Description of Four New Trypanosoma Species Infecting Small Wild Mammals from Two Brazilian Biomes: The Pantanal and Cerrado Hotspots
Abstract
1. Introduction
2. Materials and Methods
2.1. Trypanosome Isolates
2.2. Trypanosoma Isolation and Culture in Mammalian and Insect Cells
2.3. Morphological Characterization
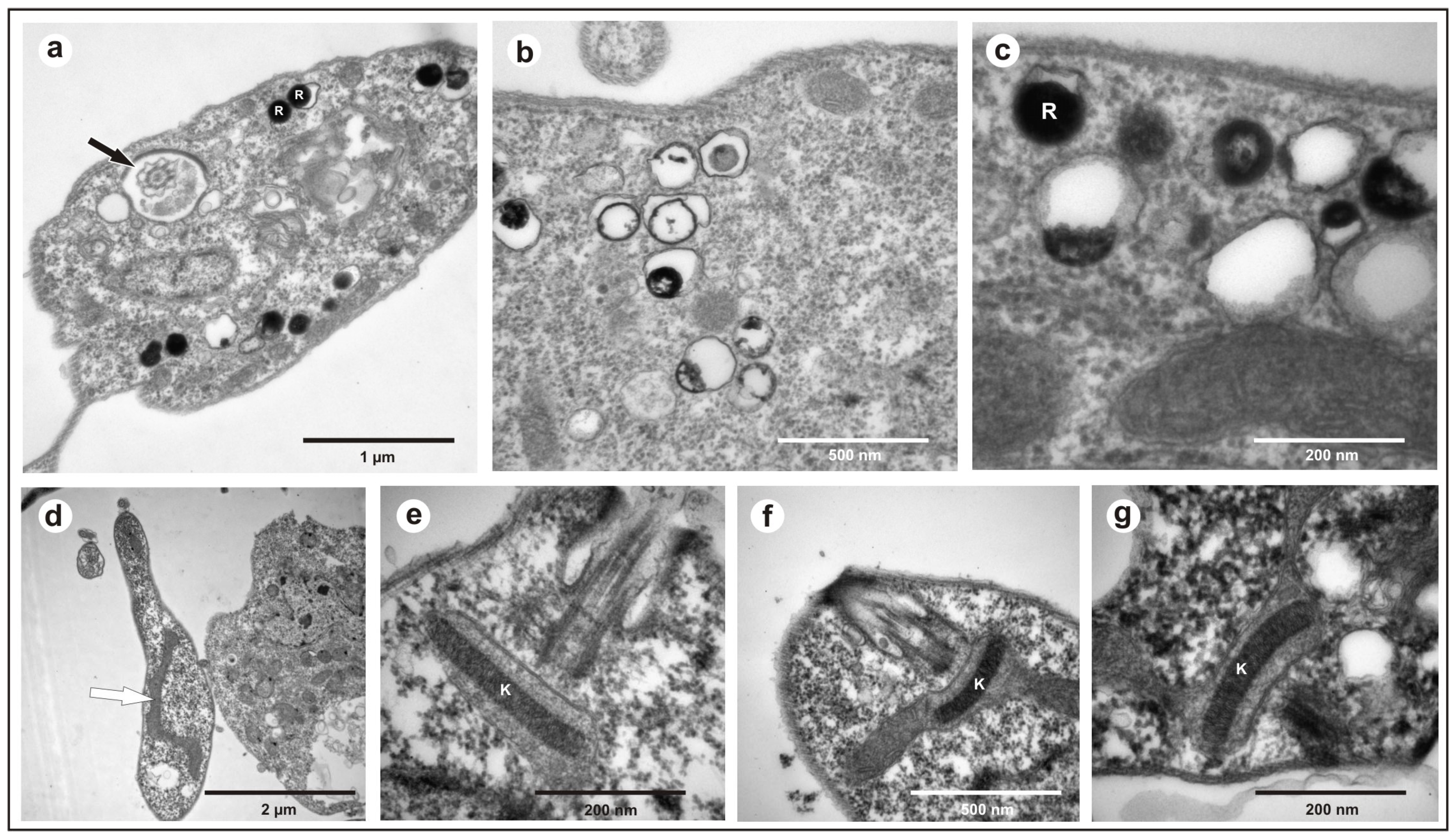
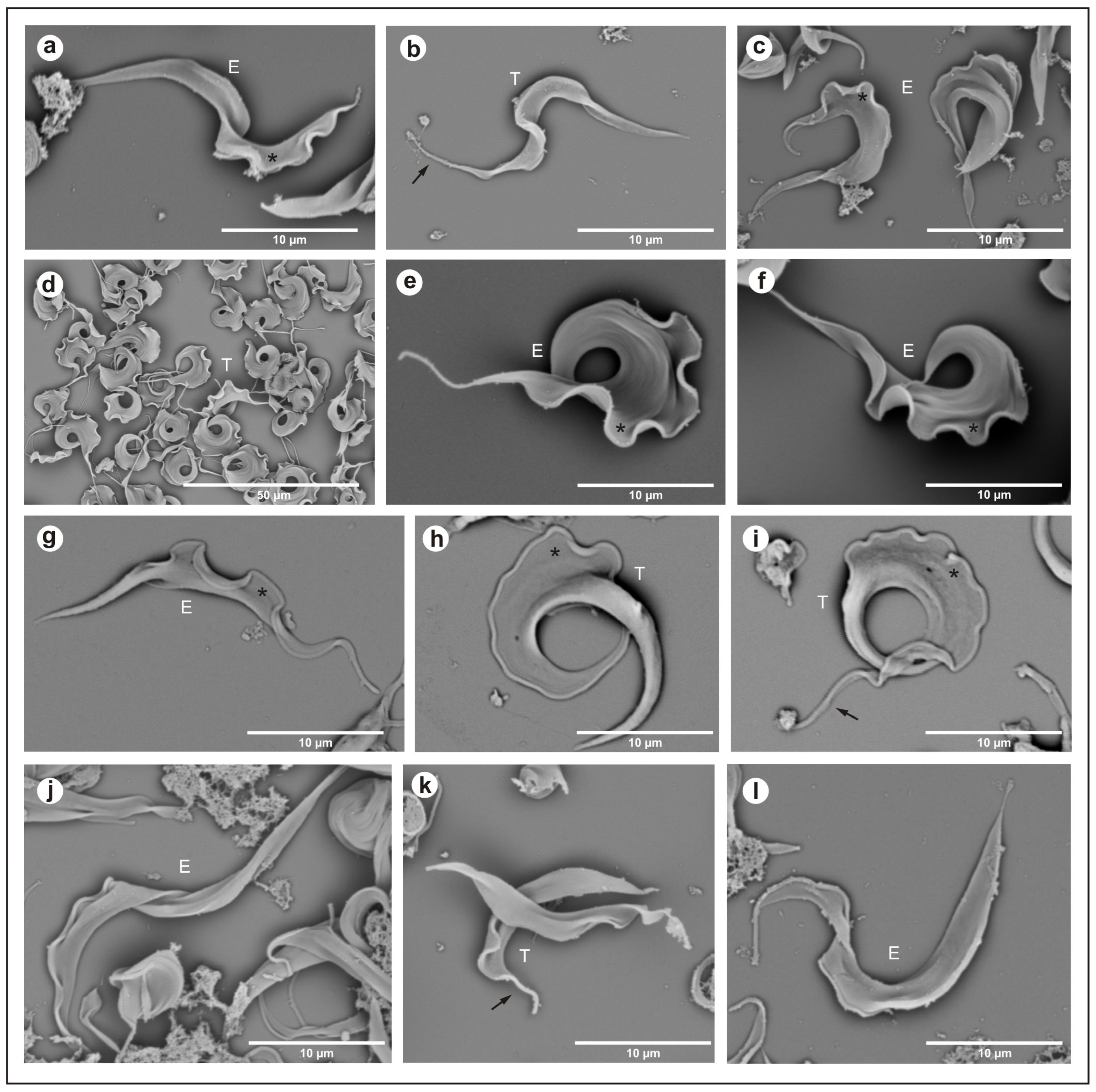
2.3.1. Scanning Electron Microscopy
2.3.2. Transmission Electron Microscopy
2.4. Molecular and Phylogenetic Analysis
3. Results
3.1. Taxonomy of Trypanosoma
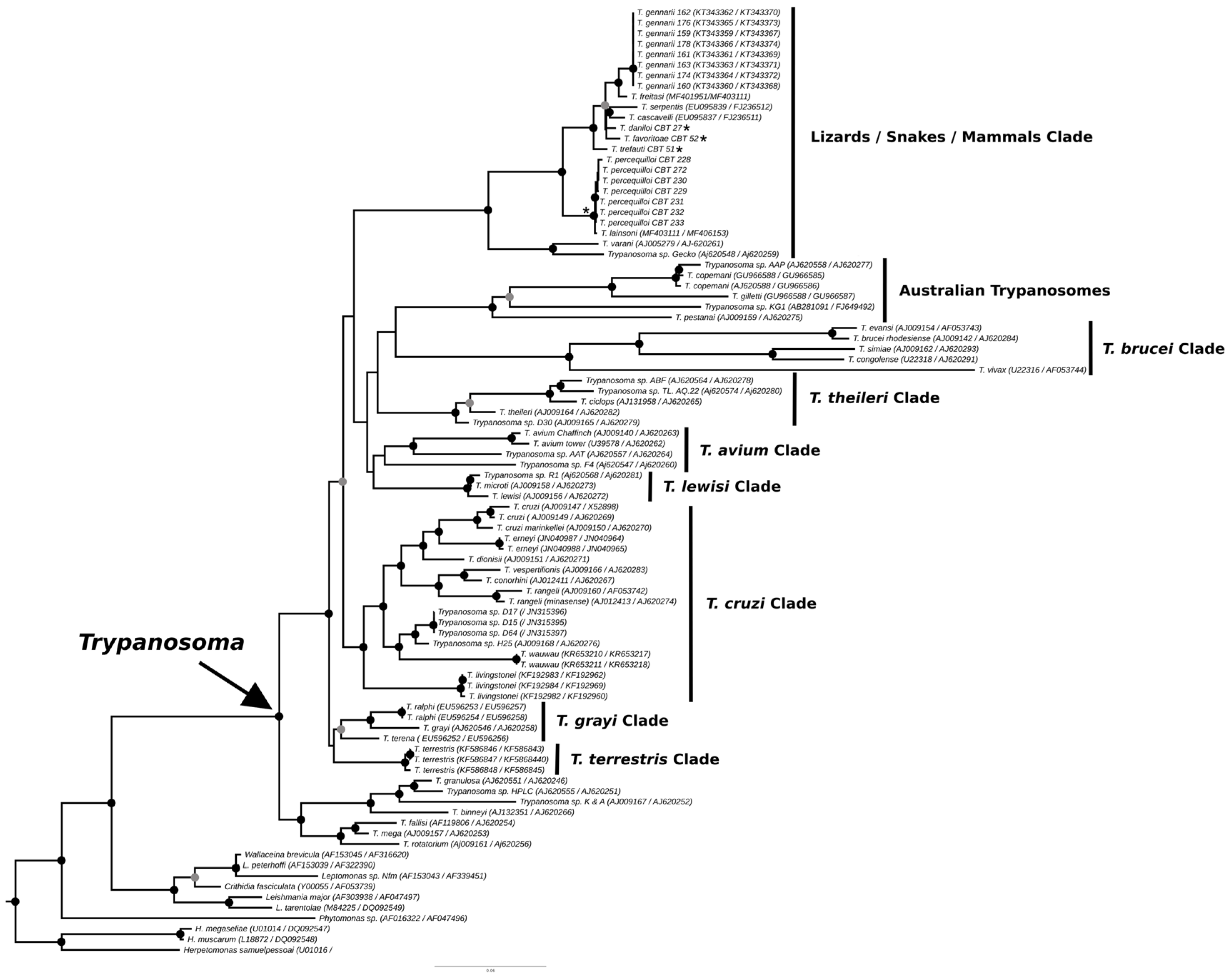
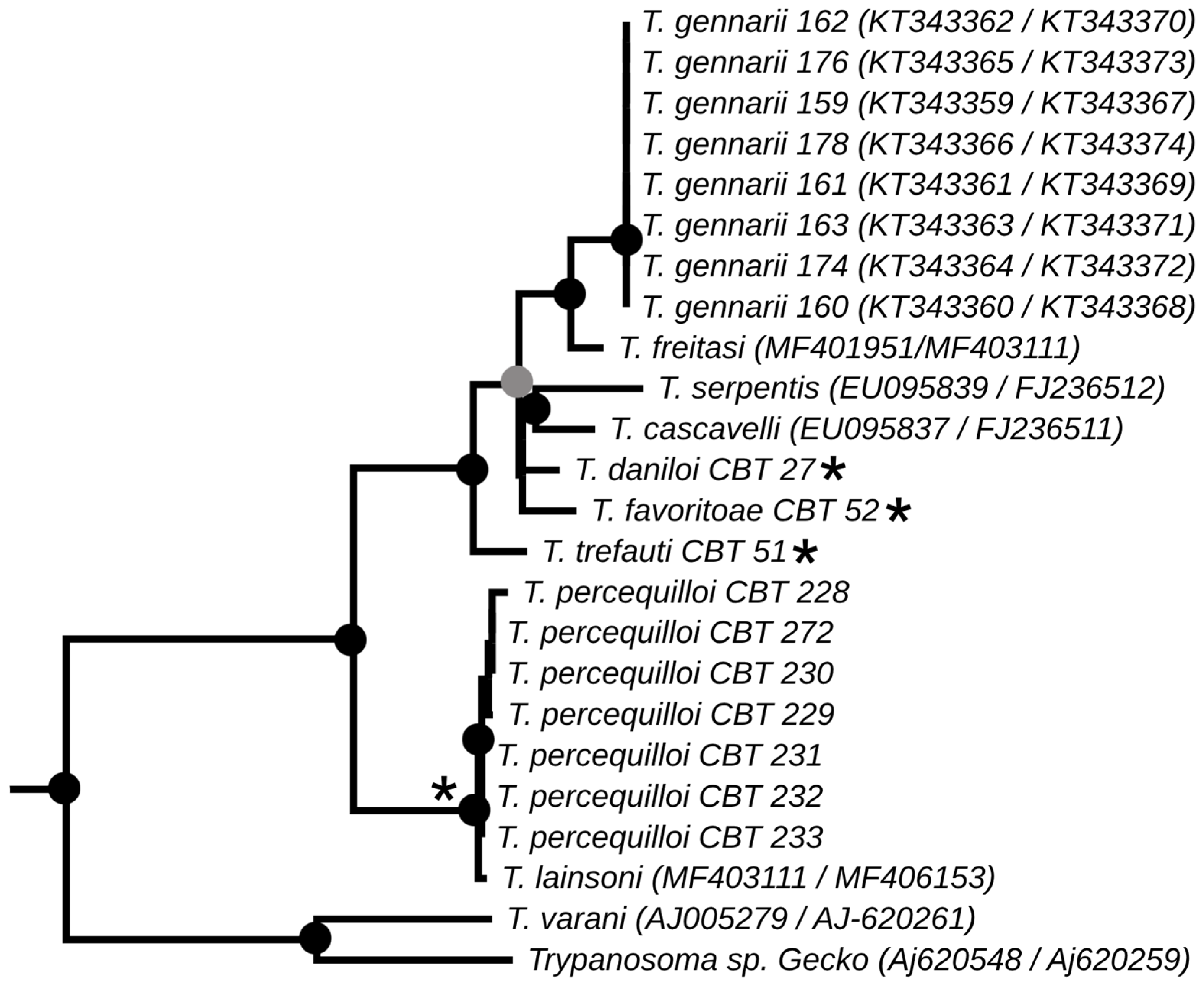
3.2. Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hamilton, P.B.; Stevens, J.R.; Gaunt, M.W.; Gidley, J.; Gibson, W.C. Trypanosomes are monophyletic: Evidence from genes for glyceraldehyde phosphate dehydrogenase and small subunit ribosomal RNA. Int. J. Parasitol. 2004, 34, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.G.; Stevens, J.R.; Lukeš, J. The evolution and diversity of kinetoplastid flagellates. Trends Parasitol. 2006, 22, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Gibson, W.C.; Stevens, J.R. Patterns of co-evolution between trypanosomes and their hosts deduced from ribosomal RNA and protein-coding gene phylogenies. Mol. Phylogenetics Evol. 2007, 44, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hoare, C.A. The Trypanosomes of Mammals; Blackwell: Oxford, UK, 1972; 749p. [Google Scholar]
- Rodrigues, A.C.; Garcia, H.A.; Batista, J.S.; Minervino, A.H.H.; Góes-Cavalcante, G.; DA Silva, F.M.; Ferreira, R.C.; Campaner, M.; Paiva, F.; Teixeira, M.M.G. Characterization of spliced leader genes of Trypanosoma (Megatrypanum) theileri: Phylogeographical analysis of Brazilian isolates from cattle supports spatial clustering of genotypes and parity with ribosomal markers. Parasitology 2010, 137, 111–122. [Google Scholar] [CrossRef]
- Milocco, C.; Kamyingkird, K.; Desquesnes, M.; Jittapalapong, S.; Herbreteau, V.; Chaval, Y.; Douangboupha, B.; Morand, S. Molecular demonstration of Trypanosoma evansi and Trypanosoma lewisi DNA in wild rodents from Cambodia, Lao PDR and Thailand. Transbound. Emerg. Dis. 2013, 60, 17–26. [Google Scholar] [CrossRef]
- Srbek-Araujo, A.C. Do female jaguars (Panthera onca Linnaeus, 1758) deliberately avoid camera traps? Mamm. Biol. 2018, 88, 26–30. [Google Scholar] [CrossRef]
- Musser, G.C.; Carleton, M.D. Superfamily Muroidea. In Mammal Species of the World: A Taxonomic and Geographic Reference; Wilson, D.E., Reeder, D.M., Eds.; The Johns Hopkins University Press: Baltimore, MD, USA, 2005; p. 2142. [Google Scholar]
- Wilson, D.E.; Reeder, D.M. (Eds.) Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2005. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- DA Silva, F.M.; Rodrigues, A.C.; Campaner, M.; Takata, C.S.A.; Brigido, M.C.; Junqueira, A.C.V.; Coura, J.R.; Takeda, G.F.; Shaw, J.J.; Teixeira, M.M.G. Randomly amplified polymorphic DNA analysis of Trypanosoma rangeli and allied species from humans, monkeys, and other sylvatic mammals of the Brazilian Amazon disclosed a new group and a species-specific marker. Parasitology 2004, 128, 283–294. [Google Scholar] [CrossRef]
- Rêgo, S.F.M.; Magalhães, A.E.A.; Siqueira, A.F. Um novo tripanossoma do gambá, Trypanosoma freitasi sp. nov. Rev. Bras. Malariologia 1957, 9, 277–284. [Google Scholar]
- Deane, M.P.; Milder, R. Ultrastructure of the “cyst-like bodies” of Trypanosoma conorhini. J. Protozool. 1972, 19, 28–42. [Google Scholar] [CrossRef]
- Mello, D.A. Trypanosoma (Megatrypanum) samueli sp. nov., a Trypanosomatidae isolated from Monodelphis domesticus (Wagner, 1842) (Marsupiala). Ann. Parasitol. Hum. Comparée 1977, 52, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Noyes, H.; Stevens, J.; Teixeira, M.; Phelan, J.; Holz, P. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999, 29, 331–339. [Google Scholar] [CrossRef]
- Gurgel-Gonçalves, R.; Ramalho, E.D.; Duarte, M.A.; Palma, A.R.T.; Abad-Franch, F.; Carranza, J.C.; Cuba, C.A.C. Enzootic transmission of Trypanosoma cruzi and T. rangeli in the Federal District of Brazil. Rev. Inst. Med. Trop. Sao Paulo 2004, 46, 323–330. [Google Scholar] [CrossRef]
- Lainson, R.; Da Silva, F.; Franco, C. Trypanosoma (Megatrypanum) saloboense sp. nov. (Kinetoplastida: Trypanosomatidae) parasite of Monodelphis emiliae (Marsupiala: Didelphidae) from Amazonian Brazil. Parasite 2008, 15, 99–103. [Google Scholar] [CrossRef]
- McINNES, L.M.; Gillett, A.; Ryan, U.M.; Austen, J.; Campbell, R.S.F.; Hanger, J.; Reid, S.A. Trypanosoma irwini n. sp (Sarcomastigophora: Trypanosomatidae) from the koala (Phascolarctos cinereus). Parasitology 2009, 136, 875–885. [Google Scholar] [CrossRef]
- Austen, J.M.; Jefferies, R.; Friend, J.A.; Ryan, U.; Adams, P.; Reid, S.A. Morphological and molecular characterization of Trypanosoma copemani sp. nov. (Trypanosomatidae) isolated from Gilbert’s potoroo (Potorous gilbertii) and quokka (Setonix brachyurus). Parasitology 2009, 136, 783–792. [Google Scholar] [CrossRef]
- McInnes, L.M.; Hanger, J.; Simmons, G.; Reid, S.A.; Ryan, U.M. Novel trypanosome Trypanosoma gilletti sp. (Euglenozoa: Trypanosomatidae) and the extension of the host range of Trypanosoma copemani to include the koala (Phascolarctos cinereus). Parasitology 2011, 138, 59–70. [Google Scholar] [CrossRef]
- Botero, A.; Cooper, C.; Thompson, C.K.; Clode, P.L.; Rose, K.; Thompson, R.A. Morphological and phylogenetic description of Trypanosoma noyesi sp. nov.: An Australian wildlife trypanosome within the T. cruzi Clade. Protist 2016, 167, 425–439. [Google Scholar] [CrossRef]
- Ferreira, J.I.; da Costa, A.P.; Nunes, P.H.; Ramirez, D.; Fournier, G.F.; Saraiva, D.; Tonhosolo, R.; Marcili, A. New Trypanosoma species, Trypanosoma gennarii sp. nov., from South American marsupial in Brazilian Cerrado. Acta Trop. 2017, 176, 249–255. [Google Scholar] [CrossRef]
- Votýpka, J.; Stříbrná, E.; Modrý, D.; Bryja, J.; Bryjová, A.; Lukeš, J. Unexpectedly high diversity of trypanosomes in small sub-Saharan mammals. Int. J. Parasitol. 2022, 52, 647–658. [Google Scholar] [CrossRef]
- Marcili, A.; da Costa, A.P.; Soares, H.S.; Acosta Ida, C.; de Lima, J.T.R.; Minervino, A.H.H.; Melo, A.T.L.; Aguiar, D.M.; Pacheco, R.C.; Gennari, S.M. Isolation and phylogenetic relationships of bat trypanosomes from different biomes in Mato Grosso, Brazil. J. Parasitol. 2013, 99, 1071–1076. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Viola, L.B.; Campaner, M.; Takata, C.S.; Ferreira, R.C.; Rodrigues, A.C.; Freitas, R.A.; Duarte, M.R.; Grego, K.F.; Barrett, T.V.; Camargo, E.P.; et al. Phylogeny of snake trypanosomes inferred by SSU rDNA sequences, their possible transmission by phlebotomines, and taxonomic appraisal by molecular, cross-infection and morphological analysis. Parasitology 2008, 135, 595–605. [Google Scholar] [CrossRef]
- Ferreira, R.C.; DE Souza, A.A.; Freitas, R.A.; Campaner, M.; Takata, C.S.A.; Barrett, T.V.; Shaw, J.J.; Teixeira, M.M.G. A phylogenetic lineage of closely related trypanosomes (Trypanosomatidae, Kinetoplastida) of anurans and sand flies (Psychodidae, Diptera) sharing the same ecotopes in Brazilian Amazonia. J. Eukaryot. Microbiol. 2008, 55, 427–435. [Google Scholar] [CrossRef]
- Lima, L.; Espinosa-Álvarez, O.; Hamilton, P.B.; Neves, L.; Takata, C.S.; Campaner, M.; Attias, M.; de Souza, W.; Camargo, E.P.; Teixeira, M.M. Trypanosoma livingstonei: A new species from African bats supports the bat seeding hypothesis for the Trypanosoma cruzi clade. Parasites Vectors 2013, 6, 221. [Google Scholar] [CrossRef]
- Nicholas, K.B.T.; Nicholas, H.B.; Deerfield, D.W. GeneDoc: Analysis and visualization of genetic variation. Embnew News. 1997, 4, 14. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Xia, X.; Lemey, P. Assessing substitution saturation with DAMBE. In The Phylo-Genetic Handbook: A Practical Approach to DNA and Protein Phylogeny, 2nd ed.; Lemey, P., Salemi, M., Vandamme, A.-M., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 615–630. [Google Scholar]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Pattengale, N.D.; Alipour, M.; Bininda-Emonds, O.R.P.; Moret, B.M.E.; Stamatakis, A. How many bootstrap replicates are necessary? In Proceedings of the 2009 IEEE International Conference on Bioinformatics and Biomedicine, Washington, DC, USA, 2–4 November 2009; pp. 184–190. [Google Scholar]
- Aberer, A.J.; Kobert, K.; Stamatakis, A. ExaBayes: Massively parallel Bayesian tree inference for the whole-genome era. Mol. Biol. Evol. 2014, 31, 2553–2556. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v1.6. 2014. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 20 November 2024).
- Acosta, I.D.C.; da Costa, A.P.; Nunes, P.H.; Gondim, M.F.N.; Gatti, A.; Rossi, J.L., Jr.; Gennari, S.M.; Marcili, A. Morphological and molecular characterization and phylogenetic relationships of a new species of trypanosome in Tapirus terrestris (lowland tapir), Trypanosoma terrestris sp. nov., from Atlantic Rainforest of southeastern Brazil. Parasites Vectors 2013, 6, 349. [Google Scholar] [CrossRef] [PubMed]
- Gruby, M. Recherches sur une espèce observations nouvelle d’hématozoaire, Trypanosoma sanguinis. C. R. Acad. Sci. 1843, 17, 1134–1136. [Google Scholar]
- Paparini, A.; Macgregor, J.; Irwin, P.J.; Warren, K.; Ryan, U.M. Novel genotypes of Trypanosoma binneyi from wild platypuses (Ornithorhynchus anatinus) and identification of a leech as a potential vector. Exp. Parasitol. 2014, 145, 42–50. [Google Scholar] [CrossRef]
- Haag, J.; O’Huigin, C.; Overath, P. The molecular phylogeny of trypanosomes: Evidence for an early divergence of the Salivaria. Mol. Biochem. Parasitol. 1998, 91, 37–49. [Google Scholar] [CrossRef]
- Palma, R.E. Evolution of American marsupials and their phylogenetic relationships with Australian metatherians. In Predators with Pouches: The Biology of Carnivorous Marsupials; Jones, M., Archer, M., Dickman, C., Eds.; CSIRO Publishing: Melbourne, Australia, 2003; pp. 21–29. [Google Scholar]
- Ferreira, R.C.; Campaner, M.; Viola, L.B.; Takata, C.S.A.; Takeda, G.F.; Teixeira, M.M.G. Morphological and molecular diversity and phylogenetic relationships among anuran trypanosomes from the Amazonia, Atlantic Forest and Pantanal biomes in Brazil. Parasitology 2007, 134, 1623–1638. [Google Scholar] [CrossRef]
- Lima, L.; da Silva, F.M.; Neves, L.; Attias, M.; Takata, C.S.; Campaner, M.; de Souza, W.; Hamilton, P.B.; Teixeira, M.M. Evolutionary insights from bat trypanosomes: Morphological, developmental and phylogenetic evidence of a new species, Trypanosoma (Schizotrypanum) erneyi sp. nov., in African bats closely related to Trypanosoma (Schizotrypanum) cruzi and allied species. Protist 2012, 163, 856–872. [Google Scholar] [CrossRef]
- García, L.; Ortiz, S.; Osorio, G.; Torrico, M.C.; Torrico, F.; Solari, A. Phylogenetic analysis of Bolivian bat trypanosomes of the subgenus Schizotrypanum based on cytochrome B sequence and minicircle analyses. PLoS ONE 2012, 7, e36578. [Google Scholar] [CrossRef]
- Minter-Goedbloed, E.; Leake, C.J.; Minter, D.M.; McNamara, J.; Kimber, C.; Bastien, P.; Evans, D.A.; Le Ray, D. Trypanosoma varani and T. grayi-like trypanosomes: Development in vivo and in insect hosts. Parasitol. Res. 1993, 79, 329–333. [Google Scholar] [CrossRef]
- Cutolo, A.A.; Teodoro, A.K.M.; Ovallos, F.G.; Allegretti, S.M.; Galati, E.A.B. Sandflies (Diptera: Psychodidae) associated with opossum nests at urban sites in southeastern Brazil: A risk factor for urban and periurban zoonotic Leishmania transmission? Mem. Inst. Oswaldo Cruz 2014, 109, 391–393. [Google Scholar] [CrossRef]
- Quaresma, P.F.; Carvalho, G.M.d.L.; Ramos, M.C.d.N.F.; Filho, J.D.A. Natural Leishmania sp. reservoirs and phlebotomine sandfly food source identification in Ibitipoca State Park, Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2012, 107, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Deane, M.P.; Lenzi, H.L.; Jansen, A. Trypanosoma cruzi: Vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem. Inst. Oswaldo Cruz 1984, 79, 513–515. [Google Scholar] [CrossRef]
- Jansen, A.M.; Xavier, S.C.d.C.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasites Vectors 2018, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.O.d.R.e.; Pattoli, D.B.G.; Camargo, J. A new finding of Trypanosoma (Megatrypanum) freitasi, parasite of the opossum. Rev. Saude Publica 1976, 10, 121–124. [Google Scholar] [CrossRef]
- Lopes, C.M.T.; Menna-Barreto, R.F.S.; Pavan, M.G.; Pereira, M.C.S.; Roque, A.L.R. Trypanosoma janseni sp. nov. (Trypanosomatida: Trypanosomatidae) isolated from Didelphis aurita (Mammalia: Didelphidae) in the Atlantic Rainforest of Rio de Janeiro, Brazil: Integrative taxonomy and phylogeography within the Trypanosoma cruzi clade. Memórias Inst. Oswaldo Cruz 2018, 113, 45–55. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Lima, L.; Xavier, S.C.d.C.; Herrera, H.M.; Rocha, F.L.; Roque, A.L.R.; Teixeira, M.M.G.; Jansen, A.M. Uncovering Trypanosoma spp. diversity of wild mammals by the use of DNA from blood clots. Int. J. Parasitol. Parasites Wildl. 2019, 8, 171–181. [Google Scholar] [CrossRef]
- Brandão, E.M.V.; Xavier, S.C.C.; Carvalhaes, J.G.; D’Andrea, P.S.; Lemos, F.G.; Azevedo, F.C.; Cássia-Pires, R.; Jansen, A.M.; Roque, A.L.R. Trypanosomatids in small mammals of an agroecosystem in central Brazil: Another piece in the puzzle of parasite transmission in an anthropogenic landscape. Pathogens 2019, 8, 190. [Google Scholar] [CrossRef]
- Herrer, A. Trypanosoma phyllotis sp. nov. e infecciones asociadas en uma titira, el Phlebotomus nogushi. Peru. Med. Exp. Salud Pública 1942, 1, 40–55. Available online: http://www.scielo.org.pe/scielo.php?pid=S1726-46341942000100002&script=sci_abstract (accessed on 20 November 2024).
- Rodrigues, V.L.; Filho, A.D.N.F. Trypanosoma (Megatrypanum) rochasilvai, sp. n., encontrada no Estado de São Paulo, Brasil, parasitando o Oryzomys laticeps (Leche, 1886) (Rodentia-Cricetidae). Rev. Bras. Biol. 1984, 44, 299–304. [Google Scholar]
- Herrera, L.; Urdaneta-Morales, S. Synanthropic rodent reservoirs of Trypanosoma cruzi in the valley of Caracas, Venezuela. Rev. Inst. Med. Trop. Sao Paulo 1997, 39, 279–282. [Google Scholar] [CrossRef]
- Naiff, R.D.; Barrett, T.V. Trypanosoma (Megatrypanum) lainsoni sp. nov. from Mesomys hispidus (Rodentia: Echimyidae) in Brazil: Trypomastigotes described from experimentally infected laboratory mice. Parasite 2013, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Pumhom, P.; Pognon, D.; Yangtara, S.; Thaprathorn, N.; Milocco, C.; Douangboupha, B.; Herder, S.; Chaval, Y.; Morand, S.; Jittapalapong, S.; et al. Molecular prevalence of Trypanosoma spp. in wild rodents of Southeast Asia: Influence of human settlement habitat. Epidemiol. Infect. 2014, 142, 1221–1230. [Google Scholar] [CrossRef]
- Hong, X.-K.; Zhang, X.; Fusco, O.A.; Lan, Y.-G.; Lun, Z.-R.; Lai, D.-H. PCR-based identification of Trypanosoma lewisi and Trypanosoma musculi using maxicircle kinetoplast DNA. Acta Trop. 2017, 171, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Barreto, W.T.G.; de Macedo, G.C.; Barros, J.H.d.S.; Xavier, S.C.d.C.; Garcia, C.M.; Mourão, G.; de Oliveira, J.; Rimoldi, A.R.; Porfírio, G.E.d.O.; et al. The reservoir system for Trypanosoma (Kinetoplastida, Trypanosomatidae) species in large neotropical wetland. Acta Trop. 2019, 199, 105098. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. A conservação do Cerrado brasileiro. Megadiversidade 2005, 1, 147–155. [Google Scholar]
- Alho, C.J.R.; Mamede, S.B.; Benites, M.; Andrade, B.S.; Sepúlveda, J.J.O. Threats to the biodiversity of the Brazilian Pantanal due to land use and occupation. Ambient. Soc. 2019, 22, e01891. [Google Scholar] [CrossRef]
- Roque, A.L.R.; D’andrea, P.S.; Jansen, A.M.; Duarte, A.C.M.; Xavier, S.C.C.; da Rocha, M.G. Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted Chagas disease outbreaks. Am. J. Trop. Med. Hyg. 2008, 79, 742–749. [Google Scholar] [CrossRef]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.E.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- Epstein, P. The threatened plague. People Planet 1997, 6, 14–17. [Google Scholar]
| Trypanosoma spp. | Isolate Code | Host | Geographic Source | |
|---|---|---|---|---|
| Trypanosoma trefauti | CBT 51 | Hylaeamys megacephalus | Poconé | MT |
| Trypanosoma favoritoae | CBT 52 | Phylander canus | Poconé | MT |
| Trypanosoma daniloi | CBT 127 | Didelphis albiventris | Chapada Guimarâes | MT |
| Trypanosoma percequilloi | CBT 228 | Hylaeamys megacephalus | Poconé | MT |
| Trypanosoma percequilloi | CBT 229 | Hylaeamys megacephalus | Poconé | MT |
| Trypanosoma percequilloi | CBT 230 | Hylaeamys megacephalus | Poconé | MT |
| Trypanosoma percequilloi | CBT 231 | Hylaeamys megacephalus | Poconé | MT |
| Trypanosoma percequilloi | CBT 232 | Hylaeamys megacephalus | Poconé | MT |
| Trypanosoma percequilloi | CBT 233 | Hylaeamys megacephalus | Poconé | MT |
| Trypanosoma percequilloi | CBT 272 | Hylaeamys megacephalus | Poconé | MT |
| Isolates | Media or Cells Monolayer Culture | ||||
|---|---|---|---|---|---|
| (CBT) | LIT | TC100 | RPMI | Vero + RPMI | SF9 + TC100 |
| 51 | - | +/- | - | - | + |
| 52 | + | - | - | - | - |
| 127 | - | - | - | + | - |
| 228 | - | - | - | + | - |
| 229 | - | - | - | + | - |
| 230 | - | - | - | + | - |
| 231 | - | - | - | + | - |
| 232 | - | - | - | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcili, A.; Costa, A.P.d.; Nunes, P.H.; Ferreira, J.I.G.d.S.; Tonhosolo, R.; Bosco, V.C.; Pesenato, I.P.; Roxo, F.F.; Aparecida Nieri Bastos, F.; Pacheco, R.C.; et al. Description of Four New Trypanosoma Species Infecting Small Wild Mammals from Two Brazilian Biomes: The Pantanal and Cerrado Hotspots. Microorganisms 2025, 13, 1257. https://doi.org/10.3390/microorganisms13061257
Marcili A, Costa APd, Nunes PH, Ferreira JIGdS, Tonhosolo R, Bosco VC, Pesenato IP, Roxo FF, Aparecida Nieri Bastos F, Pacheco RC, et al. Description of Four New Trypanosoma Species Infecting Small Wild Mammals from Two Brazilian Biomes: The Pantanal and Cerrado Hotspots. Microorganisms. 2025; 13(6):1257. https://doi.org/10.3390/microorganisms13061257
Chicago/Turabian StyleMarcili, Arlei, Andréa Pereira da Costa, Pablo Henrique Nunes, Juliana Isabel Giuli da Silva Ferreira, Renata Tonhosolo, Varley Cardoso Bosco, Isabella Pereira Pesenato, Fábio Fernandes Roxo, Fernanda Aparecida Nieri Bastos, Richard Campos Pacheco, and et al. 2025. "Description of Four New Trypanosoma Species Infecting Small Wild Mammals from Two Brazilian Biomes: The Pantanal and Cerrado Hotspots" Microorganisms 13, no. 6: 1257. https://doi.org/10.3390/microorganisms13061257
APA StyleMarcili, A., Costa, A. P. d., Nunes, P. H., Ferreira, J. I. G. d. S., Tonhosolo, R., Bosco, V. C., Pesenato, I. P., Roxo, F. F., Aparecida Nieri Bastos, F., Pacheco, R. C., Rossi, R. V., Semedo, T. B. F., Shio, M. T., & Bahia Labruna, M. (2025). Description of Four New Trypanosoma Species Infecting Small Wild Mammals from Two Brazilian Biomes: The Pantanal and Cerrado Hotspots. Microorganisms, 13(6), 1257. https://doi.org/10.3390/microorganisms13061257






