Diplodia fraxini: The Main Pathogen Involved in the Ash Dieback of Fraxinus angustifolia in Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Fungal Isolation and Identification
2.3. Pathogenicity Test
3. Results
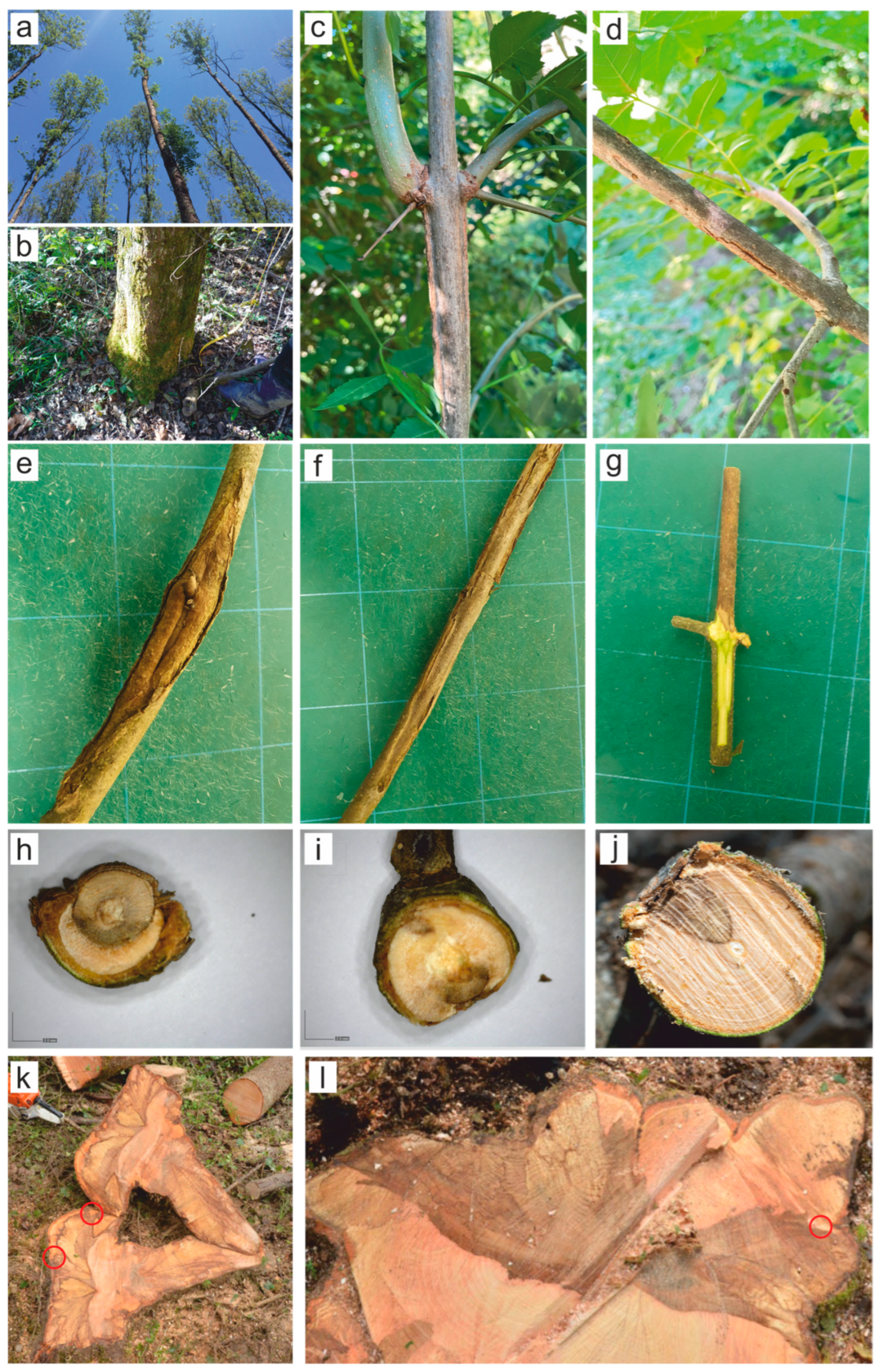
3.1. Observed Symptoms and Ocurrence of Diplodia Fraxini on Sampled Trees
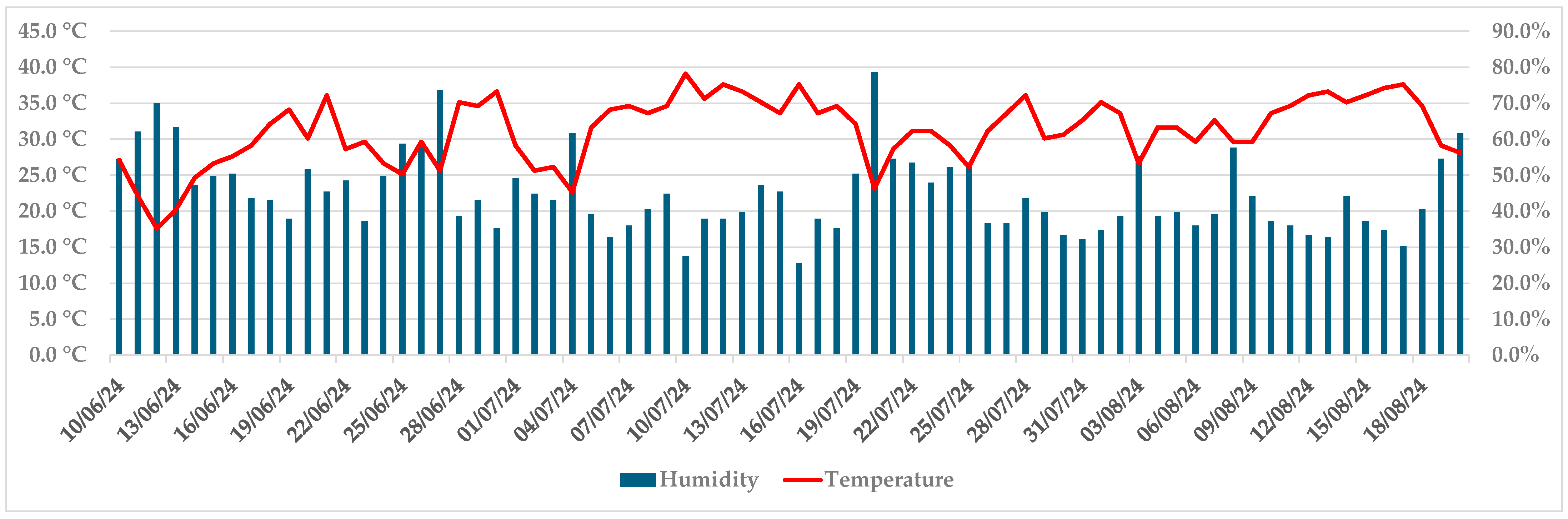
3.2. Pathogenicity of Diplodia fraxini on Fraxinus angustifolia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MEA | Malt extract agar |
| DNA | Deoxyribonucleic acid |
| MEB | Malt extract broth |
| PDA | Potato dextrose agar |
| ICP-Forests | International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests |
| ITS | Internal transcribed spacer |
| PCR | Polymerase chain reaction |
| dNTPs | Deoxynucleotide triphosphates |
| NCBI | National Center for Biotechnology Information |
| BLAST | Basic Local Alignment Search Tool |
| DBH | Diameter at breast height |
Appendix A
| Location (Forest Stand) | Tree | Height (m)/DBH (cm) | Crown Defoliation (%) | Number of Shoots and Branches Positive to D. fraxini | Observed Symptoms on a Stem Base Cross-Section | Number of Cross-Section Wood Sub-Samples Positive to D. fraxini | Other Fungi Present in Sampled Trees |
|---|---|---|---|---|---|---|---|
| L1 Strizivojna 10a | 1 | 28.4/35 | 45 | 5/7 | rot and necrosis on 40% of surface | 0/5 | DE in crown HF in stem base |
| 2 | 29.1/34 | 55 | 0/7 | four smaller individual necroses | 0/4 | DS in stem base | |
| 3 | 28.2/35 | 60 | 8/10 | central irregular necrosis on 20% of surface | 0/2 | DE in crown | |
| 4 | 27.9/36 | 70 | 6/10 | rot and necrosis on 30% of surface | 1/4 | DE in crown HF in stem base | |
| L2 Strizivojna 21c | 1 | 15.3/14 | 30 | 2/8 | none | 0/1 | HF in crown |
| 2 | 15.1/15 | 20 | 1/7 | none | 0/1 | - | |
| 3 | 15.6/15 | 40 | 0/9 | three individual necroses on 15% of surface | 0/3 | HF in both crown and stem base AG in stem base | |
| L3 Kutina 19a | 1 | 19.7/21 | 30 | 3/9 | rot and necrosis on 20% of surface | 0/3 | DE in crown |
| 2 | 14.3/16 | 70 | 2/9 | multiple necroses on 25% of surface | 0/5 | DE and BD in crown | |
| 3 | 18.4/19 | 15 | 0/7 | small necroses on 5% of surface | 0/2 | DE in crown | |
| L4 Radinje 16c | 1 | 14.8/15 | 25 | 2/8 | central irregular discoloration on 50% of surface | 0/4 | DS in crown |
| 2 | 13.9/14 | 45 | 2/7 | rot and necrosis on 80% of surface | 0/5 | DE in crown AG in stem base | |
| L5 Radinje 49c | 1 | 24.7/33 | 50 | 3/8 | small central discoloration on 5% of surface | 0/1 | DE in crown |
| 2 | 24.9/34 | 30 | 2/7 | small central discoloration on 3% of surface | 0/1 | - | |
| L6 Sunja 53d | 1 | 26.2/36 | 20 | 7/10 | rot and necrosis on 70% of surface | 0/5 | DE in crown HF and LT in stem base |
| 2 | 23.5/35 | 55 | 4/7 | rot and necrosis on 30% of surface | 0/5 | DE in crown HF and LT in stem base | |
| L7 Sunja 39b | 1 | 28.3/34 | 65 | 4/7 | one small central necroses on 3% of surface | 0/1 | DE in crown |
| 2 | 28.4/42 | 35 | 3/8 | two small necroses on 5% of surface | 0/2 | DE in crown | |
| L8 Mirna 4d | 1 | 29.8/28 | 50 | 8/10 | rot and necrosis on 90% of surface | 2/5 | DE in crown HF and AG in stem base |
| 2 | 27.4/27 | 40 | 3/7 | four small necroses on 5% of surface | 0/4 | DE in crown |
References
- Boshier, D.; Cordero, J.; Harris, S.; Pannell, J.; Rendell, S.; Savill, P.; Stewart, J.; Cundall, N.; Hubert, J.; Samuel, S.; et al. Ash Species in Europe: Biological Characteristics and Practical Guidelines for Sustainable Use; Department of Plant Sciences, Oxford Forestry Institute, University of Oxford: Oxford, UK, 2005; ISBN 0-85074-163-7. [Google Scholar]
- Hill, L.; Jones, G.; Atkinson, N.; Hector, A.; Hemery, G.; Brown, N. The £ 15 Billion Cost of Ash Dieback in Britain. Curr. Biol. 2019, 29, R315–R316. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T. Chalara fraxinea sp. nov. Associated with Dieback of Ash (Fraxinus excelsior) in Poland. For. Pathol. 2006, 36, 264–270. [Google Scholar] [CrossRef]
- Kranjec Orlović, J.; Moro, M.; Diminić, D. Role of Root and Stem Base Fungi in Fraxinus angustifolia (Vahl) Dieback in Croatian Floodplain Forests. Forests 2020, 11, 607. [Google Scholar] [CrossRef]
- Carroll, D.; Boa, E. Ash Dieback: From Asia to Europe. Plant Pathol. 2024, 73, 741–759. [Google Scholar] [CrossRef]
- Kabiljo, M.; Bobinac, M.; Andrašev, S.; Milenković, I.; Šušić, N. The Importance of Stand Structure in Narrow-Leaved Ash (Fraxinus angustifolia Vahl) Dieback—Insights from an Extensively Managed Stand on a Humogley Soil in Serbia. Forests 2025, 16, 36. [Google Scholar] [CrossRef]
- Bengtsson, S.B.K.; Barklund, P.; von Brömssen, C.; Stenlid, J. Seasonal Pattern of Lesion Development in Diseased Fraxinus excelsior Infected by Hymenoscyphus pseudoalbidus. PLoS ONE 2014, 9, e76429. [Google Scholar] [CrossRef]
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. Hymenoscyphus pseudoalbidus, the Causal Agent of E Uropean Ash Dieback. Mol. Plant Pathol. 2014, 15, 5–21. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Hosoya, T.; Baral, H.-O.; Hosaka, K.; Kakishima, M. Hymenoscyphus pseudoalbidus, the Correct Name for Lambertella Albida Reported from Japan. Mycotaxon 2013, 122, 25–41. [Google Scholar] [CrossRef]
- Nielsen, L.R.; McKinney, L.V.; Hietala, A.M.; Kjær, E.D. The Susceptibility of Asian, European and North American Fraxinus Species to the Ash Dieback Pathogen Hymenoscyphus fraxineus Reflects Their Phylogenetic History. Eur. J. For. Res. 2017, 136, 59–73. [Google Scholar] [CrossRef]
- Coker, T.L.; Rozsypálek, J.; Edwards, A.; Harwood, T.P.; Butfoy, L.; Buggs, R.J. Estimating Mortality Rates of European Ash (Fraxinus excelsior) under the Ash Dieback (Hymenoscyphus fraxineus) Epidemic. Plants People Planet 2019, 1, 48–58. [Google Scholar] [CrossRef]
- Bakys, R.; Vasiliauskas, A.; Ihrmark, K.; Stenlid, J.; Menkis, A.; Vasaitis, R. Root Rot, Associated Fungi and Their Impact on Health Condition of Declining Fraxinus excelsior Stands in Lithuania. Scand. J. For. Res. 2011, 26, 128–135. [Google Scholar] [CrossRef]
- Lenz, H.D.; Bartha, B.; Straßer, L.; Lemme, H. Development of Ash Dieback in South-Eastern Germany and the Increasing Occurrence of Secondary Pathogens. Forests 2016, 7, 41. [Google Scholar] [CrossRef]
- Meyn, R.; Langer, G.J.; Gross, A.; Langer, E.J. Fungal Colonization Patterns in Necrotic Rootstocks and Stem Bases of Dieback-Affected Fraxinus excelsior L. For. Pathol. 2019, 49, e12520. [Google Scholar] [CrossRef]
- Peters, S.; Fuchs, S.; Bien, S.; Bußkamp, J.; Langer, G.J.; Langer, E.J. Fungi Associated with Stem Collar Necroses of Fraxinus excelsior Affected by Ash Dieback. Mycol. Prog. 2023, 22, 52. [Google Scholar] [CrossRef]
- Enderle, R.; Peters, F.; Nakou, A.; Metzler, B. Temporal Development of Ash Dieback Symptoms and Spatial Distribution of Collar Rots in a Provenance Trial of Fraxinus excelsior. Eur. J. For. Res. 2013, 132, 865–876. [Google Scholar] [CrossRef]
- Spiegel, P.; Hintze, T.; Kopp, A.; Sahli, M.; Detter, A.; Queloz, V.; Prospero, S.; Heinzelmann, R. Synergistic Negative Effects of Ash Dieback and Armillaria Root Rot on Health and Stability of Mature Ash Trees. For. Ecol. Manag. 2025, 580, 122476. [Google Scholar] [CrossRef]
- Kowalski, T.; Kraj, W.; Bednarz, B. Fungi on Stems and Twigs in Initial and Advanced Stages of Dieback of European Ash (Fraxinus excelsior) in Poland. Eur. J. For. Res. 2016, 135, 565–579. [Google Scholar] [CrossRef]
- Kowalski, T.; Bilański, P.; Kraj, W. Pathogenicity of Fungi Associated with Ash Dieback towards Fraxinus excelsior. Plant Pathol. 2017, 66, 1228–1238. [Google Scholar] [CrossRef]
- Vemić, A.; Tomšovský, M.; Jung, T.; Milenković, I. Pathogenicity of Fungi Associated with Ash Dieback Symptoms of One-Year-Old Fraxinus excelsior in Montenegro. For. Pathol. 2019, 49, e12539. [Google Scholar] [CrossRef]
- Alves, A.; Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Phillips, A. The Complex of Diplodia Species Associated with Fraxinus and Some Other Woody Hosts in Italy and Portugal. Fungal Divers. 2014, 67, 143–156. [Google Scholar] [CrossRef]
- Saccardo, P.A. Sylloge Fungorum Omnium Hucusque Cognitorum; Edwards Brothers Inc.: Ann Arbor, MI, USA, 1884; Volume 3. [Google Scholar]
- Elena, G.; León, M.; Abad-Campos, P.; Armengol, J.; Mateu-Andrés, I.; Güemes-Heras, J. First Report of Diplodia fraxini Causing Dieback of Fraxinus angustifolia in Spain. Plant Dis. 2018, 102, 2645. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Bottecchia, F.; Bregant, C.; Maddau, L.; Montecchio, L. Diplodia fraxini and Diplodia subglobosa: The Main Species Associated with Cankers and Dieback of Fraxinus excelsior in North-Eastern Italy. Forests 2020, 11, 883. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Bregant, C.; Montecchio, L.; Brglez, A.; Piškur, B.; Ogris, N. First Report of Diplodia fraxini and Diplodia subglobosa Causing Canker and Dieback of Fraxinus excelsior in Slovenia. Plant Dis. 2022, 106, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Benigno, A.; Bregant, C.; Aglietti, C.; Rossetto, G.; Tolio, B.; Moricca, S.; Linaldeddu, B.T. Pathogenic Fungi and Oomycetes Causing Dieback on Fraxinus Species in the Mediterranean Climate Change Hotspot Region. Front. For. Glob. Change 2023, 6, 1253022. [Google Scholar] [CrossRef]
- Cimmino, A.; Maddau, L.; Masi, M.; Linaldeddu, B.T.; Pescitelli, G.; Evidente, A. Fraxitoxin, a New Isochromanone Isolated from Diplodia fraxini. Chem. Biodivers. 2017, 14, e1700325. [Google Scholar] [CrossRef]
- Benigno, A.; Aglietti, C.; Rossetto, G.; Bregant, C.; Linaldeddu, B.T.; Moricca, S. Botryosphaeriaceae Species Associated with Stem Canker, Shoot Blight and Dieback of Fraxinus ornus in Italy. Forests 2023, 15, 51. [Google Scholar] [CrossRef]
- Langer, G. Collar Rots in Forests of Northwest Germany Affected by Ash Dieback. Balt. For. 2017, 23, 4–19. [Google Scholar]
- Peters, S.; Gruschwitz, N.; Bien, S.; Fuchs, S.; Bubner, B.; Blunk, V.; Langer, G.J.; Langer, E.J. The Fungal Predominance in Stem Collar Necroses of Fraxinus excelsior: A Study on Hymenoscyphus fraxineus Multilocus Genotypes. J. Plant Dis. Prot. 2024, 131, 1341–1353. [Google Scholar] [CrossRef]
- Čavlović, J. Prva Nacionalna Inventura Šuma Republike Hrvatske; Ministarstvo Regionalnog Razvoja, Šumarstva i Vodnoga Gospodarstva: Zagreb, Hrvatska, 2010; ISBN 978-953-292-016-1.
- Potočić, N.; Seletković, I.; Jakovljević, T.; Marjanović, H.; Indir, K.; Medak, J.; Ognjenović, M.; Zorić, N. Oštećenost Šumskih Ekosustava Republike Hrvatske—Izvješće Za 2019. Godinu; Croatian Forest Research Institute: Jastrebarsko, Croatia, 2020; pp. 1–91. [Google Scholar]
- Potočić, N.; Seletković, I.; Medak, J.; Jakovljević, T.; Marjanović, H.; Indir, K.; Marušić, M.; Zorić, N.; Bogdanić, R.; Lovrić, V. Oštećenost Šumskih Ekosustava Republike Hrvatske—Izvješće Za 2024. Godinu; Croatian Forest Research Institute: Jastrebarsko, Croatia, 2025; p. 77. [Google Scholar]
- Barić, L.; Županić, M.; Pernek, M.; Diminić, D. First Records of Chalara fraxinea in Croatia–a New Agent of Ash Dieback (Fraxinus Spp.). Šumar. List 2012, 136, 461–468. [Google Scholar]
- Anić, I. Uspijevanje i Pomlađivanje Sastojina Poljskog Jasena (Fraxinus angustifolia Vahl) u Posavini. Ph.D. Thesis, University of Zagreb Faculty of Forestry, Zagreb, Croatia, 2001. [Google Scholar]
- Eichhorn, J.; Roskams, P.; Potocic, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletkovic, I.; Schroeck, H.-W. Part IV Visual Assessment of Crown Condition and Damaging Agents. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; p. 49. [Google Scholar]
- Cenis, J.L. Rapid Extraction of Fungal DNA for PCR Amplification. Nucleic Acids Res. 1992, 20, 2380. [Google Scholar] [CrossRef]
- Kranjec Orlović, J.; Diminić, D.; Ištok, I.; Volenec, I.; Hodak, L.; Grubešić, M.; Tomljanović, K. Fungal Presence and Changes of Wood Structure in Bark Stripping Wounds Made by Red Deer (Cervus elaphus L.) on Stems of Fraxinus angustifolia (Vahl). Forests 2024, 15, 314. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Elsevier B.V.: Amsterdam, The Netherlands, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Grosdidier, M.; Scordia, T.; Ioos, R.; Marçais, B. Landscape Epidemiology of Ash Dieback. J. Ecol. 2020, 108, 1789–1799. [Google Scholar] [CrossRef]
- Madsen, C.L.; Kosawang, C.; Thomsen, I.M.; Hansen, L.N.; Nielsen, L.R.; Kjær, E.D. Combined Progress in Symptoms Caused by Hymenoscyphus fraxineus and Armillaria Species, and Corresponding Mortality in Young and Old Ash Trees. For. Ecol. Manag. 2021, 491, 119177. [Google Scholar] [CrossRef]
- Barta, M.; Pastirčáková, K.; Ostrovský, R.; Kobza, M.; Kádasi Horáková, M. Culturable Endophytic Fungi in Fraxinus excelsior and Their Interactions with Hymenoscyphus fraxineus. Forests 2022, 13, 1098. [Google Scholar] [CrossRef]
- Skovsgaard, J.; Thomsen, I.; Skovgaard, I.; Martinussen, T. Associations among Symptoms of Dieback in Even-aged Stands of Ash (Fraxinus excelsior L.). For. Pathol. 2010, 40, 7–18. [Google Scholar] [CrossRef]
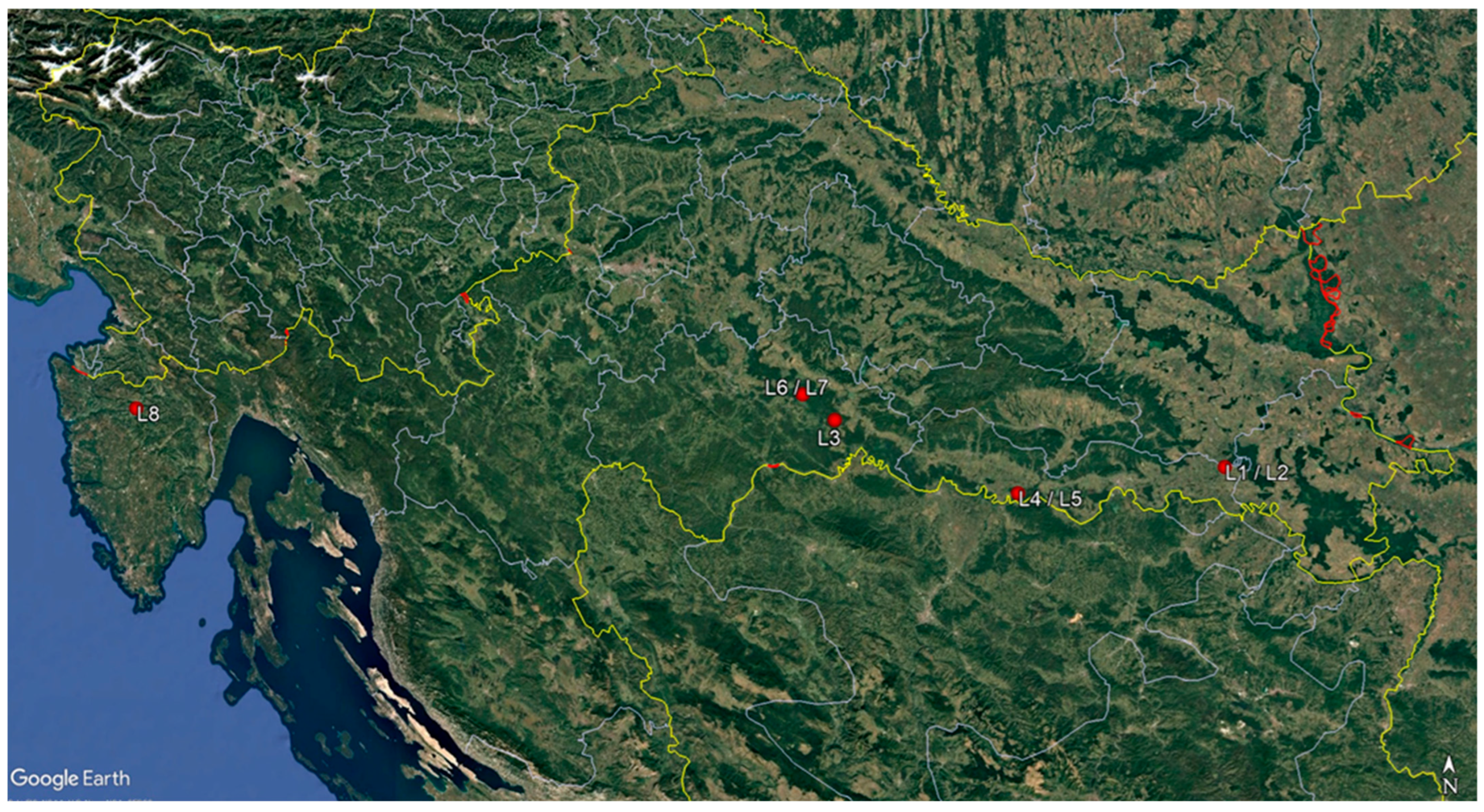

| Sampling Site | Share of F. angustifolia in Total Wood Stock (%) | Age (Years) | Height Above Sea Level (m) | Sampling Date | No. of Sampled Trees | No. of Sampled Shoots and Branches Per Tree | No. of Subsamples Taken from Each Stem Base Cross-Section |
|---|---|---|---|---|---|---|---|
| L1 Strizivojna 10a | 80 | 62 | 83 | 11 October 2021 | 4 | 7 | 5 |
| 7 | 4 | ||||||
| 10 | 2 | ||||||
| 10 | 4 | ||||||
| L2 Strizivojna 21c | 95 | 20 | 83 | 28 October 2021 | 3 | 8 | 1 |
| 7 | 1 | ||||||
| 9 | 3 | ||||||
| L3 Kutina 19a | 97 | 25 | 95 | 11 July 2023 | 3 | 9 | 3 |
| 9 | 5 | ||||||
| 7 | 2 | ||||||
| L4 Radinje 16c | 45 | 26 | 88 | 26 October 2023 | 2 | 8 | 4 |
| 7 | 5 | ||||||
| L5 Radinje 49c | 99 | 70 | 0 | 27 November 2023 | 2 | 8 | 1 |
| 7 | 1 | ||||||
| L6 Sunja 53d | 79 | 35 | 94 | 6 November 2024 | 2 | 10 | 5 |
| 7 | 5 | ||||||
| L7 Sunja 39b | 92 | 68 | 94 | 8 December 2024 | 2 | 7 | 1 |
| 8 | 2 | ||||||
| L8 Mirna 4d | 96 | 60 | 14 | 27 March 2023 | 2 | 10 | 5 |
| 7 | 4 |
| Fungal Species | Accession No. | Positive Samples | No. of Trees | No. of Sites | |
|---|---|---|---|---|---|
| Shoots/Branches | Stem Base | ||||
| Diplodia fraxini | PV490087 | 65 | 5 | 17 | 8 |
| Diaporthe eres | PV492149 | 33 | - | 14 | 7 |
| Hymenoscyphus fraxineus | PV492150 | 4 | 6 | 7 | 4 |
| Armillaria gallica | PV492157 | - | 9 | 3 | 3 |
| Lentinus tigrinus | PV492168 | - | 7 | 2 | 1 |
| Diplodia seriata | PV492152 | 1 | 1 | 2 | 2 |
| Botryosphaeria dothidea | PV492159 | 1 | - | 1 | 1 |
| Monitoring Dates: | 24.6 (20) | 1.7 (28) | 8.7 (35) | 15.7 (42) | 22.7 (49) | 29.7 (56) | 5.8 (63) | 12.8 (70) | 19.8 (77) |
|---|---|---|---|---|---|---|---|---|---|
| Isolate D27 | 15.6/10.7 | 18.7/13.3 | 19.9/14.0 | 20.3/14.4 | 20.6/15.0 | 20.9/15.3 | 21.4/15.7 | 21.8/16.0 | 22.4/16.4 |
| Isolate D44 | 15.4/11.3 | 19.4/13.1 | 19.7/14.0 | 20.3/15.0 | 20.7/15.1 | 21.0/15.4 | 21.3/15.9 | 21.5/15.9 | 21.7/15.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlović, J.K.; Bregant, C.; Linaldeddu, B.T.; Montecchio, L.; Volenec, I.; Uidl, K.; Diminić, D. Diplodia fraxini: The Main Pathogen Involved in the Ash Dieback of Fraxinus angustifolia in Croatia. Microorganisms 2025, 13, 1238. https://doi.org/10.3390/microorganisms13061238
Orlović JK, Bregant C, Linaldeddu BT, Montecchio L, Volenec I, Uidl K, Diminić D. Diplodia fraxini: The Main Pathogen Involved in the Ash Dieback of Fraxinus angustifolia in Croatia. Microorganisms. 2025; 13(6):1238. https://doi.org/10.3390/microorganisms13061238
Chicago/Turabian StyleOrlović, Jelena Kranjec, Carlo Bregant, Benedetto T. Linaldeddu, Lucio Montecchio, Ida Volenec, Katarina Uidl, and Danko Diminić. 2025. "Diplodia fraxini: The Main Pathogen Involved in the Ash Dieback of Fraxinus angustifolia in Croatia" Microorganisms 13, no. 6: 1238. https://doi.org/10.3390/microorganisms13061238
APA StyleOrlović, J. K., Bregant, C., Linaldeddu, B. T., Montecchio, L., Volenec, I., Uidl, K., & Diminić, D. (2025). Diplodia fraxini: The Main Pathogen Involved in the Ash Dieback of Fraxinus angustifolia in Croatia. Microorganisms, 13(6), 1238. https://doi.org/10.3390/microorganisms13061238









