Actinobacteria Emerge as Novel Dominant Soil Bacterial Taxa in Long-Term Post-Fire Recovery of Taiga Forests
Abstract
1. Introduction
2. Materials and Methods
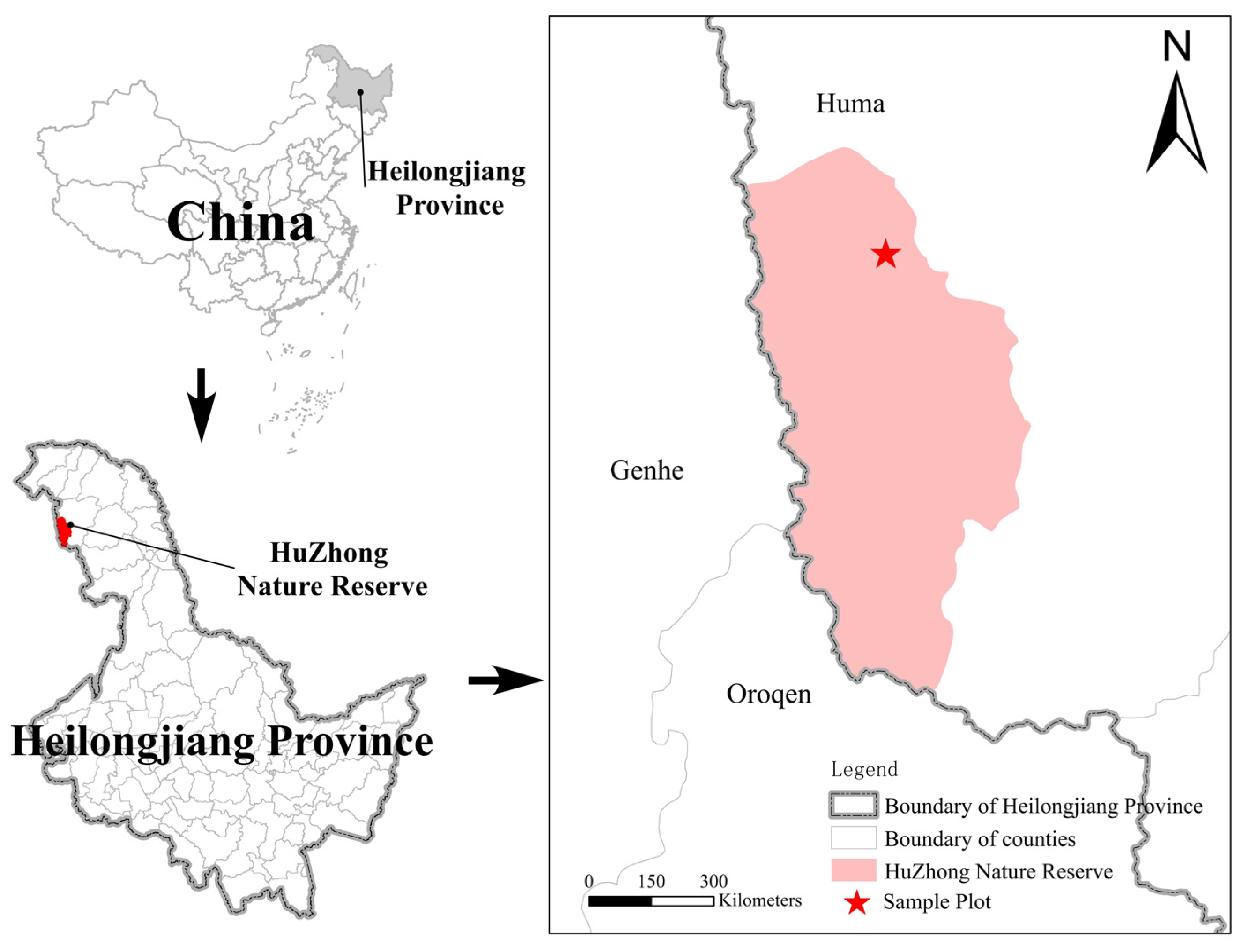
2.1. Study Area
2.2. Sample Plots and Sample Collection
2.3. Determination of Soil Physicochemical Properties and Enzyme Activities
2.4. Extraction and Sequencing of Bacterial DNA
2.5. Data Analysis
3. Results
3.1. Changes and Differences in Soil Physicochemical Properties and Enzyme Activities After Long-Term Restoration of Burned Forest Land
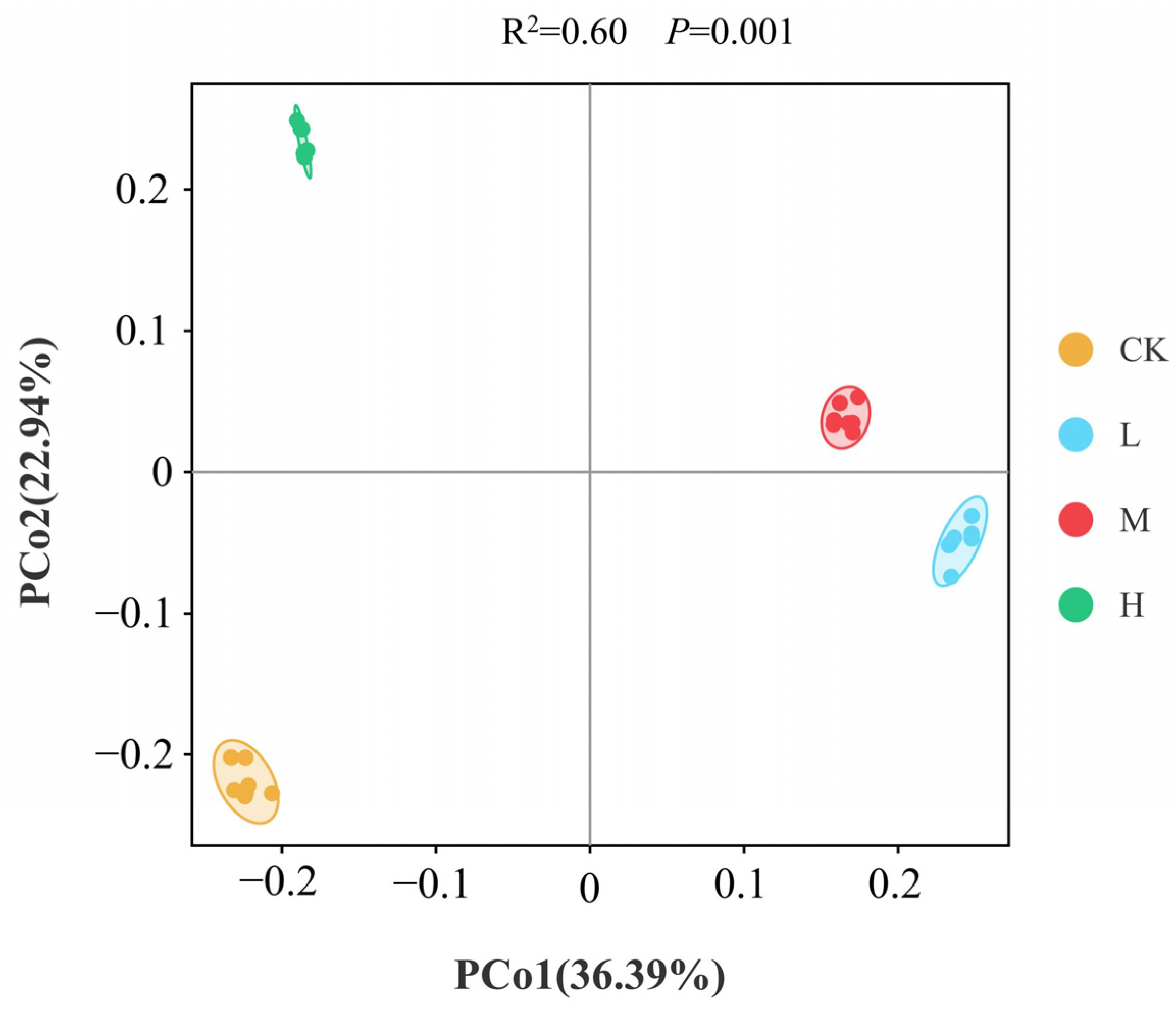
3.2. Characterizing the RESToration of Soil Microbial Community Diversity
3.3. Microbial Community Composition and Restoration Characteristics of Community Types
3.4. Differences Correlation Analysis
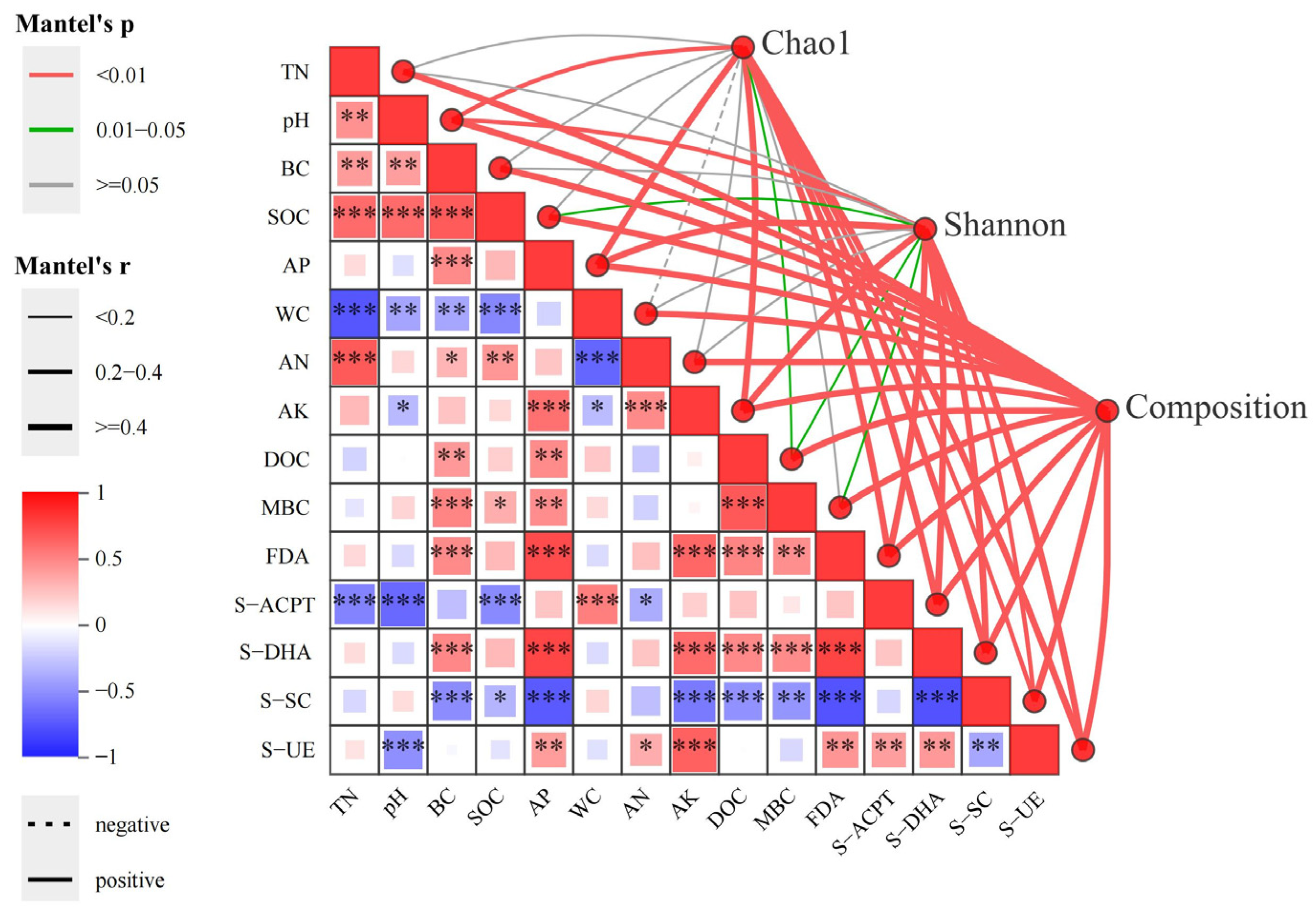
3.4.1. Microbial Community Composition and Diversity Correlated with Soil Physicochemical Properties and Enzyme Activities
3.4.2. Species Composition Correlates with Soil Physicochemical Properties and Enzyme Activities
3.5. Co-Occurrence Network Analysis of Soil Microbial Communities After Long-Term Restoration of Burned Forests
4. Discussion
4.1. Effects on Soil Physicochemical Properties and Enzyme Activities After Long-Term Restoration of Burned Forest Land
4.2. Impacts on Soil Bacterial Diversity After Long-Term Restoration of Burned Forest Land
4.3. Relationship Between Soil Microbial Community Characteristics and Soil Physicochemical Properties and Enzyme Activities
4.4. Co-Occurrence Network Analysis of Soil Microbial Communities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansoor, S.; Farooq, I.; Kachroo, M.M.; Mahmoud, A.E.D.; Fawzy, M.; Popescu, S.M.; Alyemeni, M.; Sonne, C.; Rinklebe, J.; Ahmad, P. Elevation in wildfire frequencies with respect to the climate change. J. Environ. Manag. 2022, 301, 113769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, L.Z.; Li, H.R.; Wei, C.L.; Zhao, H.K.; Jie, X. Grass-Larix gmelinii Forest in Permafrost Region of Cold Temperate Zone Community Characteristics of Natural Regeneration After Fire. J. Temp. For. Res. 2021, 4, 25–31. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Martínez-Hidalgo, P.; Cobo-Díaz, J.F.; Villadas, P.J.; Martínez-Molina, E.; Toro, N.; Tringe, S.G.; Fernández-López, M. The rhizosphere microbiome of burned holm-oak: Potential role of the genus Arthrobacter in the recovery of burned soils. Sci. Rep. 2017, 7, 6008. [Google Scholar] [CrossRef]
- Fultz, L.M.; Moore-Kucera, J.; Dathe, J.; Davinic, M.; Perry, G.; Wester, D.; Schwilk, D.W.; Rideout-Hanzak, S. Forest wildfire and grassland prescribed fire effects on soil biogeochemical processes and microbial communities: Two case studies in the semi-arid Southwest. Appl. Soil Ecol. 2016, 99, 118–128. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Wang, L.H.; Xin, Y.; Zhao, Y.S.; Hou, D.Z.; Sun, T.; Guan, Y.J. Soil Microbial Biomass and Enzyme Activity in the Process of Vegetation Restoration in Burned Area of Great Xing’an Mountains. J. Soil Water Conserv. 2015, 29, 184–189. [Google Scholar] [CrossRef]
- You, Y.; Wang, J.; Huang, X.; Tang, Z.; Liu, S.; Sun, O.J. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 2014, 4, 633–647. [Google Scholar] [CrossRef]
- Dooley, S.R.; Treseder, K.K. The effect of fire on microbial biomass: A meta-analysis of field studies. Biogeochemistry 2012, 109, 49–61. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Putten, W.H.V.D. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Heijden, M.G.A.V.D.; Bardgett, R.D.; Straalen, N.M.V. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lv, X.; Ma, B.; Chen, N.; Chang, S.X.; Lin, J.; Wang, X.; Su, W.; Liu, H.; Huang, Y.; et al. Concurrent and rapid recovery of bacteria and protist communities in Canadian boreal forest ecosystems following wildfire. Soil Biol. Biochem. 2021, 163, 108452. [Google Scholar] [CrossRef]
- Sun, H.; Santalahti, M.; Pumpanen, J.; Koster, K.; Berninger, F.; Raffaello, T.; Asiegbu, F.O.; Heinonsalo, J. Bacterial community structure and function shift across a northern boreal forest fire chronosequence. Sci. Rep. 2016, 6, 32411. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.C.; Deluca, T.H.; Newman, G.S.; Mackenzie, M.D.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Pressler, Y.; Moore, J.C.; Cotrufo, M.F. Belowground community responses to fire: Meta-analysis reveals contrasting responses of soil microorganisms and mesofauna. Oikos 2019, 128, 309–327. [Google Scholar] [CrossRef]
- Whitman, T.; Woolet, J.; Sikora, M.; Johnson, D.B.; Whitman, E. Resilience in soil bacterial communities of the boreal forest from one to five years after wildfire across a severity gradient. Soil Biol. Biochem. 2022, 172, 108755. [Google Scholar] [CrossRef]
- Xiong, Y.; D’atri, J.J.; Fu, S.; Xia, H.; Seastedt, T.R. Rapid soil organic matter loss from forest dieback in a subalpine coniferous ecosystem. Soil Biol. Biochem. 2011, 43, 2450–2456. [Google Scholar] [CrossRef]
- Walker, X.J.; Rogers, B.M.; Baltzer, J.L.; Cumming, S.G.; Day, N.J.; Goetz, S.J.; Johnstone, J.F.; Schuur, E.A.G.; Turetsky, M.R.; Mack, M.C. Cross-scale controls on carbon emissions from boreal forest megafires. Glob. Chang. Biol. 2018, 24, 4251–4265. [Google Scholar] [CrossRef]
- Mack, M.C.; Walker, X.J.; Johnstone, J.F.; Alexander, H.D.; Melvin, A.M.; Jean, M.; Miller, S.N. Carbon loss from boreal forest wildfires offset by increased dominance of deciduous trees. Science 2021, 372, 280–283. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Wu, S.; Zhou, T. Warming changes the composition and diversity of fungal communities in permafrost. Ann. Microbiol. 2023, 73, 7. [Google Scholar] [CrossRef]
- Su, W.Q.; Tang, C.; Lin, J.; Yu, M.; Dai, Z.; Luo, Y.; Li, Y.; Xu, J. Recovery patterns of soil bacterial and fungal communities in Chinese boreal forests along a fire chronosequence. Sci. Total Environ. 2022, 805, 150372. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.R.; Gutierrez, A.; Treseder, K.K. Changes in Soil Fungal Communities, Extracellular Enzyme Activities, and Litter Decomposition Across a Fire Chronosequence in Alaskan Boreal Forests. Ecosystems 2013, 16, 34–46. [Google Scholar] [CrossRef]
- Zhong, C.; Guo, M.; Zhou, F.; Li, J.; Yu, F.; Guo, F.; Li, W. Forest succession trajectories after fires in valleys and on slopes in the Greater Khingan Mountains, China. J. For. Res. 2023, 34, 623–640. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, S.; Du, J.; Liu, Y.; Sui, X.; Yang, L. Reduced Arbuscular Mycorrhizal Fungi (AMF) Diversity in Light and Moderate Fire Sites in Taiga Forests, Northeast China. Microorganisms 2023, 11, 1836. [Google Scholar] [CrossRef]
- Hou, G.; Shi, P.; Zhou, T.; Sun, J.; Zong, N.; Song, M.; Zhang, X. Dominant species play a leading role in shaping community stability in the northern Tibetan grasslands. J. Plant Ecol. 2023, 16, rtac110. [Google Scholar] [CrossRef]
- Wear, E.K.; Wilbanks, E.G.; Nelson, C.E.; Carlson, C.A. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ. Microbiol. 2018, 20, 2709–2726. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Jung, J.Y.; Yergeau, E.; Hwang, C.Y.; Hinzman, L.; Nam, S.; Hong, S.G.; Kim, O.-S.; Chun, J.; Lee, Y.K. Bacterial community structure and soil properties of a subarctic tundra soil in Council, Alaska. FEMS Microbiol. Ecol. 2014, 89, 465–475. [Google Scholar] [CrossRef]
- Shang, W.; Wu, X.; Zhao, L.; Yue, G.; Zhao, Y.; Qiao, Y.; Li, Y. Seasonal variations in labile soil organic matter fractions in permafrost soils with different vegetation types in the central Qinghai–Tibet Plateau. Catena 2016, 137, 670–678. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Q.; Li, D.; Cheng, G.; Mu, J.; Wu, Q.; Niu, F.; An, L.; Feng, H. Diversity and community structure of fungi through a permafrost core profile from the Qinghai-Tibet Plateau of China. J. Basic Microbiol. 2014, 54, 1331–1341. [Google Scholar] [CrossRef]
- Ade, L.J.; Hu, L.; Zi, H.B.; Wang, C.T.; Lerdau, M.; Dong, S.K. Effect of snowpack on the soil bacteria of alpine meadows in the Qinghai-Tibetan Plateau of China. Catena 2018, 164, 13–22. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Frankeberger, W.T.; Johanson, J.B. Method of measuring invertase activity in soils. Plant Soil 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil Dehydrogenase Activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, 10–1128. [Google Scholar] [CrossRef]
- Chen, W.; Pan, Y.; Yu, L.; Yang, J.; Zhang, W. Patterns and Processes in Marine Microeukaryotic Community Biogeography from Xiamen Coastal Waters and Intertidal Sediments, Southeast China. Front. Microbiol. 2017, 8, 1912. [Google Scholar] [CrossRef]
- Dai, T.; Wen, D.; Bates, C.T.; Wu, L.; Guo, X.; Liu, S.; Su, Y.; Lei, J.; Zhou, J.; Yang, Y. Nutrient supply controls the linkage between species abundance and ecological interactions in marine bacterial communities. Nat. Commun. 2022, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Certini, G. Effects of Fire on Properties of Forest Soils: A Review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Wu, Z.; Shen, Y.; Cai, Y. Effect of annual prescribed burning of wetlands on soil organic carbon fractions: A 5-year study in Poyang, China. Ecol. Eng. 2019, 138, 219–226. [Google Scholar] [CrossRef]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Soil organic carbon and total nitrogen stocks under different land uses in a semi-arid watershed in Tigray, Northern Ethiopia. Agric. Ecosyst. Environ. 2014, 188, 256–263. [Google Scholar] [CrossRef]
- Wang, C.; Morrissey, E.M.; Mau, R.L.; Hayer, M.; Pieiro, J.; Mack, M.C.; Marks, J.C.; Bell, S.L.; Miller, S.N.; Schwartz, E.; et al. The temperature sensitivity of soil: Microbial biodiversity, growth, and carbon mineralization. ISME J. 2021, 15, 2738–2747. [Google Scholar] [CrossRef]
- Ellingson, L.; Kauffman, J.; Cummings, D.; Sanford, R.; Jaramillo, V. Soil N dynamics associated with deforestation, biomass burning, and pasture conversion in a Mexican tropical dry forest. For. Ecol. Manag. 2000, 137, 41–51. [Google Scholar] [CrossRef]
- Kara, O.; Bolat, I. Short-term effects of wildfire on microbial biomass and abundance in black pine plantation soils in Turkey. Ecol. Indic. 2009, 9, 1151–1155. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Nix, B.; Jacobs, K.A.; Bowles, M.L. Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern, USA oak (Quercus) forest. Geoderma 2012, 183, 80–91. [Google Scholar] [CrossRef]
- Li, W.K.; Liu, X.D.; Niu, S.K.; Li, B.Y.; Liu, G.H.; Chu, Y.Q. Impact of fire on soil microbial biomass of Pinus tabuliformis forest in Pingquan County, Hebei of northern China. J. Beijing For. Univ. 2017, 39, 70–77. [Google Scholar] [CrossRef]
- Moya, D.; Vega, S.G.-D.; Lozano, E.; García-Orenes, F.; Mataix-Solera, J.; Lucas-Borja, M.; Heras, J.d.L. The burn severity and plant recovery relationship affect the biological and chemical soil properties of Pinus halepensis Mill. stands in the short and mid-terms after wildfire. J. Environ. Manag. 2019, 235, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ulery, A.; Graham, R.; Amrhein, C. Wood-ash composition and soil pH following intense burning. Soil Sci. 1993, 156, 358–364. [Google Scholar] [CrossRef]
- Wang, W.X.; Luo, M.; Pan, C.D. Microorganisms and their biological activity in rhizospheric soil around desert plants at the lower reaches of Tarim River, Xinjiang, China. J. Desert Res. 2010, 30, 571–576. [Google Scholar]
- Lan, J.J.; Liu, Y.H. Species Composition and Diversity of Larix gmelinii Community in Burned Area. J. Northeast. For. Univ. 2022, 50, 22–27. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, L.J.; Zhang, Y.L.; Wu, Z.J. Research advances in catalytic kinetics of soil oxidoreductase. Yingyong Shengtai Xuebao 2005, 16, 371. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.M.; Zheng, R.; Zhou, M.; TaI, T.Y. Studies on soil microbial biomass and enzyme activities of burned areas in great hinggan mountains. J. Inn. Mong. Norm. Univ. (Nat. Sci.) 2016, 37, 77–83. [Google Scholar] [CrossRef]
- Wang, L.; Yao, T.; Guo, C.X.; Han, F.G.; Wang, F.L.; Sun, T.; Zhang, Y.H. Research Progress of Soil Microbiology. Agric. Sci. Technol. 2015, 11, 2367. [Google Scholar]
- Ma, X.; Geng, Q.; Zhang, H.; Bian, C.; Chen, H.Y.H.; Jiang, D.; Xu, X. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytol. 2021, 229, 2957–2969. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Zhu, W.; Wang, Z.; Wu, L.; Du, A. Effects of enrichmemt planting with native tree species on bacterial community structure and potential impact on Eucalyptus plantations in southern China. J. For. Res. 2022, 33, 1349–1363. [Google Scholar] [CrossRef]
- O’Rourke, S.M.; Angers, D.A.; Holden, N.M.; McBratney, A.B. Soil organic carbon across scales. Glob. Change Biol. 2015, 21, 3561–3574. [Google Scholar] [CrossRef]
- Kong, C.C.; Zhang, S.W.; Wang, W.R.; Yan, F.; Song, X.X.; Guo, D.D. Characteristics of soil organic carbon and bacterial communities functional in agricultural lands of facilities with different continuous cropping periods. Trans. Chin. Soc. Agric. Mach. 2024, 55, 326–337. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Jones, S.E.; Caporaso, J.G.; Handelsman, J.; Knight, R.; Fierer, N.; Gilbert, J.A. Conditionally rare taxa disproportionately contribute to temporal changes in microbial diversity. mBio 2014, 5, e01371-14. [Google Scholar] [CrossRef]
- Mouillot, D.; Bellwood, D.R.; Baraloto, C.; Chave, J.; Galzin, R.; Harmelin-Vivien, M.; Kulbicki, M.; Lavergne, S.; Lavorel, S.; Mouquet, N.; et al. Rare Species Support Vulnerable Functions in High-Diversity Ecosystems. PLoS Biol. 2013, 11, e1001569. [Google Scholar] [CrossRef]
- Hillerislambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking Community Assembly through the Lens of Coexistence Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 227–248. [Google Scholar] [CrossRef]
- Yang, N.; Ji, L.; Yang, Y.; Yang, L. The influence of tree species on soil properties and microbial communities following afforestation of abandoned land in northeast China. Eur. J. Soil Biol. 2018, 85, 73–78. [Google Scholar] [CrossRef]
- Hartmann, M.; Lee, S.; Hallam, S.J.; Mohn, W.W. Bacterial, archaeal and eukaryal community structures throughout soil horizons of harvested and naturally disturbed forest stands. Environ. Microbiol. 2009, 11, 3045–3062. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.; Yan, W.; Zhong, Y.; Shangguan, Z. Changes in soil microbial community structure during long-term secondary succession. Land Degrad. Dev. 2020, 31, 1151–1166. [Google Scholar] [CrossRef]
- Dong, X.; Liu, C.; Ma, D.; Wu, Y.; Man, H.; Wu, X.; Li, M.; Zang, S. Organic carbon mineralization and bacterial community of active layer soils responses to short-term warming in the Great Hing’an Mountains of Northeast China. Front. Microbiol. 2021, 12, 802213. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhu, Z.J.; Qian, X.Q.; Wang, G.L. Effects of year-round rice-wheat straw return on soil bacterial community structure in paddy fields. Soil Fertil. Sci. China 2022, 161, 108393. [Google Scholar] [CrossRef]
- Morrissey, E.M.; Mau, R.L.; Schwartz, E.; A McHugh, T.; Dijkstra, P.; Koch, B.J.; Marks, J.C.; Hungate, B.A. Bacterial carbon use plasticity, phylogenetic diversity and the priming of soil organic matter. ISME J. 2017, 11, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yin, Y.; Zhu, W.; Zhou, Y. Variations in Soil Bacterial Community Diversity and Structures Among Different Revegetation Types in the Baishilazi Nature Reserve. Front. Microbiol. 2018, 9, 2874. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Bailey, M.; Whiteley, A.S. The bacterial biogeography of British soils. Environ. Microbiol. 2011, 13, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef]
- Shen, C.; Shi, Y.; Fan, K.; He, J.-S.; Adams, J.M.; Ge, Y.; Chu, H. Soil pH dominates elevational diversity pattern for bacteria in high elevation alkaline soils on the Tibetan Plateau. FEMS Microbiol. Ecol. 2019, 95, fiz003. [Google Scholar] [CrossRef]
- Ivarsson, E.; Roos, S.; Liu, H.; Lindberg, J. Fermentable non-starch polysaccharides increases the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Wubs, E.R.J.; Melchers, P.D.; Bezemer, T.M. Potential for synergy in soil inoculation for nature restoration by mixing inocula from different successional stages. Plant Soil 2018, 433, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, J.; Chen, X.; Meng, Z.; Xu, R.; Duoji, D.; Zhang, J.; He, J.; Wang, Z.; Chen, J.; et al. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Mo, Y.; Peng, F.; Gao, X.; Xiao, P.; Logares, R.; Jeppesen, E.; Ren, K.; Xue, Y.; Yang, J. Low shifts in salinity determined assembly processes and network stability of microeukaryotic plankton communities in a subtropical urban reservoir. Microbiome 2021, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Sun, Y.; Wang, C. Influence of the cold bottom water on taxonomic and functional composition and complexity of microbial communities in the southern Yellow Sea during the summer. Sci. Total Environ. 2020, 759, 143496. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Hartmann, M.; Howes, C.G.; Vaninsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Henrik Nilsson, R.; Hallam, S.J.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Fu, Y.; Jeewani, P.H.; Tang, C.; Pan, S.; Reid, B.J.; Gunina, A.; Li, Y.; Li, Y.; Cai, Y.; et al. Organic matter chemistry and bacterial community structure regulate decomposition processes in post-fire forest soils. Soil Biol. Biochem. 2021, 160, 108311. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]




| Recovery Stage | Time Scale | Character |
|---|---|---|
| Short Term | 0–5 years | Sharp decline in bacterial diversity, outbreaks of actinomycetes, carbon cycle dysfunction |
| Medium Term | 5–20 years | Re-establishment of nitrogen-fixing bacterial communities and gradual recovery of bacterial mutualistic networks |
| Long Term | >20 years | Microbial diversity stabilizes, but functional genes still lag behind |
| Fire Intensity | Flame Height | Victimization of Standing Timber | Severity |
|---|---|---|---|
| Low fire (L) | ≤1.5 m | ≤30% | Burned bark and stems, tree with green leaf cover |
| Moderate fire (M) | 1.5–3 m | 30–70% | Charred stems, trees still have green leaf cover |
| High fire (H) | ≥3 m | ≥70% | Burned canopy, no green leaf cover |
| Index | CK | L | M | H |
|---|---|---|---|---|
| TN g/kg | 1.65 ± 0.03 d | 3.99 ± 0.06 b | 4.47 ± 0.10 a | 3.22 ± 0.02 c |
| pH | 4.31 ± 0.01 c | 4.78 ± 0.00 a | 4.38 ± 0.01 b | 4.20 ± 0.01 d |
| BC g/kg | 7.07 ± 0.40 c | 51.39 ± 0.88 a | 33.97 ± 0.91 b | 34.12 ± 1.77 b |
| SOC g/kg | 12.00 ± 0.28 d | 78.81 ± 1.21 a | 52.02 ± 0.94 b | 48.02 ± 0.78 c |
| AP mg/kg | 20.46 ± 0.17 d | 39.66 ± 0.24 b | 29.35 ± 0.31 c | 59.63 ± 0.09 a |
| WC % | 41.14 ± 1.46 a | 29.77 ± 1.51 c | 18.77 ± 0.76 d | 32.20 ± 3.10 b |
| AN mg/kg | 50.93 ± 0.52 c | 104.43 ± 2.60 b | 143.82 ± 2.34 a | 108.12 ± 7.02 b |
| DOC mg/kg | 145.95 ± 3.76 c | 282.34 ± 10.79 a | 125.49 ± 2.57 d | 273.30 ± 5.24 b |
| AK mg/kg | 172.44 ± 2.41 d | 267.83 ± 3.09 c | 276.83 ± 2.63 b | 444.96 ± 3.39 a |
| MBC mg/kg | 368.84 ± 6.65 c | 579.24 ± 13.49 a | 351.59 ± 12.36 d | 493.66 ± 7.00 b |
| FDA μmol/d | 1.20 ± 0.06 c | 3.93 ± 0.22 b | 1.41 ± 0.09 c | 13.03 ± 0.32 a |
| S-ACPT mg/d | 1.57 ± 0.10 b | 1.22 ± 0.07 c | 1.20 ± 0.10 c | 3.39 ± 0.25 a |
| S-DHA μg/d | 40.55 ± 1.25 d | 100.53 ± 6.11 b | 62.4 ± 4.06 c | 160.26 ± 7.20 a |
| S-SC mg/d | 61.54 ± 2.98 a | 44.71 ± 2.10 c | 54.15 ± 0.99 b | 39.85 ± 1.94 d |
| S-UE μg/d | 168.67 ± 1.75 c | 168.58 ± 9.55 c | 302.39 ± 15.65 b | 1088.76 ± 17.18 a |
| Index | CK | L | M | H |
|---|---|---|---|---|
| AAT | 10 (0.28%) | 7 (0.16%) | 9 (0.31%) | 7 (0.18%) |
| CAT | 6 (0.17%) | 11 (0.26%) | 13 (0.45%) | 9 (0.23%) |
| MT | 296 (8.27%) | 301 (7.08%) | 271 (9.48%) | 354 (8.92%) |
| CRAT | 0 (0.00%) | 2 (0.05%) | 0 (0.00%) | 0 (0.00%) |
| CRT | 1731 (48.37%) | 2311 (54.35%) | 1208 (42.24%) | 1904 (47.96%) |
| ART | 1536 (42.92%) | 1620 (38.10%) | 1359 (47.52%) | 1696 (42.72%) |
| DT | 16 (0.45%) | 20 (0.47%) | 22 (0.77%) | 16 (0.40%) |
| RT | 3267(91.28%) | 3931 (92.45%) | 2567 (89.76%) | 3600 (90.68%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Qu, H.; Cheng, Z.; Fu, X.; Yang, L.; Zhou, J. Actinobacteria Emerge as Novel Dominant Soil Bacterial Taxa in Long-Term Post-Fire Recovery of Taiga Forests. Microorganisms 2025, 13, 1262. https://doi.org/10.3390/microorganisms13061262
Jiang S, Qu H, Cheng Z, Fu X, Yang L, Zhou J. Actinobacteria Emerge as Novel Dominant Soil Bacterial Taxa in Long-Term Post-Fire Recovery of Taiga Forests. Microorganisms. 2025; 13(6):1262. https://doi.org/10.3390/microorganisms13061262
Chicago/Turabian StyleJiang, Siyu, Huijiao Qu, Zhichao Cheng, Xiaoyu Fu, Libin Yang, and Jia Zhou. 2025. "Actinobacteria Emerge as Novel Dominant Soil Bacterial Taxa in Long-Term Post-Fire Recovery of Taiga Forests" Microorganisms 13, no. 6: 1262. https://doi.org/10.3390/microorganisms13061262
APA StyleJiang, S., Qu, H., Cheng, Z., Fu, X., Yang, L., & Zhou, J. (2025). Actinobacteria Emerge as Novel Dominant Soil Bacterial Taxa in Long-Term Post-Fire Recovery of Taiga Forests. Microorganisms, 13(6), 1262. https://doi.org/10.3390/microorganisms13061262






