, B.; Cantor, M.D.; Nielsen, B.; Brockmann, E.; Derkx, P.M.F.; Jespersen, L. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl. Microbiol. Biotechnol. 2014, 98, 1105–1118. [Google Scholar] [CrossRef]
- Mazur-Kuśnirek, M.; Lipiński, K.; Jørgensen, J.N.; Hansen, L.H.B.; Antoszkiewicz, Z.; Zabielski, R.; Konieczka, P. The Effect of a Bacillus-Based Probiotic on Sow and Piglet Performance in Two Production Cycles. Animals 2023, 13, 3163. [Google Scholar] [CrossRef]
- Barbosa, A.M.S.; Carvalho, M.P.S.; Naves, L.d.P.; da Motta, S.A.B.; Chaves, R.F.; Resende, M.; de Lima, D.; Hansen, L.H.B.; Cantarelli, V.d.S. Performance and Health Parameters of Sows and Their Litters Using a Probiotic Supplement Composed of Bacillus subtilis 541 and Bacillus amyloliquefaciens 516. Animals 2024, 14, 3511. [Google Scholar] [CrossRef]
- Konieczka, P.; Ferenc, K.; Jørgensen, J.N.; Hansen, L.H.; Zabielski, R.; Olszewski, J.; Gajewski, Z.; Mazur-Kuśnirek, M.; Szkopek, D.; Szyryńska, N.; et al. Feeding Bacillus-based probiotics to gestating and lactating sows is an efficient method for improving immunity, gut functional status and biofilm formation by probiotic bacteria in piglets at weaning. Anim. Nutr. 2023, 13, 361–372. [Google Scholar] [CrossRef]
- Saladrigas-García, M.; Solà-Oriol, D.; López-Vergé, S.; D’angelo, M.; Collado, M.C.; Nielsen, B.; Faldyna, M.; Pérez, J.F.; Martín-Orúe, S.M. Potential effect of two Bacillus probiotic strains on performance and fecal microbiota of breeding sows and their piglets. J. Anim. Sci. 2022, 100, skac163. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, D.P.; O’doherty, J.V.; Ryan, M.T.; Sweeney, T. Effects of Maternal Probiotics and Piglet Dietary Tryptophan Level on Gastric Function Pre- and Post-Weaning. Agriculture 2025, 15, 310. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.-H.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. An Insight into the Roles of Dietary Tryptophan and Its Metabolites in Intestinal Inflammation and Inflammatory Bowel Disease. Mol. Nutr. Food Res. 2021, 65, 2000461. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.C.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef]
- Liu, G.; Lu, J.; Sun, W.; Jia, G.; Zhao, H.; Chen, X.; Kim, I.H.; Zhang, R.; Wang, J. Tryptophan supplementation enhances intestinal health by improving gut barrier function, alleviating inflammation, and modulating intestinal microbiome in lipopolysaccharide-challenged piglets. Front. Microbiol. 2022, 13, 919431. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tao, J.; Lu, J.; Jia, G.; Zhao, H.; Chen, X.; Tian, G.; Cai, J.; Zhang, R.; Wang, J. Dietary Tryptophan Supplementation Improves Antioxidant Status and Alleviates Inflammation, Endoplasmic Reticulum Stress, Apoptosis, and Pyroptosis in the Intestine of Piglets after Lipopolysaccharide Challenge. Antioxidants 2022, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Li, Y.; Yan, H.; Zhang, H. L-Tryptophan Enhances Intestinal Integrity in Diquat-Challenged Piglets Associated with Improvement of Redox Status and Mitochondrial Function. Animals 2019, 9, 266. [Google Scholar] [CrossRef]
- Zhang, L.; Nichols, R.G.; Correll, J.; Murray, I.A.; Tanaka, N.; Smith, P.B.; Hubbard, T.D.; Sebastian, A.; Albert, I.; Hatzakis, E.; et al. Persistent organic pollutants modify gut microbiota–host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ. Health Perspect. 2015, 123, 679–688. [Google Scholar] [CrossRef]
- Tossou, M.C.B.; Liu, H.; Bai, M.; Chen, S.; Cai, Y.; Duraipandiyan, V.; Liu, H.; Adebowale, T.O.; Al-Dhabi, N.A.; Long, L.; et al. Effect of High Dietary Tryptophan on Intestinal Morphology and Tight Junction Protein of Weaned Pig. BioMed Res. Int. 2016, 2016, 2912418. [Google Scholar] [CrossRef]
- Beaumont, M.; Roura, E.; Lambert, W.; Turni, C.; Michiels, J.; Chalvon-Demersay, T. Selective nourishing of gut microbiota with amino acids: A novel prebiotic approach? Front. Nutr. 2022, 9, 1066898. [Google Scholar]
- Kiernan, D.P.; O’doherty, J.V.; Sweeney, T. The effect of prebiotic supplements on the gastrointestinal microbiota and associated health parameters in the pig. Animals 2023, 13, 3012. [Google Scholar] [CrossRef]
- Liang, H.; Dai, Z.; Liu, N.; Ji, Y.; Chen, J.; Zhang, Y.; Yang, Y.; Li, J.; Wu, Z.; Wu, G. Dietary L-Tryptophan Modulates the Structural and Functional Composition of the Intestinal Microbiome in Weaned Piglets. Front. Microbiol. 2018, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Li, J.; Shi, B.; Zeng, Y.; Liu, Y.; Sun, Z.; Wu, L.; Sun, W.; Tang, Z. Dietary Tryptophan Levels Impact Growth Performance and Intestinal Microbial Ecology in Weaned Piglets via Tryptophan Metabolites and Intestinal Antimicrobial Peptides. Animals 2021, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, H.; Li, Z.; Qi, M.; Qiu, Y.; Hu, Z.; Feng, F.; Tang, W.; Diao, H.; Sun, W.; et al. Dietary tryptophan improves growth and intestinal health by promoting the secretion of intestinal β-defensins against enterotoxigenic Escherichia coli F4 in weaned piglets. J. Nutr. Biochem. 2024, 129, 109637. [Google Scholar] [CrossRef]
- Sauvant, D.; Perez, J.-M.; Tran, G. Tables of Composition and Nutritional Value of Feed Materials: Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses and Fish; BRILL, Wagening Academic Publishers: Wagening, The Netherlands, 2023. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 2016, e2584. [Google Scholar] [CrossRef]
- Eren, A.M.; Maignien, L.; Sul, W.J.; Murphy, L.G.; Grim, S.L.; Morrison, H.G.; Sogin, M.L. Oligotyping: Differentiating between closely related microbial taxa using 16S rRNA gene data. Methods Ecol. Evol. 2013, 4, 1111–1119. [Google Scholar] [CrossRef]
- Eren, A.M.; Morrison, H.G.; Lescault, P.J.; Reveillaud, J.; Vineis, J.H.; Sogin, M.L. Minimum entropy decomposition: Unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J. 2015, 9, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Angly, F.E.; Dennis, P.G.; Skarshewski, A.; Vanwonterghem, I.; Hugenholtz, P.; Tyson, G.W. CopyRighter: A rapid tool for improving the accuracy of microbial community profiles through lineage-specific gene copy number correction. Microbiome 2014, 2, 11. [Google Scholar] [CrossRef]
- Dowley, A.; Sweeney, T.; Conway, E.; Vigors, S.; Yadav, S.; Wilson, J.; Gabrielli, W.; O’doherty, J.V. Effects of Dietary Supplementation with Mushroom or Vitamin D2-Enriched Mushroom Powders on Gastrointestinal Health Parameters in the Weaned Pig. Animals 2021, 11, 3603. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.C.; Sweeney, T.; Curley, E.; Gath, V.; Duffy, S.K.; Vigors, S.; Rajauria, G.; O’Doherty, J.V. Effect of β-glucanase and β-xylanase enzyme supplemented barley diets on nutrient digestibility, growth performance and expression of intestinal nutrient transporter genes in finisher pigs. Anim. Feed. Sci. Technol. 2018, 238, 98–110. [Google Scholar]
- Dowley, A.; O’doherty, J.V.; Mukhopadhya, A.; Conway, E.; Vigors, S.; Maher, S.; Ryan, M.T.; Sweeney, T. Maternal Supplementation With a Casein Hydrolysate and Yeast Beta-glucan From Late Gestation Through Lactation Improves Gastrointestinal Health of Piglets at Weaning. Sci. Rep. 2022, 12, 17407. [Google Scholar] [CrossRef]
- Hu, Z.; Feng, L.; Jiang, Q.; Wang, W.; Tan, B.; Tang, X.; Yin, Y. Intestinal tryptophan metabolism in disease prevention and swine production. Anim. Nutr. 2023, 15, 364–374. [Google Scholar] [CrossRef]
- Fabà, L.; de Groot, N.; Ramis, G.; Cabrera-Gómez, C.G.; Doelman, J. Serotonin receptors and their association with the immune system in the gastrointestinal tract of weaning piglets. Porc. Health Manag. 2022, 8, 8. [Google Scholar] [CrossRef]
- Ryan, M.T.; O’doherty, J.V.; Sweeney, T. A Transcriptomic Evaluation of Neuroactive Receptors in the Colon of a Dextran Sodium Sulphate Pig Model of Colitis. Nutraceuticals 2024, 4, 395–408. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Harrell, M.F.E., Jr. Package ‘hmisc’. CRAN2018 2019, 2019, 235–236. [Google Scholar]
- Barret Schloerke, D.C.; Joseph, L.; Francois, B.; Moritz, M.; Edwin, T.; Amos, E.; Ott, T.; Jason, C. GGally: Extension to ‘ggplot2’. R Package Version 2.2.1. 2024. Available online: https://archive.softwareheritage.org/browse/directory/16636441710e633dfb96108e0b723390a0740073/?origin_url=https://hal.archives-ouvertes.fr/halshs-03354562&release=HEAD&snapshot=a670af2ebeeeae775b0c9573c9571ffaca8af228 (accessed on 25 May 2025).
- Abe, K.; Ueki, A.; Ohtaki, Y.; Kaku, N.; Watanabe, K.; Ueki, K. Anaerocella delicata gen. nov., sp. nov., a strictly anaerobic bacterium in the phylum Bacteroidetes isolated from a methanogenic reactor of cattle farms. J. Gen. Appl. Microbiol. 2012, 58, 405–412. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Ma, Y.-W.; Feng, S.-X.; Zhang, Y.-X.; Kou, H.-J.; Sun, Y. The synergistic effect of chemical oxidation and microbial activity on improving volatile fatty acids (VFAs) production during the animal wastewater anaerobic digestion process treated with persulfate/biochar. Sci. Total Environ. 2023, 857, 159276. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L.; Blauwiekel, R.; Bunn, J.Y.; Jetton, T.L.; Frankel, W.L.; Holst, J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr. 2007, 137, 916–922. [Google Scholar] [CrossRef]
- Zeng, X.; Sunkara, L.T.; Jiang, W.; Bible, M.; Carter, S.; Ma, X.; Qiao, S.; Zhang, G. Induction of Porcine Host Defense Peptide Gene Expression by Short-Chain Fatty Acids and Their Analogs. PLoS ONE 2013, 8, e72922. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Niu, Q.; Li, P.; Hao, S.; Kim, S.W.; Du, T.; Hua, J.; Huang, R. Characteristics of Gut Microbiota in Sows and Their Relationship with Apparent Nutrient Digestibility. Int. J. Mol. Sci. 2019, 20, 870. [Google Scholar] [CrossRef]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Betancur, C.; Martínez, Y.; Tellez-Isaias, G.; Castillo, R.; Ding, X. Effect of oral administration with Lactobacillus plantarum CAM6 strain on sows during gestation-lactation and the derived impact on their progeny performance. Mediat. Inflamm. 2021, 2021, 6615960. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Ma, Y.; Cai, S.; Yang, L.; Fan, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri improves the development and maturation of fecal microbiota in piglets through mother-to-infant microbe and metabolite vertical transmission. Microbiome 2022, 10, 211. [Google Scholar] [CrossRef]
- Gao, T.; Li, R.; Hu, L.; Hu, Q.; Wen, H.; Zhou, R.; Yuan, P.; Zhang, X.; Huang, L.; Zhuo, Y.; et al. Probiotic Lactobacillus rhamnosus GG improves insulin sensitivity and offspring survival via modulation of gut microbiota and serum metabolite in a sow model. J. Anim. Sci. Biotechnol. 2024, 15, 89. [Google Scholar] [CrossRef]
- Kelly, J.; Daly, K.; Moran, A.W.; Ryan, S.; Bravo, D.; Shirazi-Beechey, S.P. Composition and diversity of mucosa-associated microbiota along the entire length of the pig gastrointestinal tract; dietary influences. Environ. Microbiol. 2017, 19, 1425–1438. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, Y.; Ma, L.; Chen, Q.; Hu, C.; Yang, H.; Hong, Q.; Xiao, Y. Comparative analysis of fecal microbiota between diarrhea and non-diarrhea piglets reveals biomarkers of gut microbiota associated with diarrhea. Anim. Nutr. 2024, 19, 401–410. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, Y.; Zhang, G.; Liu, L.; Lai, C. Relationship between Dietary Fiber Fermentation and Volatile Fatty Acids’ Concentration in Growing Pigs. Animals 2020, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, X.; Zhao, J.; Wang, Z.; Ye, H.; Pi, Y.; Che, D.; Han, D.; Zhang, S.; Wang, J. Sources of dietary fiber affect the SCFA production and absorption in the hindgut of growing pigs. Front. Nutr. 2022, 8, 719935. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Jørgensen, H.; Larsen, T.; Zhao, X.-Q.; Eggum, B.O. The energy value of short-chain fatty acids infused into the caecum of pigs. Br. J. Nutr. 1997, 77, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019, 14, 4. [Google Scholar] [CrossRef]
- Liu, X.-F.; Shao, J.-H.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.-J.; Liu, Z.-Z.; He, D.-D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- La, A.L.T.Z.; Feng, Y.; Hu, D.; Feng, Y.; Jin, X.; Liu, D.; Guo, Y.; Cheng, G.; Hu, Y. Enzymatically prepared alginate oligosaccharides improve broiler chicken growth performance by modulating the gut microbiota and growth hormone signals. J. Anim. Sci. Biotechnol. 2023, 14, 96. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kishino, E.; Inoue, R.; Nakanishi, N.; Nakayama, K.; Ito, T.; Ushida, K. Correlation between villous height and the disaccharidase activity in the small intestine of piglets from nursing to growing. Anim. Sci. J. 2013, 84, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, C.; Wang, Q.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Yang, H.; Yin, Y. The relationship between villous height and growth performance, small intestinal mucosal enzymes activities and nutrient transporters expression in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 606–615. [Google Scholar] [CrossRef]
- Pluske, J.R. Morphological and functional changes in the small intestine of the newly-weaned pig. In Gut Environment of Pigs; Nottingham University Press: Nottingham, UK, 2001; pp. 1–27. ISBN 9781897676776. [Google Scholar]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.-T.; Carlson, E.J.; Melov, S.; Ursell, P.C.; Olson, J.L.; Noble, L.J.; Yoshimura, M.P.; Berger, C.; Chan, P.H.; et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995, 11, 376–381. [Google Scholar] [CrossRef]
- Velarde, M.C.; Flynn, J.M.; Day, N.U.; Melov, S.; Campisi, J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging 2012, 4, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Elahi, S.; Buchanan, R.M.; Attah-Poku, S.; Townsend, H.G.G.; Babiuk, L.A.; Gerdts, V. The Host Defense Peptide Beta-Defensin 1 Confers Protection against Bordetella pertussis in Newborn Piglets. Infect. Immun. 2006, 74, 2338–2352. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning from Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Menon, B.B.; Kaiser-Marko, C.; Spurr-Michaud, S.; Tisdale, A.S.; Gipson, I.K. Suppression of Toll-like receptor-mediated innate immune responses at the ocular surface by the membrane-associated mucins MUC1 and MUC16. Mucosal Immunol. 2015, 8, 1000–1008. [Google Scholar] [CrossRef]
- Layunta, E.; Buey, B.; Mesonero, J.E.; Latorre, E. Crosstalk Between Intestinal Serotonergic System and Pattern Recognition Receptors on the Microbiota–Gut–Brain Axis. Front. Endocrinol. 2021, 12, 748254. [Google Scholar] [CrossRef]
- Liu, Y.D.; Yu, L.; Ying, L.; Balic, J.; Gao, H.; Deng, N.T.; West, A.; Yan, F.; Ji, C.B.; Gough, D.; et al. Toll-like receptor 2 regulates metabolic reprogramming in gastric cancer via superoxide dismutase 2. Int. J. Cancer 2019, 144, 3056–3069. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.L.; Ma, C.; Rosenberger, C.M.; Bergstrom, K.S.B.; Valdez, Y.; Huang, J.T.; Khan, M.A.; Vallance, B.A. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell. Microbiol. 2008, 10, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, E.; Gardiner, G.E.; Chombart, M.; Doherty, J.V.O.; Sweeney, T.; Lawlor, P.G. Effect of creep feeding pelleted starter diet, liquid milk replacer and a liquid mixture of starter diet and milk replacer to suckling pigs on their growth and medication usage. Transl. Anim. Sci. 2024, 8, txae041. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.V.; Kiernan, D.P.; Sweeney, T. Gastrointestinal development in pigs: Implications for nutrition and performance. In Advances in Pig Nutrition; Wiseman, J., Ed.; Burleigh Dodds Science Publishing: Sawston, UK, 2024; ISBN 9781801466943. [Google Scholar]
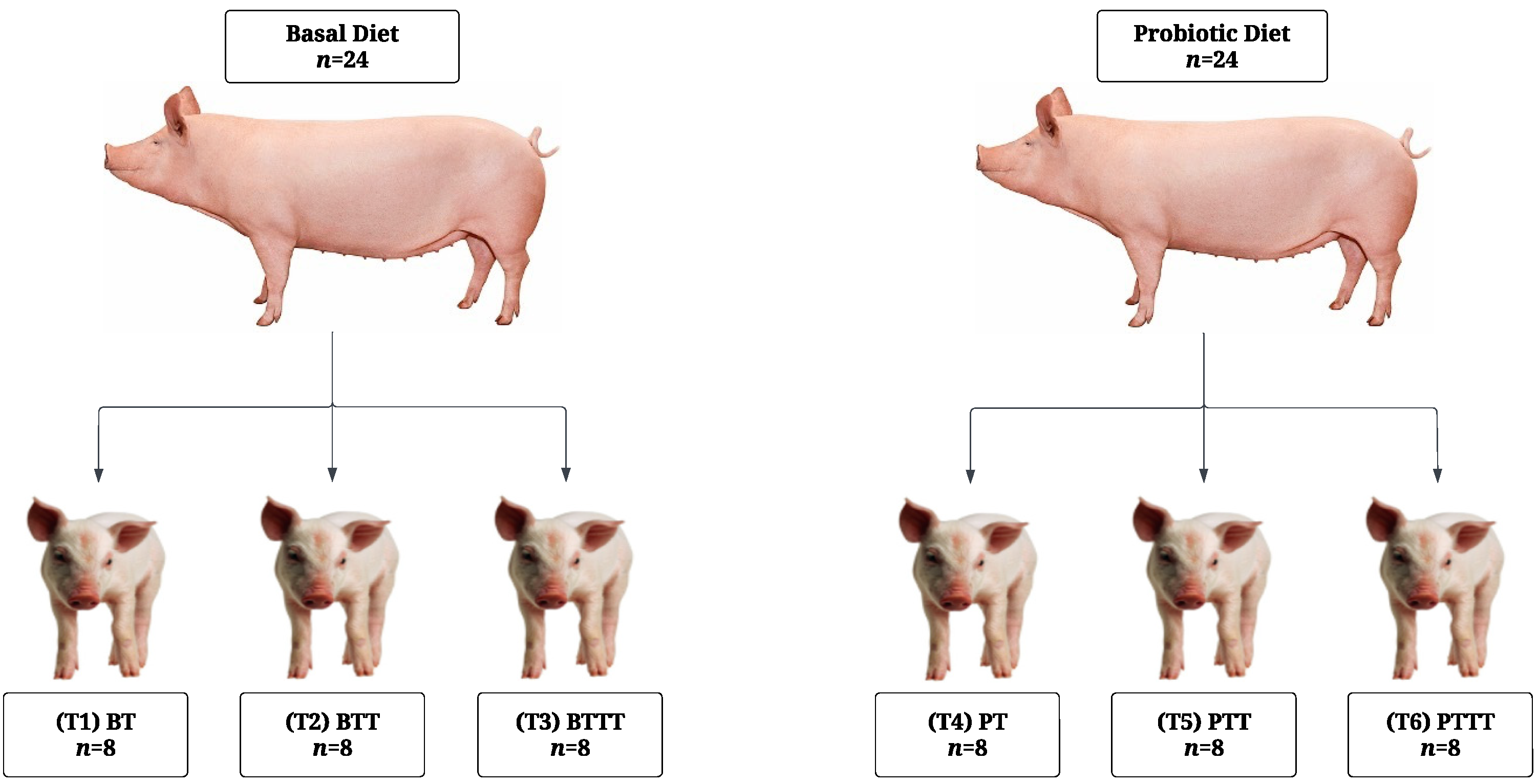


| Ingredients (g/kg) | Gestation Sow Diet a | Lactation Sow Diet a | Creep | ||
|---|---|---|---|---|---|
| 0.22% Trp b | 0.27% Trp b | 0.33% Trp b | |||
| Wheat | - | 380 | 472 | 472 | 472 |
| Barley | 750 | 250 | 100 | 100 | 100 |
| Maize | - | - | 120 | 120 | 120 |
| Soyabean meal | 90 | 170 | - | - | - |
| Soya bean 50 | - | - | 90 | 90 | 90 |
| Full-fat soya | - | 80 | 90 | 90 | 90 |
| Soycomil | - | - | 30 | 30 | 30 |
| Whey protein | - | - | 40 | 40 | 40 |
| Soya oil | 12 | 25 | 30 | 30 | 30 |
| Soya hulls | 120 | 10 | - | - | - |
| Beet pulp | 4 | 10 | - | - | - |
| Pollard | - | 40 | - | - | - |
| Vitamins and mineral premix c | 1.5 | 1.5 | 3 | 3 | 3 |
| Salt | 4 | 5 | 2 | 2 | 2 |
| Monocalcium phosphate | 6 | 8 | 4.2 | 4.2 | 4.2 |
| Limestone | 9 | 12 | 4.5 | 4.5 | 4.5 |
| Lysine-HCL 78.8% | 2.2 | 4 | 5.8 | 5.8 | 5.8 |
| Methionine | 0.6 | 1.3 | 2.5 | 2.5 | 2.5 |
| Threonine | 0.7 | 2.5 | 2.8 | 2.8 | 2.8 |
| Tryptophan | 0 | 0.7 | 0.2 | 0.7 | 1.2 |
| Ingredients (g/kg) | Gestation Sow Diet a | Lactation Sow Diet a | Creep | ||
|---|---|---|---|---|---|
| 0.22% Trp b | 0.27% Trp b | 0.33% Trp b | |||
| Dry matter | 870 | 870 | 900 | 880 | 900 |
| Crude protein (N × 6.25) | 141.5 | 170.3 | 180.5 | 177.0 | 182.5 |
| Gross energy (MJ/kg) | 15.91 | 15.95 | 16.56 | 16.84 | 16.82 |
| Ash | 50.5 | 52.6 | 50 | 60 | 50 |
| Neutral detergent fiber | 240.0 | 135.0 | 145.1 | 135.2 | 141.2 |
| Crude oil | 26.6 | 51.0 | 38.2 | 46.0 | 42.4 |
| Arginine | 9.3 | 11.0 | 10.7 | 11.4 | 10.6 |
| Histidine | 3.5 | 4.1 | 4.3 | 4.3 | 4.2 |
| Isoleucine | 5.1 | 7.2 | 7.4 | 7.8 | 7.5 |
| Leucine | 11.3 | 12.6 | 13.1 | 13.5 | 14.4 |
| Lysine | 7.5 | 11.3 | 13.9 | 14.0 | 14.1 |
| Methionine | 2.5 | 2.5 | 4.8 | 4.5 | 4.5 |
| Phenylalanine | 6.1 | 8.0 | 8.2 | 8.0 | 8.3 |
| Threonine | 5.7 | 6.9 | 8.9 | 8.5 | 9.1 |
| Tryptophan | 1.8 | 2.2 | 2.4 | 2.8 | 3.2 |
| Valine | 6.6 | 7.9 | 9.7 | 8.2 | 9.3 |
| Target Bacteria | Forward and Reverse Primers (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| Escherichia coli | F: CATGCCGCGTGTATGAAGAA R: CGGGTAACGTCAATGAGCAAA | 112 |
| Target Gene | Gene Name | Accession No. | Forward Primer (5′–3′) Reverse Primer (5′–3′) | Amplicon Length (bp) |
|---|---|---|---|---|
| Immune response | ||||
| AHR | Aryl Hydrocarbon Receptor | NM_001303026.1 | F: GCAGCGCCAACATCACCT R: GGGATTGGCTTGACAGTTTTC | 70 |
| DEFB1 | Beta Defensin 1 | NM_214442.2 | F: CCTGCCCGCTCTTCAACA R: GTCAGCGGATGCAGCACTT | 69 |
| DEFB3 | Beta Defensin 3 | XM_021074698.1 | F: GCACGCCTTCCTATCCAGTCT R: GGCAAAGAGAAGGTAGTGGATCCT | 72 |
| IL6 | Interleukin 6 | NM_214399.1 | F: GACAAAGCCACCACCCCTAA R: CTCGTTCTGTGACTGCAGCTTATC | 69 |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 | NM_213867.1 | F: TGCACTTACTCTTGCCAGAACTG R: CAAACTGGCTGTTGCCTTCTT | 82 |
| IL10 | Interleukin 10 | NM_214041.1 | F: GCCTTCGGCCCAGTGAA R: AGAGACCCGGTCAGCAACAA | 71 |
| IL22 | Interleukin 22 | XM_021091968.1 | F: GATGAGAGAGCGCTGCTACCTGG R: GAAGGACGCCACCTCCTGCATGT | 112 |
| TLR2 | Toll Like Receptor 2 | NM_213761.1 | F: CATCTTCGTGCTTTCCGAGAAC R: AAAGAGACGGAAGTGGGAGAAGT | 79 |
| TLR4 | Toll Like Receptor 4 | NM_001293317.1 | F: TGCATGGAGCTGAATTTCTACAA R: GATAAATCCAGCACCTGCAGTTC | 140 |
| TNF | Tumor Necrosis Factor | NM_214022.1 | F: TGGCCCCTTGAGCATCA R: CGGGCTTATCTGAGGTTTGAG | 68 |
| Intestinal barrier | ||||
| CLDN3 | Claudin 3 | NM_001160075.1 | F: GAGGGCCTGTGGATGAACTG R: GAGTCGTACACTTTGCACTGCAT | 65 |
| TJP1 | Tight Junction Protein 1 | XM_021098827.1 | F: TGAGAGCCAACCATGTCTTGAA R: CTCAGACCCGGCTCTCTGTCT | 76 |
| Mucin | ||||
| MUC1 | Mucin 1 | XM_001926883.1 | F: ACACCCATGGGCGCTATGT R: GCCTGCAGAAACCTGCTCAT | 68 |
| MUC2 | Mucin 2 | AK231524 | F: CAACGGCCTCTCCTTCTCTGT R: GCCACACTGGCCCTTTGT | 70 |
| Nutrient transporters | ||||
| Fatty acid transporters | ||||
| FABP2 | Fatty Acid-Binding Protein 2 | NM_001031780.1 | F: CAGCCTCGCAGACGGAACTGAA R: GTGTTCTGGGCTGTGCTCCAAGA | 102 |
| Monosaccharide transporters | ||||
| SLC2A1 (GLUT1) | Solute Carrier Family 2 Member 1 | XM_021098317.1 | F: TGCTCATCAACCGCAATGA R: GTTCCGCGCAGCTTCTTC | 70 |
| SLC2A2 (GLUT2) | Solute Carrier Family 2 Member 2 | NM_001097417.1 | F: CCAGGCCCCATCCCCTGGTT R: GCGGGTCCAGTTGCTGAATGC | 96 |
| Peptide and amino acid transporters | ||||
| SLC15A1 (PEPT1) | Solute Carrier Family 15 Member 1 | NM_214347.1 | F: GGATAGCCTGTACCCCAAGCT R: CATCCTCCACGTGCTTCTTGA | 73 |
| SLC7A5 | Solute Carrier Family 7 Member 5 | XR_002344446.1 | F: CGGTCCTTTGCCAGAAGCT R: CCTTGGCTCCTGCTGCTTAT | 63 |
| SLC7A6 | Solute Carrier Family 7 Member 6 | XM_021094151.1 | F: AGCGCGACAGAGCATCCT R: ACGTGTCTGTTTTGGCCAATT | 66 |
| SLC7A7 | Solute Carrier Family 7 Member 7 | NM_001110421.1 | F: TGATTCATGTTGAGCGGTTCA R: ACAAGTAGATCAGCGCCATGAG | 72 |
| Oxidative status | ||||
| SOD2 | Superoxide Dismutase 2 | NM_214127.2 | F: GCTTGTTCTAACCAGGATCCC R: TAATACGCATGCTCCCACAC | 83 |
| NOX1 | NAPDH Oxidase 1 | XM_003484140.3 | F: AGCCATGCTGAGATCCCAAT R: TGCTTTATGGCAGGCTTTCA | 68 |
| Serotonin receptor | ||||
| HTR4 | 5-Hydroxytryptamine Receptor 4 | NM_001001267.1 | F: TGAGCGCTACCGAAGACCTT R: TTGACGGTTGTGGTTGAACAG | 63 |
| Reference Genes | ||||
| ACTB | Beta Actin | XM_001927228.1 | F: GGACATCGGATACCCAAGGA R: AAGTTGGAAGGCCGGTTAATTT | 71 |
| HMBS | Hydroxymethylbilane Synthase | NM_001097412.1 | F: CTGAACAAAGGTGCCAAGAACA R: GCCCCGCAGACCAGTTAGT | 74 |
| H375A | H3.3 histone A (H3-3A) | NM_213930.1 | F: CATGGCTCGTACAAAGCAGA R: ACCAGGCCTGTAACGATGAG | 136 |
| YWHAZ | Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein Zeta | NM_001315726.1 | F: GGACATCGGATACCCAAGGA R: AAGTTGGAAGGCCGGTTAATTT | 71 |
| Maternal Diet | Control | Probiotic | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Creep Diet | 0.22% Trp | 0.27% Trp | 0.33% Trp | 0.22% Trp | 0.27% Trp | 0.33% Trp | M | C | M × C | |
| Litter size (n) | ||||||||||
| Born alive | 13.88 | 14.13 | 14.50 | 15.63 | 14.38 | 15.25 | 1.00 | 0.269 | 0.805 | 0.749 |
| Stillborn | 1.63 | 1.38 | 0.63 | 0.63 | 0.63 | 1.88 | 0.49 | 0.678 | 0.878 | 0.052 |
| Post-cross fostering | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 | 0.00 | 1.000 | 1.000 | 1.000 |
| D7 | 13.38 | 13.25 | 13.13 | 13.38 | 13.13 | 13.25 | 0.35 | 0.607 | 0.980 | 0.159 |
| D21 | 13.00 | 12.50 | 12.38 | 12.20 | 12.88 | 13.25 | 0.38 | 0.578 | 0.831 | 0.049 |
| D26 (weaning) | 13.00 | 12.00 | 12.00 | 12.00 | 12.75 | 13.25 | 0.48 | 0.399 | 0.873 | 0.058 |
| Piglet mortality% | 7.14 | 14.29 | 14.29 | 14.29 | 8.93 | 5.36 | 3.42 | 0.398 | 0.873 | 0.058 |
| Piglet weight (kg) | ||||||||||
| Birth | 1.53 | 1.44 | 1.43 | 1.45 | 1.59 | 1.48 | 0.06 | 0.431 | 0.643 | 0.215 |
| D7 | 2.70 | 2.58 | 2.35 | 2.50 | 2.57 | 2.80 | 0.16 | 0.607 | 0.980 | 0.159 |
| D21 | 6.10 | 6.17 | 5.63 | 5.90 | 6.69 | 6.07 | 0.31 | 0.343 | 0.196 | 0.477 |
| D26 (weaning) | 7.37 | 7.48 | 6.93 | 7.58 | 7.41 | 7.16 | 0.38 | 0.710 | 0.486 | 0.931 |
| Weaned litter weight (kg) | 95.30 | 86.90 | 82.96 | 91.64 | 91.63 | 94.75 | 4.67 | 0.301 | 0.581 | 0.279 |
| Piglet daily gain (kg) | ||||||||||
| D0–7 | 0.17 | 0.16 | 0.13 | 0.14 | 0.14 | 0.19 | 0.02 | 0.929 | 0.917 | 0.075 |
| D7–21 | 0.24 | 0.26 | 0.24 | 0.25 | 0.28 | 0.23 | 0.02 | 0.578 | 0.273 | 0.884 |
| D21–26 | 0.25 | 0.26 | 0.26 | 0.23 | 0.28 | 0.22 | 0.01 | 0.349 | 0.191 | 0.173 |
| D0–26 | 0.22 | 0.23 | 0.21 | 0.24 | 0.24 | 0.22 | 0.02 | 0.527 | 0.423 | 0.982 |
| Total creep intake per litter (kg) | 1.02 | 0.62 | 0.58 | 0.93 | 1.17 | 0.84 | 0.24 | 0.227 | 0.536 | 0.422 |
| Sow backfat loss (mm) | −7.06 | −6.00 | −5.13 | −6.94 | −3.06 | −3.94 | 1.20 | 0.156 | 0.071 | 0.503 |
| Sow lactation feed intake (kg/day) | 8.44 | 8.26 | 8.22 | 7.66 | 8.18 | 7.82 | 0.29 | 0.078 | 0.761 | 0.483 |
| Gestation length (days) | 117.38 | 117.38 | 118.50 | 117.13 | 117.25 | 117.00 | 0.54 | 0.164 | 0.605 | 0.380 |
| Lactation length (days) | 26.38 | 26.25 | 25.63 | 25.63 | 25.13 | 26.88 | 0.62 | 0.685 | 0.668 | 0.136 |
| Weaning to service interval (days) | 4.81 | 4.52 | 4.92 | 4.50 | 5.00 | 4.58 | 0.22 | 0.812 | 0.846 | 0.119 |
| Maternal | SEM | Creep | SEM | p-Value * | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Probiotic | 0.22% Trp | 0.27% Trp | 0.33% Trp | M | C | |||
| VH (μm) | 389.63 | 450.04 | 20.81 | 419.68 | 443.27 | 396.56 | 25.78 | 0.047 | 0.435 |
| CD (μm) | 138.80 | 110.62 | 5.45 | 126.62 | 125.28 | 122.23 | 6.75 | 0.001 | 0.891 |
| VH/CD | 2.89 | 4.17 | 0.22 | 3.50 | 3.72 | 3.36 | 0.28 | <0.001 | 0.624 |
| Maternal Diet | Control | Probiotic | SEM | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Creep Diet | 0.22% Trp | 0.27% Trp | 0.33% Trp | 0.22% Trp | 0.27% Trp | 0.33% Trp | M | C | M × C | ||
| Role | Gene | ||||||||||
| Immune response | AHR | 1.10 | 1.49 | 1.32 | 1.36 | 1.11 | 1.27 | 0.15 | 0.641 | 0.869 | 0.127 |
| DEFB1 | 0.74 | 0.90 | 0.63 | 0.80 | 1.18 | 0.84 | 0.19 | 0.247 | 0.229 | 0.841 | |
| DEFB3 | 1.27 | 1.62 | 1.34 | 1.25 | 1.59 | 1.63 | 0.23 | 0.677 | 0.336 | 0.748 | |
| IL6 | 1.11 | 1.08 | 1.08 | 1.10 | 1.04 | 1.84 | 0.21 | 0.168 | 0.125 | 0.103 | |
| CXCL8 | 1.02 | 0.76 | 1.10 | 0.76 | 0.82 | 1.11 | 0.18 | 0.669 | 0.239 | 0.645 | |
| IL10 | 1.37 | 1.09 | 1.07 | 1.01 | 0.88 | 1.76 | 0.18 | 0.788 | 0.078 | 0.012 | |
| IL22 | 1.06 | 0.58 | 0.72 | 0.98 | 1.35 | 1.80 | 0.43 | 0.112 | 0.783 | 0.405 | |
| TLR2 | 1.32 | 0.99 | 1.23 | 0.94 | 1.08 | 1.47 | 0.18 | 0.912 | 0.205 | 0.200 | |
| TLR4 | 2.06 | 1.90 | 2.18 | 2.04 | 1.98 | 2.21 | 0.24 | 0.883 | 0.594 | 0.977 | |
| TNF | 1.15 | 1.27 | 1.20 | 1.24 | 1.31 | 1.46 | 0.19 | 0.405 | 0.783 | 0.835 | |
| Intestinal barrier | CLDN3 | 1.10 | 1.23 | 1.11 | 1.09 | 1.12 | 1.09 | 0.10 | 0.582 | 0.662 | 0.845 |
| TJP1 | 1.02 | 1.24 | 1.14 | 1.14 | 0.96 | 1.05 | 0.11 | 0.367 | 0.978 | 0.183 | |
| Mucin | MUC1 | 0.71 | 1.21 | 1.04 | 0.76 | 0.70 | 0.92 | 0.13 | 0.073 | 0.117 | 0.146 |
| MUC2 | 1.15 | 1.12 | 1.06 | 0.88 | 1.05 | 1.10 | 0.21 | 0.561 | 0.926 | 0.753 | |
| Nutrient transporters | FABP2 | 1.62 | 1.80 | 1.31 | 1.47 | 2.17 | 1.33 | 0.49 | 0.846 | 0.407 | 0.870 |
| SLC2A1 | 1.06 | 1.44 | 0.93 | 0.87 | 1.04 | 1.00 | 0.17 | 0.222 | 0.202 | 0.400 | |
| SLC2A2 | 1.37 | 1.81 | 1.50 | 1.57 | 1.39 | 1.41 | 0.17 | 0.484 | 0.669 | 0.209 | |
| SLC15A1 | 1.10 | 1.22 | 1.07 | 1.27 | 1.02 | 1.17 | 0.17 | 0.866 | 0.909 | 0.556 | |
| SLC7A5 | 0.67 | 0.71 | 0.69 | 0.67 | 0.69 | 0.94 | 0.10 | 0.339 | 0.311 | 0.334 | |
| SLC7A6 | 0.79 | 0.90 | 0.86 | 0.97 | 0.84 | 1.04 | 0.11 | 0.285 | 0.715 | 0.470 | |
| SLC7A7 | 1.59 | 1.80 | 1.55 | 1.60 | 1.70 | 1.75 | 0.20 | 0.641 | 0.869 | 0.127 | |
| Oxidative status | SOD2 | 0.93 | 1.06 | 1.21 | 1.24 | 1.31 | 1.60 | 0.13 | 0.247 | 0.229 | 0.841 |
| NOX1 | 0.96 | 0.76 | 0.68 | 0.70 | 0.92 | 1.26 | 0.21 | 0.677 | 0.336 | 0.748 | |
| Serotonin receptor | HTR4 | 1.02 | 1.83 | 1.16 | 1.12 | 1.05 | 1.20 | 0.23 | 0.168 | 0.125 | 0.103 |
| Maternal Diet | Control | Probiotic | SEM | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Creep Diet | 0.22% Trp | 0.27% Trp | 0.33% Trp | 0.22% Trp | 0.27% Trp | 0.33% Trp | M | C | M x C | ||
| Role | Gene | ||||||||||
| Immune response | AHR | 1.14 | 1.23 | 1.03 | 0.93 | 0.99 | 1.13 | 0.08 | 0.054 | 0.586 | 0.054 |
| DEFB1 | 1.02 | 0.89 | 0.81 | 0.70 | 0.69 | 0.83 | 0.12 | 0.045 | 0.759 | 0.217 | |
| DEFB3 | 0.93 | 1.16 | 1.00 | 0.94 | 1.08 | 0.94 | 0.09 | 0.578 | 0.127 | 0.899 | |
| IL6 | 1.31 | 1.63 | 1.22 | 1.27 | 1.45 | 1.45 | 0.18 | 0.980 | 0.386 | 0.540 | |
| CXCL8 | 0.79 | 0.74 | 0.88 | 0.63 | 0.53 | 0.70 | 0.16 | 0.149 | 0.617 | 0.984 | |
| IL10 | 1.38 | 1.46 | 1.09 | 0.90 | 1.05 | 1.41 | 0.18 | 0.185 | 0.738 | 0.044 | |
| IL22 | 1.03 | 0.89 | 0.79 | 0.90 | 0.68 | 1.19 | 0.20 | 0.904 | 0.554 | 0.288 | |
| TLR2 | 0.98 | 0.92 | 1.03 | 0.70 | 0.69 | 0.70 | 0.11 | 0.003 | 0.850 | 0.887 | |
| TLR4 | 0.90 | 1.13 | 1.12 | 1.01 | 0.92 | 1.11 | 0.16 | 0.760 | 0.662 | 0.622 | |
| TNF | 1.43 | 1.35 | 1.13 | 1.25 | 1.61 | 1.81 | 0.34 | 0.381 | 0.899 | 0.459 | |
| Intestinal barrier | CLDN3 | 0.95 | 0.88 | 1.15 | 0.61 | 0.46 | 0.67 | 0.17 | 0.003 | 0.343 | 0.920 |
| TJP1 | 0.95 | 0.98 | 1.01 | 0.91 | 1.00 | 0.99 | 0.05 | 0.774 | 0.266 | 0.803 | |
| Mucin | MUC1 | 0.97 | 0.84 | 0.78 | 0.61 | 0.56 | 0.61 | 0.10 | 0.003 | 0.598 | 0.685 |
| MUC2 | 0.84 | 0.85 | 0.63 | 0.45 | 0.61 | 0.96 | 0.17 | 0.490 | 0.692 | 0.100 | |
| Nutrient transporters | SLC7A5 | 1.13 | 1.27 | 0.89 | 1.12 | 1.43 | 1.56 | 0.14 | 0.025 | 0.321 | 0.055 |
| SLC7A6 | 1.06 | 1.25 | 0.97 | 0.98 | 1.09 | 1.12 | 0.07 | 0.648 | 0.125 | 0.128 | |
| SLC7A7 | 1.06 | 0.93 | 1.13 | 0.76 | 0.62 | 0.66 | 0.19 | 0.031 | 0.760 | 0.893 | |
| Oxidative status | SOD2 | 0.97 | 0.94 | 0.86 | 0.73 | 0.67 | 0.77 | 0.08 | 0.007 | 0.855 | 0.571 |
| NOX1 | 1.27 | 1.33 | 0.93 | 0.93 | 0.99 | 1.17 | 0.11 | 0.108 | 0.636 | 0.016 | |
| Serotonin receptor | HTR4 | 0.79 | 1.00 | 0.97 | 0.77 | 0.64 | 0.60 | 0.15 | 0.050 | 0.956 | 0.460 |
| Maternal Diet | SEM | Creep Diet | SEM | p-Value * | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Probiotic | 0.22% Trp | 0.27% Trp | 0.33% Trp | Maternal | Creep | |||
| Cecum (mmol/g digesta) | |||||||||
| Total VFA | 176.22 | 187.71 | 23.98 | 173.28 | 186.15 | 186.49 | 28.05 | 0.708 | 0.926 |
| Acetate | 93.28 | 122.50 | 16.18 | 104.34 | 105.55 | 112.46 | 18.92 | 0.152 | 0.938 |
| Propionate | 47.15 | 42.64 | 7.03 | 38.92 | 51.47 | 44.31 | 8.23 | 0.616 | 0.537 |
| Butyrate | 18.67 | 15.41 | 2.05 | 17.36 | 17.44 | 16.33 | 2.40 | 0.217 | 0.922 |
| Iso-butyrate | 5.71 | 1.77 | 0.76 | 3.69 | 3.51 | 4.02 | 0.89 | <0.001 | 0.901 |
| Iso-valerate | 6.63 | 3.17 | 0.72 | 4.54 | 4.67 | 5.49 | 0.84 | 0.001 | 0.642 |
| Valerate | 5.67 | 2.22 | 0.54 | 4.44 | 3.52 | 3.88 | 0.59 | <0.001 | 0.569 |
| Branch-chain fatty acids a | 18.01 | 7.16 | 1.51 | 12.67 | 11.69 | 13.39 | 1.76 | <0.001 | 0.756 |
| Colon (mmol/g digesta) | |||||||||
| Total VFA | 104.15 | 151.19 | 14.00 | 155.32 | 115.38 | 112.31 | 17.85 | 0.018 | 0.155 |
| Acetate | 55.37 | 89.60 | 8.63 | 88.95 | 47.71 | 60.79 | 10.79 | 0.005 | 0.151 |
| Propionate | 27.34 | 33.40 | 3.38 | 37.46 | 27.83 | 25.82 | 4.31 | 0.193 | 0.117 |
| Butyrate | 10.58 | 11.27 | 1.23 | 13.70 | 9.26 | 9.80 | 1.57 | 0.679 | 0.089 |
| Iso-butyrate | 2.87 | 3.30 | 0.31 | 3.59 | 2.78 | 2.88 | 0.40 | 0.309 | 0.271 |
| Iso-valerate | 3.53 | 4.32 | 0.40 | 4.54 | 3.45 | 3.79 | 0.51 | 0.138 | 0.265 |
| Valerate | 4.18 | 5.05 | 0.57 | 6.09 x | 3.79 y | 3.96 y | 0.71 | 0.260 | 0.041 |
| Branch-chain fatty acids a | 10.61 | 12.38 | 1.23 | 13.32 | 10.55 | 10.63 | 1.60 | 0.283 | 0.344 |
| Control | Probiotic | SEM | p-Value | |
|---|---|---|---|---|
| Observed | 70.09 | 71.83 | 6.22 | 0.842 |
| Chao1 | 70.09 | 71.83 | 6.22 | 0.842 |
| Shannon | 3.71 | 3.67 | 0.08 | 0.730 |
| Simpson | 0.96 | 0.95 | 0.01 | 0.146 |
| InvSimpson | 27.76 | 23.44 | 2.28 | 0.187 |
| Fisher | 12.02 | 12.20 | 1.35 | 0.926 |
| Control | Probiotic | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Phylum | |||||||
| Bacteroidetes * | 27.11 | 33.34 | 1.67 | 0.011 | |||
| Actinobacteria * | 1.22 | 0.44 | 0.32 | 0.050 | |||
| Phylum | Family | ||||||
| Bacteroidetes * | Rikenellaceae | 17.55 | 22.50 | 1.37 | 0.013 | ||
| Firmicutes * | Lactobacillaceae | 6.24 | 3.13 | 0.72 | 0.002 | ||
| Firmicutes * | Oscillospiraceae | 5.06 | 3.42 | 0.65 | 0.066 | ||
| Firmicutes * | Christensenellaceae | 3.04 | 1.63 | 0.50 | 0.037 | ||
| Firmicutes * | Hungateiclostridiaceae | 1.64 | 0.56 | 0.37 | 0.025 | ||
| Phylum | Family | Genus | |||||
| Bacteroidetes * | Rikenellaceae | Anaerocella | 14.14 | 17.98 | 1.22 | 0.028 | |
| Firmicutes * | Ruminococcaceae | Sporobacter | 7.16 | 9.97 | 0.91 | 0.029 | |
| Firmicutes * | Lactobacillaceae | Lactobacillus | 6.28 | 3.09 | 0.72 | 0.002 | |
| Firmicutes * | Oscillospiraceae | Oscillibacter | 5.04 | 3.34 | 0.07 | 0.056 | |
| Firmicutes * | Ruminococcaceae | Ruminococcus | 4.66 | 2.91 | 0.62 | 0.039 | |
| Firmicutes * | Christensenellaceae | Christensenella | 2.94 | 1.60 | 0.50 | 0.043 | |
| Firmicutes * | Hungateiclostridiaceae | Ruminiclostridium | 1.05 | 0.43 | 0.32 | 0.100 | |
| Firmicutes * | Lachnospiraceae | Coprococcus | 0.08 | 0.61 | 0.23 | 0.073 | |
| Firmicutes * | Lachnospiraceae | Eisenbergiella | 0.57 | 0.08 | 0.22 | 0.087 | |
| Control | Probiotic | SEM | p-Value | |
|---|---|---|---|---|
| Observed | 80.00 | 73.25 | 7.54 | 0.537 |
| Chao1 | 80.00 | 73.25 | 7.54 | 0.537 |
| Shannon | 3.89 | 3.81 | 0.10 | 0.558 |
| Simpson | 0.97 | 0.97 | 0.00 | 0.944 |
| InvSimpson | 34.95 | 32.08 | 3.77 | 0.598 |
| Fisher | 12.63 | 11.35 | 1.44 | 0.539 |
| Control | Probiotic | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Phylum | |||||||
| Firmicutes * | 66.12 | 76.96 | 3.10 | 0.023 | |||
| Bacteroidetes * | 21.51 | 13.36 | 1.64 | 0.002 | |||
| Proteobacteria | 6.27 | 2.81 | 0.89 | 0.007 | |||
| Phylum | Family | ||||||
| Firmicutes * | Lachnospiraceae | 13.99 | 18.57 | 1.53 | 0.040 | ||
| Proteobacteria * | Enterobacteriaceae | 6.21 | 2.94 | 0.88 | 0.010 | ||
| Firmicutes * | Oscillospiraceae | 4.82 | 2.82 | 0.78 | 0.062 | ||
| Firmicutes * | Hungateiclostridiaceae | 2.34 | 4.86 | 0.78 | 0.021 | ||
| Actinobacteria * | Coriobacteriaceae | 0.81 | 1.96 | 0.50 | 0.079 | ||
| Bacteroidetes * | Muribaculaceae | 2.20 | 0.41 | 0.52 | 0.014 | ||
| Phylum | Family | Genus | |||||
| Firmicutes * | Lachnospiraceae | Dorea | 2.95 | 5.60 | 0.84 | 0.025 | |
| Firmicutes * | Oscillospiraceae | Oscillibacter | 4.80 | 2.73 | 0.77 | 0.054 | |
| Firmicutes * | Ruminococcaceae | Sporobacter | 1.53 | 3.17 | 0.44 | 0.050 | |
| Firmicutes * | Hungateiclostridiaceae | Anaerobacterium | 0.65 | 4.02 | 0.71 | 0.002 | |
| Firmicutes * | Ruminococcaceae | Papillibacter | 1.28 | 2.70 | 0.58 | 0.070 | |
| Firmicutes * | Ruminococcaceae | Ruminococcus | 2.65 | 0.61 | 0.58 | 0.011 | |
| Bacteroidetes * | Prevotellaceae | Prevotellamassilia | 2.88 | 0.22 | 0.60 | 0.005 | |
| Actinobacteria * | Coriobacteriaceae | Colllinsella | 0.91 | 1.99 | 0.50 | 0.100 | |
| Firmicutes * | Ruminococcaceae | Faecalibacterium | 2.25 | 0.44 | 0.53 | 0.014 | |
| Proteobacteria * | Enterobacteriaceae | Escherichia | 0.83 | 0.08 | 0.31 | 0.091 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiernan, D.P.; O’Doherty, J.V.; Ryan, M.T.; Sweeney, T. Effect of Maternal Probiotic and Piglet Dietary Tryptophan Level on Performance and Piglet Intestinal Health Parameters Pre-Weaning. Microorganisms 2025, 13, 1264. https://doi.org/10.3390/microorganisms13061264
Kiernan DP, O’Doherty JV, Ryan MT, Sweeney T. Effect of Maternal Probiotic and Piglet Dietary Tryptophan Level on Performance and Piglet Intestinal Health Parameters Pre-Weaning. Microorganisms. 2025; 13(6):1264. https://doi.org/10.3390/microorganisms13061264
Chicago/Turabian StyleKiernan, Dillon P., John V. O’Doherty, Marion T. Ryan, and Torres Sweeney. 2025. "Effect of Maternal Probiotic and Piglet Dietary Tryptophan Level on Performance and Piglet Intestinal Health Parameters Pre-Weaning" Microorganisms 13, no. 6: 1264. https://doi.org/10.3390/microorganisms13061264
APA StyleKiernan, D. P., O’Doherty, J. V., Ryan, M. T., & Sweeney, T. (2025). Effect of Maternal Probiotic and Piglet Dietary Tryptophan Level on Performance and Piglet Intestinal Health Parameters Pre-Weaning. Microorganisms, 13(6), 1264. https://doi.org/10.3390/microorganisms13061264






