Alleviating Overgrazing Stress and Promoting Grassland Plant Regeneration via Root Exudate-Mediated Recruitment of Beneficial Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Amplicon Sequencing of 16S rRNA Gene of L. chinensis Rhizosphere Soil
2.3. Bacterial Isolation, Identification, and Testing of Growth-Promoting Properties
2.4. Metabolomic Profiling of L. chinensis Roots and Root Exudates
2.5. In Vitro Chemotaxis and Biofilm Formation Assays
2.6. Pot Experiments
2.7. Determination of Physiological Indices
2.8. Transcriptome Sequencing and qRT-PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of Long-Term Overgrazing on the Rhizosphere Bacterial Communities
3.2. Paraburkholderia graminis Isolation and Growth-Promoting Characteristics
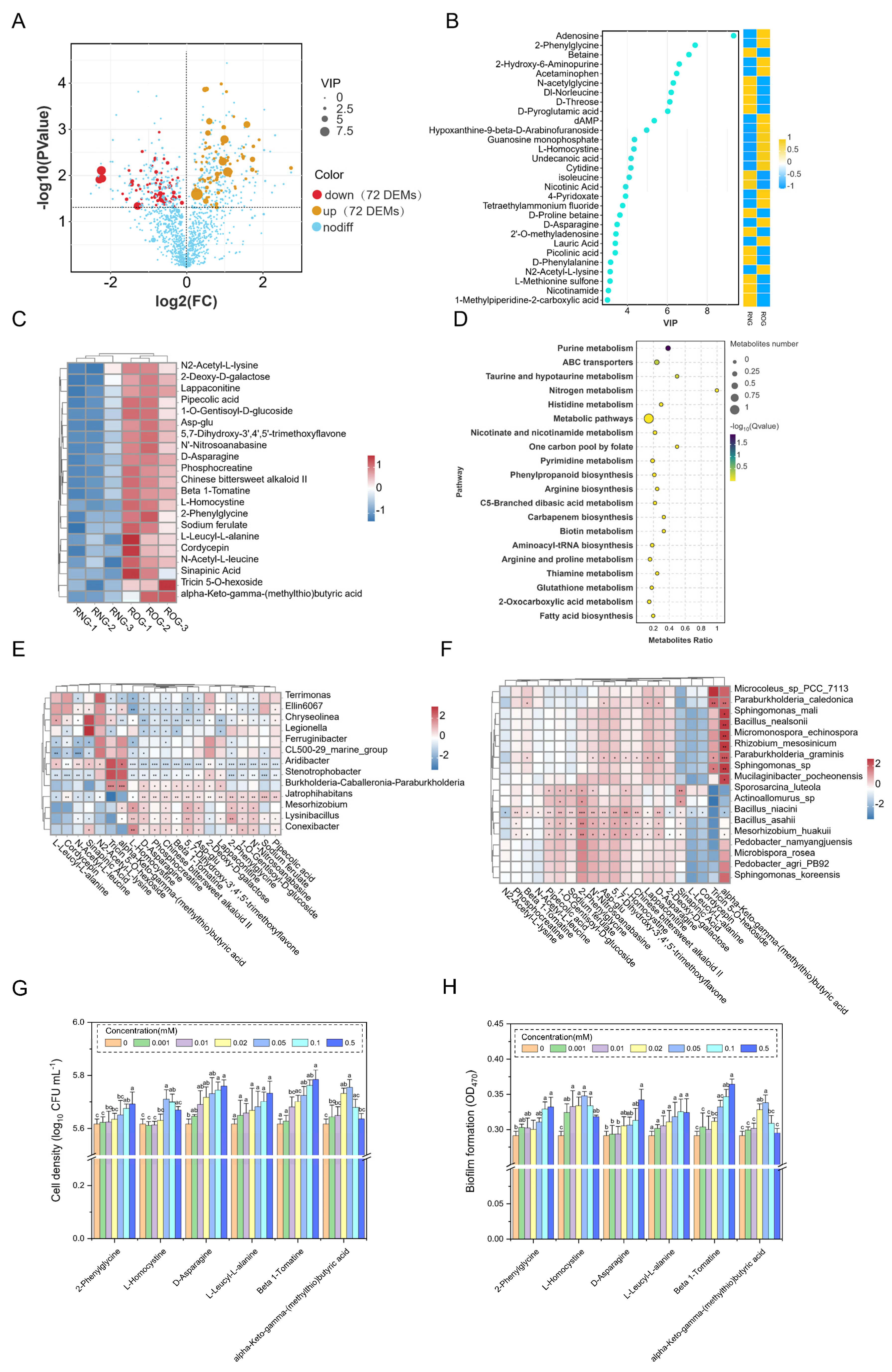
3.3. Identification of Key Root Exudates and the Analysis of Their Association with Specific Rhizosphere Bacteria
3.4. Effects of L. chinensis Specific Root Exudate Compounds on B24 Chemotaxis and Biofilm Formation
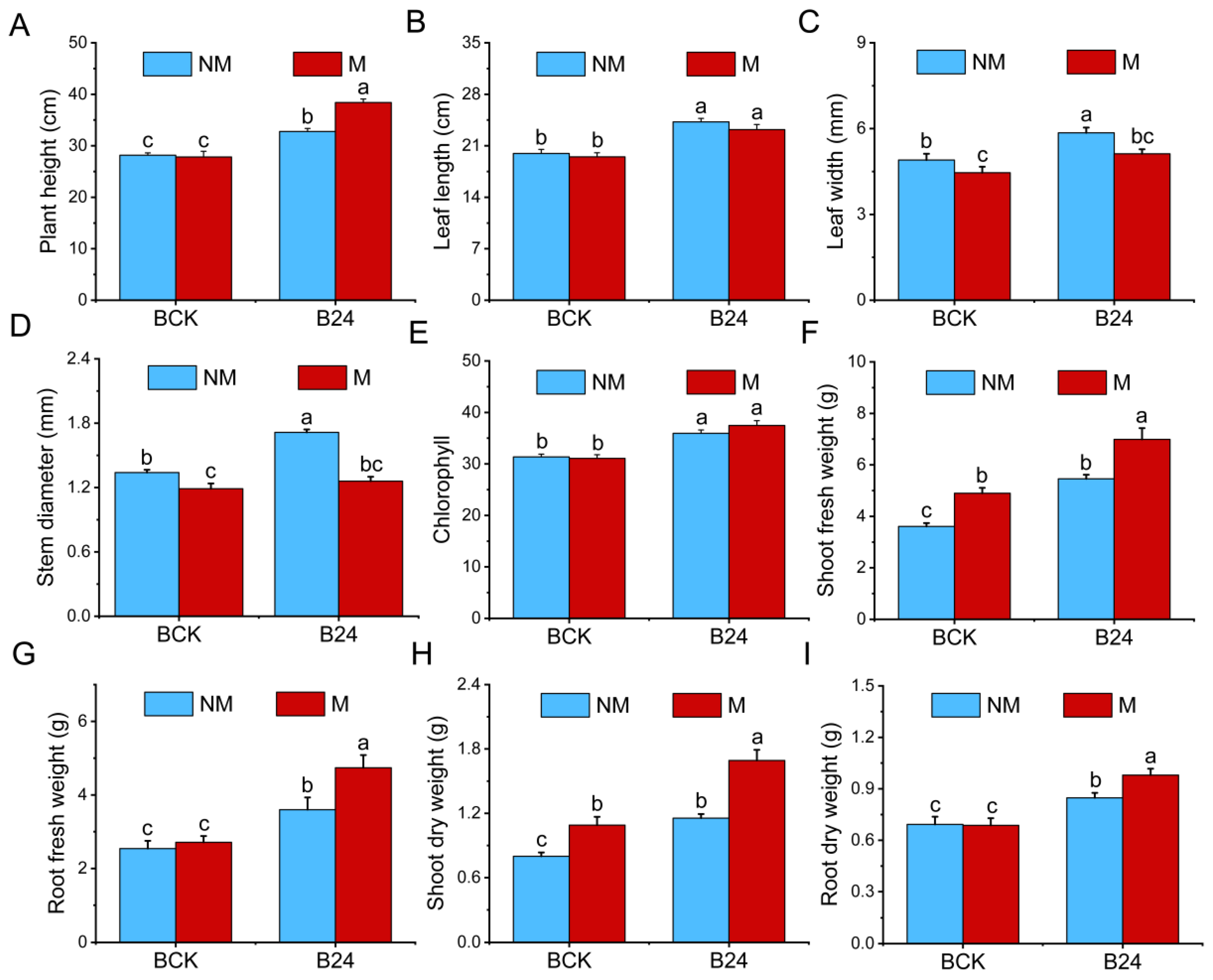
3.5. Effects of B24 Inoculation on Plant Growth
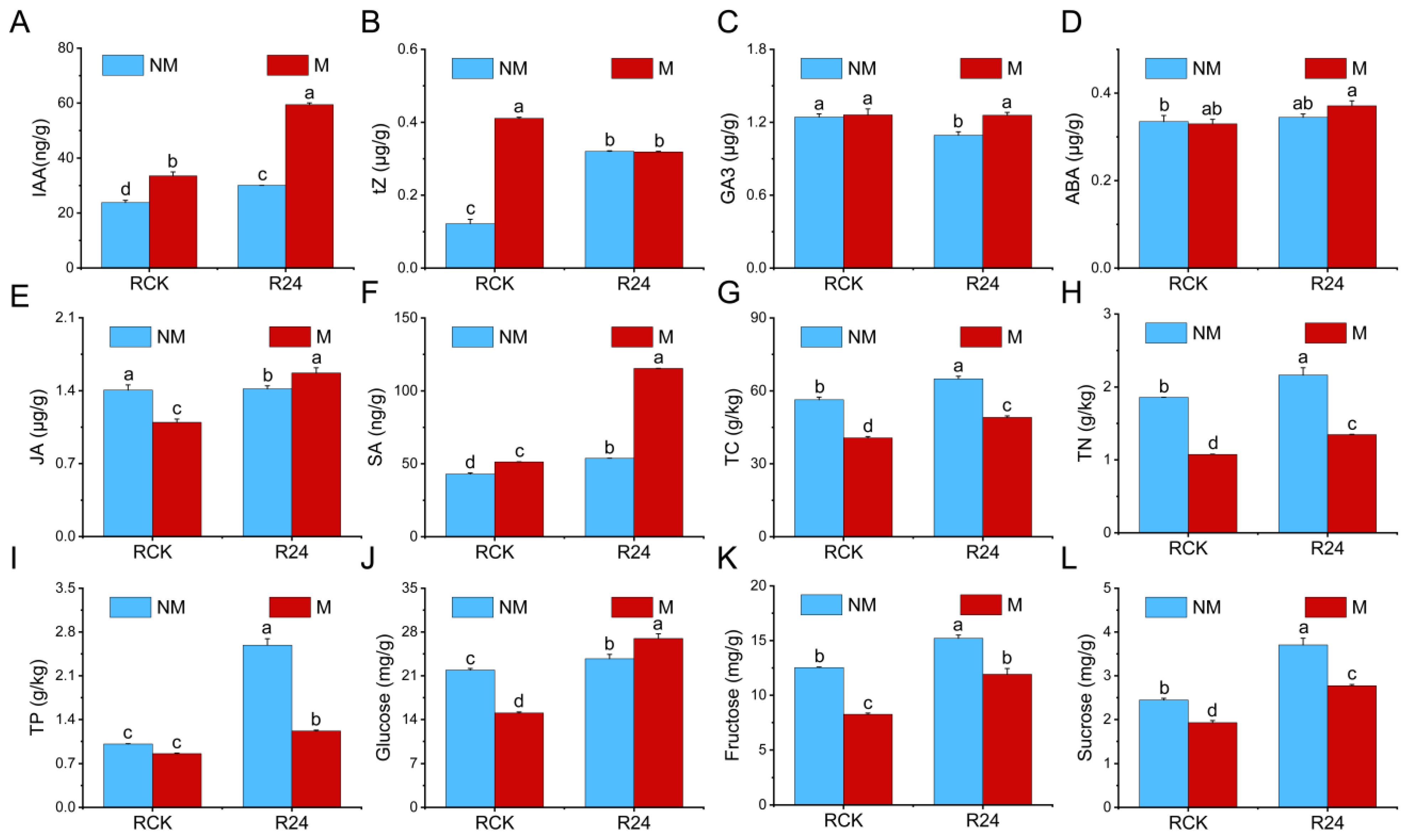
3.6. Effects of B24 Inoculation on the Physiological Indices of L. chinensis
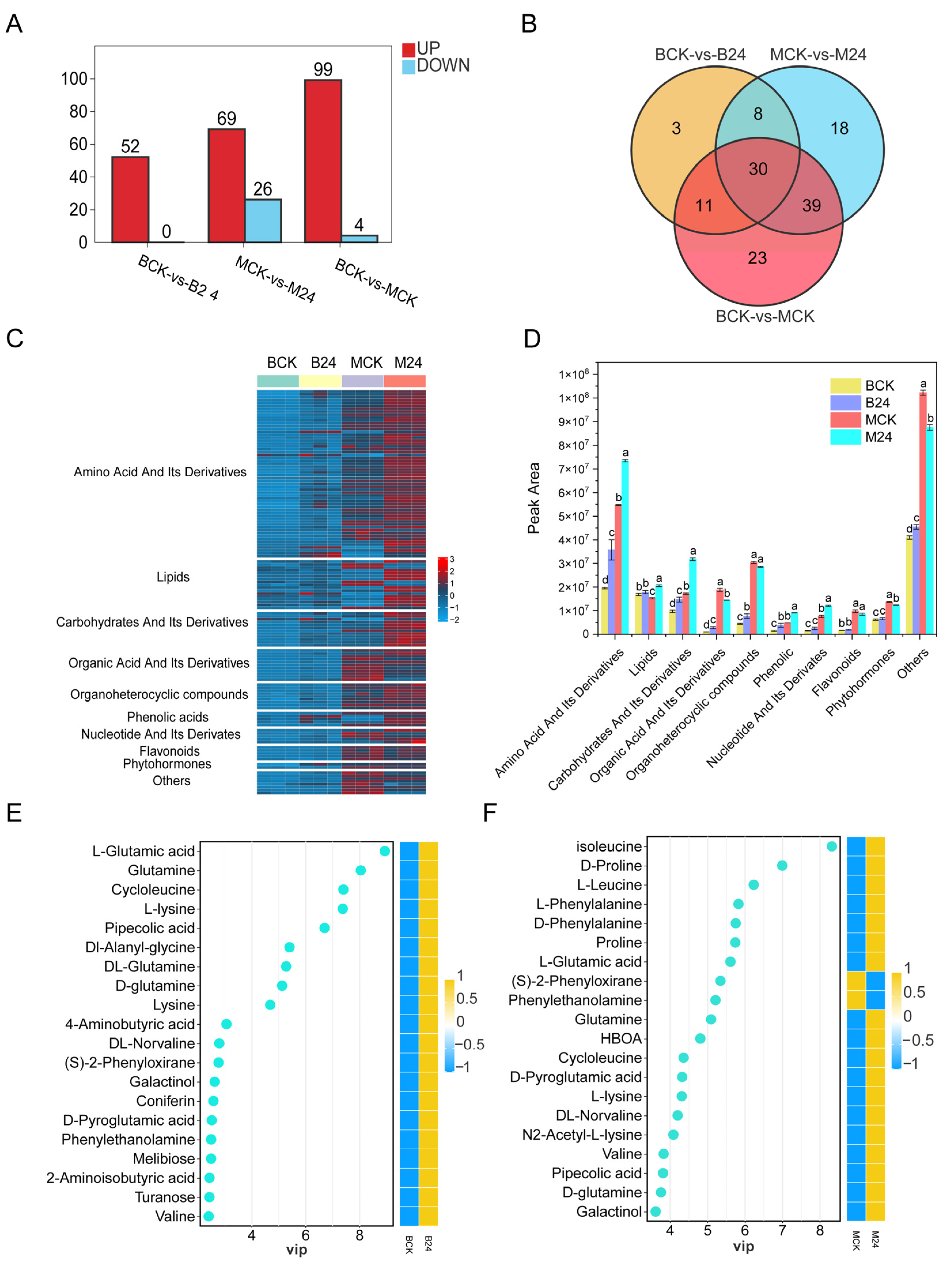
3.7. Characterization of Plant Metabolome in Response to B24 Inoculation
3.8. Transcriptomic Analysis
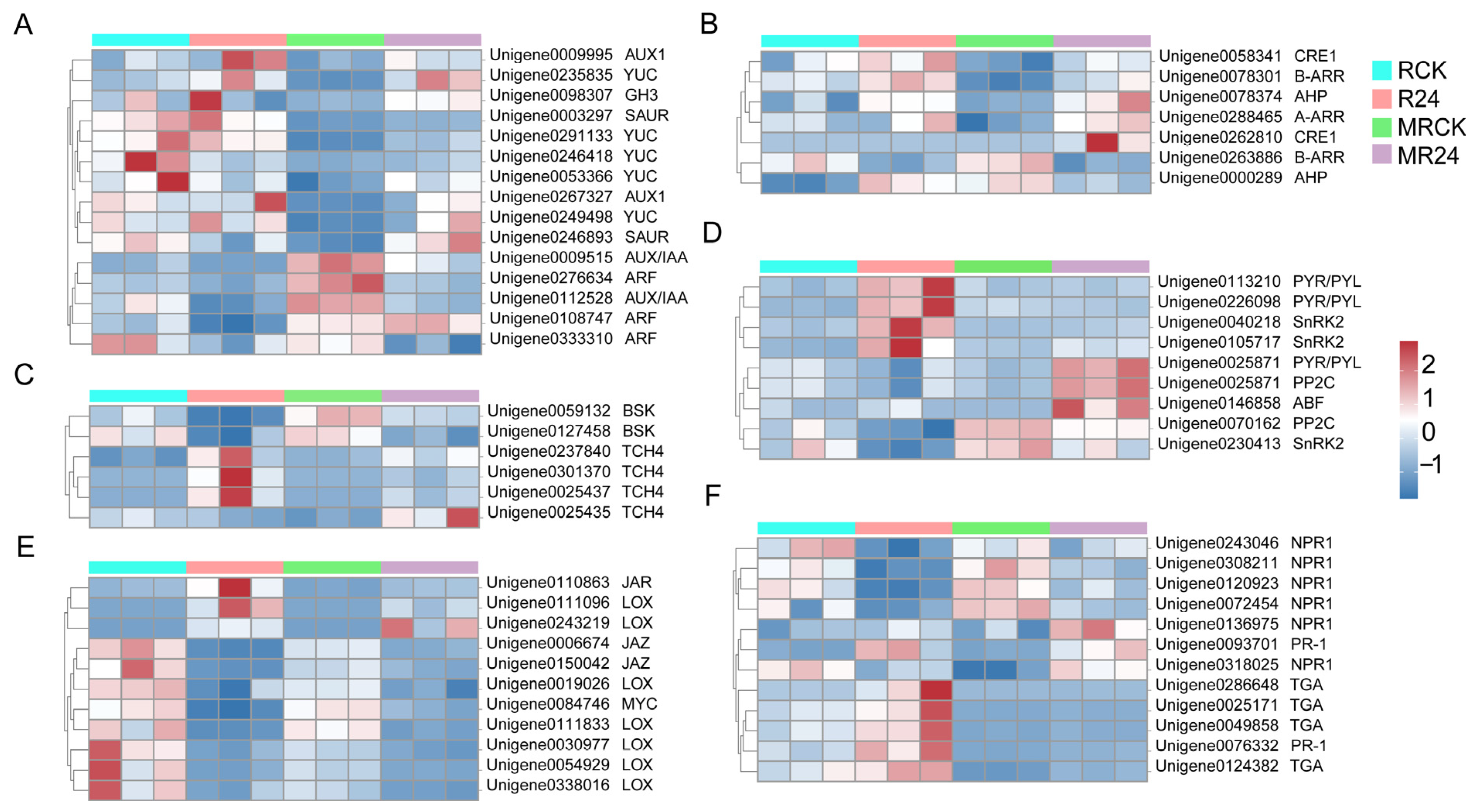
3.9. Regulation of DEGs Related to Phytohormone Signaling
3.10. Regulation of DEGs Related to Plant Growth and Development
3.11. Integrated Metabolomic and Transcriptomic Analysis to Explore Important Biological Pathways
3.12. qRT-PCR Analysis
4. Discussion
4.1. L. chinensis Recruits Key PGPR by Regulating Specific Root Exudates Under Overgrazing Stress
4.2. PGPR Inoculation Promoted the Growth and Regeneration of L. chinensis After Mowing by Promoting Nutrient Absorption, Transport, and Cell Wall Expansion
4.3. PGPR Inoculation Responds to Mowing of L. chinensis by Regulating Plant Hormone Signaling Pathways
4.4. PGPR Inoculation Responds to Mowing Stress by Regulating Amino Acid Metabolism, Energy Metabolism, and Carbohydrate Metabolism of L. chinensis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, H.; Zhang, J.; Baoyin, T.; Zhang, L.; Yuan, T. The effects of different grazing periods on the functional traits of Leymus chinensis (Trin.) Tzvelev in a typical Inner Mongolia steppe. Agronomy 2024, 14, 2370. [Google Scholar] [CrossRef]
- Li, S.G.; Harazono, Y.; Oikawa, T.; Zhao, H.L.; He, Z.Y.; Chang, X.L. Grassland desertification by grazing and the resulting micrometeorological changes in Inner Mongolia. Agric. For. Meteorol. 2000, 102, 125–137. [Google Scholar]
- Eldridge, D.J.; Delgado-Baquerizo, M.; Travers, S.K.; Val, J.; Oliver, I.; Hamonts, K.; Singh, B.K. Competition drives the response of soil microbial diversity to increased grazing by vertebrate herbivores. Ecology 2017, 98, 1922–1931. [Google Scholar] [CrossRef]
- Huhe; Chen, X.; Hou, F.; Wu, Y.; Cheng, Y. Bacterial and fungal community structures in loess plateau grasslands with different grazing intensities. Front. Microbiol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Chung, Y.A.; Rudgers, J.A. Plant-soil feedbacks promote negative frequency dependence in the coexistence of two aridland grasses. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160608. [Google Scholar] [CrossRef]
- Mueller, P.; Granse, D.; Nolte, S.; Do, H.T.; Weingartner, M.; Hoth, S.; Jensen, K. Top-down control of carbon sequestration: Grazing affects microbial structure and function in salt marsh soils. Ecol. Appl. 2017, 27, 1435–1450. [Google Scholar] [CrossRef]
- Zhao, T.; Suo, R.; Alemu, A.W.; Zheng, J.; Zhang, F.; Iwaasa, A.D.; Guo, J.; Zhao, M.; Zhang, B. Mowing increased community stability in semiarid grasslands more than either fencing or grazing. Ecol. Appl. 2024, 34, e2985. [Google Scholar] [CrossRef]
- Turner, C.; Seastedt, T.; Dyer, M. Maximization of aboveground grassland production: The role of defoliation frequency, intensity, and history. Ecol. Appl. 1993, 3, 175–186. [Google Scholar] [CrossRef]
- Robson, T.M.; Lavorel, S.; Clement, J.-C.; Le Roux, X. Neglect of mowing and manuring leads to slower nitrogen cycling in subalpine grasslands. Soil Biol. Biochem. 2007, 39, 930–941. [Google Scholar] [CrossRef]
- Ye, J.; Wu, S.; Mo, Y.; Yang, S.; Zhao, Y.; Zhang, J.; Lü, X.; Yang, G.; Han, X.; Liang, C. Non-linear response of plant caloric value to N addition and mowing treatments in a meadow steppe. Ecol. Process. 2024, 13, 67. [Google Scholar] [CrossRef]
- Pereira-Silva, E.; Casals, P.; Sodek, L.; Delitti, W.; Vallejo, V. Post-fire nitrogen uptake and allocation by two resprouting herbaceous species with contrasting belowground traits. Environ. Exp. Bot. 2019, 159, 157–167. [Google Scholar] [CrossRef]
- Ullah, S.; Bano, A.; Ullah, A.; Shahid, M.A.; Khan, N. A comparative study of plant growth promoting rhizobacteria (PGPR) and sowing methods on nutrient availability in wheat and rhizosphere soil under salinity stress. Rhizosphere 2022, 23, 100571. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Huang, S.; Li, L.; Gao, Q.; Wang, Y.; Zhang, S.; Huang, S.; Yuan, L.; Wen, Y.; et al. A highly conserved core bacterial microbiota with nitrogen-fixation capacity inhabits the xylem sap in maize plants. Nat. Commun. 2022, 13, 3361. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, R.; Li, Y.; Li, M.; Ma, W.; Zheng, L.; Wang, C.; Zhang, K.; Tong, Y.; Huang, G.; et al. Benzoic acid facilitates ANF in monocot crops by recruiting nitrogen-fixing Paraburkholderia. ISME J. 2024, 18, wrae210. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant Cell Environ. 2019, 42, 2028–2044. [Google Scholar] [CrossRef] [PubMed]
- Kula, A.A.; Hartnett, D.C.; Wilson, G.W. Effects of mycorrhizal symbiosis on tallgrass prairie plant-herbivore interactions. Ecol. Lett. 2005, 8, 61–69. [Google Scholar] [CrossRef]
- Yuan, T.; Ren, W.; Zhang, J.; Mahmood, M.; Jia, Z.; Zhang, S.; Wang, M.; Liang, S.; Yuan, F.; Liu, Y. Synergistic effect of grassland plants and beneficial rhizosphere bacteria helps plants cope with overgrazing stress. BMC Plant Biol. 2025, 25, 614. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant–microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Van Der Heijden, M.G. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Hou, S.; Wolinska, K.W.; Hacquard, S. Microbiota-root-shoot-environment axis and stress tolerance in plants. Curr. Opin. Plant Biol. 2021, 62, 102028. [Google Scholar] [CrossRef]
- Li, X.; Png, G.K.; Sun, S.; Shi, H.; Jin, K.; Li, Y. Positive microbial legacy and short-term clonal plasticity aid grazing tolerance of a widespread grass species. Plant Soil 2022, 473, 291–303. [Google Scholar] [CrossRef]
- Jingjing, Y.; Huiqin, G.; Fry, E.L.; Jonathan, R.; Shiming, T.; Ting, Y.; Weibo, R. Plant roots send metabolic signals to microbes in response to long-term overgrazing. Sci. Total Environ. 2022, 842, 156241. [Google Scholar] [CrossRef]
- Yuan, T.; Ren, W.; Wang, Z.; Fry, E.L.; Tang, S.; Yin, J.; Zhang, J.; Jia, Z. How does the pattern of root metabolites regulating beneficial microorganisms change with different grazing pressures? Front. Plant Sci. 2023, 14, 1180576. [Google Scholar] [CrossRef]
- Chen, L.; Wang, K.; Baoyin, T. Effects of grazing and mowing on vertical distribution of soil nutrients and their stoichiometry (C: N: P) in a semi-arid grassland of North China. Catena 2021, 206, 105507. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, S.; Wang, H.; Li, X.; Li, X.; Zhang, H. Distribution of fungal endophytes in roots of Stipa krylovii across six vegetation types in grassland of northern China. Fungal Ecol. 2018, 31, 47–53. [Google Scholar] [CrossRef]
- Chen, Y.; Bonkowski, M.; Shen, Y.; Griffiths, B.S.; Jiang, Y.; Wang, X.; Sun, B. Root ethylene mediates rhizosphere microbial community reconstruction when chemically detecting cyanide produced by neighbouring plants. Microbiome 2020, 8, 4. [Google Scholar] [CrossRef]
- Ren, L.; Su, S.; Yang, X.; Xu, Y.; Huang, Q.; Shen, Q. Intercropping with aerobic rice suppressed Fusarium wilt in watermelon. Soil Biol. Biochem. 2008, 40, 834–844. [Google Scholar] [CrossRef]
- Luo, L.-F.; Yang, L.; Yan, Z.-X.; Jiang, B.-B.; Li, S.; Huang, H.-C.; Liu, Y.-X.; Zhu, S.-S.; Yang, M. Ginsenosides in root exudates of Panax notoginseng drive the change of soil microbiota through carbon source different utilization. Plant Soil 2020, 455, 139–153. [Google Scholar] [CrossRef]
- Deng, L.; Luo, L.; Li, Y.; Wang, L.; Zhang, J.; Zi, B.; Ye, C.; Liu, Y.; Huang, H.; Mei, X. Autotoxic ginsenoside stress induces changes in root exudates to recruit the beneficial Burkholderia strain B36 as revealed by transcriptomic and metabolomic approaches. J. Agric. Food Chem. 2023, 71, 4536–4549. [Google Scholar] [CrossRef]
- Imparato, V.; Hansen, V.; Santos, S.S.; Nielsen, T.K.; Giagnoni, L.; Hauggaard-Nielsen, H.; Johansen, A.; Renella, G.; Winding, A. Gasification biochar has limited effects on functional and structural diversity of soil microbial communities in a temperate agroecosystem. Soil Biol. Biochem. 2016, 99, 128–136. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Abdelkefi, N.; Louati, I.; Mechichi, H.-Z.; Sayahi, N.; El-Sayed, W.S.; El Nayal, A.; Ismail, W.; Hanin, M.; Mechichi, T. Enhanced salt stress tolerance in tomato plants following inoculation with newly isolated plant growth-promoting rhizobacteria. Sci. Hortic. 2024, 328, 112921. [Google Scholar] [CrossRef]
- Yuan, T.; Ren, W.; Zhang, J.; Mahmood, M.; Fry, E.L.; Meng, R. Combined Transcriptomics and Metabolomics Uncover the Potential Mechanism of Plant Growth-Promoting Rhizobacteria on the Regrowth of Leymus chinensis After Mowing. Int. J. Mol. Sci. 2025, 26, 565. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lou, X.; Lu, Y.; Huang, H.; Yang, Q.; Zhang, Z.; Zhao, W.; Li, Z.; Liu, H.; Du, S. Suitable light combinations enhance cadmium accumulation in Bidens pilosa L. by regulating the soil microbial communities. Environ. Exp. Bot. 2023, 205, 105128. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Paré, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, M.; Tan, H.; Wang, Z.; Meng, M.; Sun, F.; Zhang, C.; Xi, Y. RNA sequencing reveals dynamic carbohydrate metabolism and phytohormone signaling accompanying post-mowing regeneration of forage winter wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 664933. [Google Scholar] [CrossRef]
- Castro-Valdecantos, P.; Puértolas, J.; Albacete, A.; Dodd, I.C. Girdling changes root and shoot hormonal balance but does not alter drought-induced stomatal closure in soybean. Environ. Exp. Bot. 2021, 192, 104657. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Sun, M.-Q.; Chen, Z.-L.; Xiao, Y.-T.; Wei, H.; Zhang, J.-Q.; Huang, L.; Zou, Q. Effect of foliage applied chitosan-based silicon nanoparticles on arsenic uptake and translocation in rice (Oryza sativa L.). J. Hazard. Mater. 2022, 433, 128781. [Google Scholar] [CrossRef]
- Gordillo, F.; Chávez, F.P.; Jerez, C.A. Motility and chemotaxis of Pseudomonas sp. B4 towards polychlorobiphenyls and chlorobenzoates. FEMS Microbiol. Ecol. 2007, 60, 322–328. [Google Scholar] [CrossRef]
- Hamon, M.A.; Lazazzera, B.A. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 2001, 42, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.M.; Joshi, P.; Belwalkar, M.; Archana, G. Root exudates influence chemotaxis and colonization of diverse plant growth promoting rhizobacteria in the pigeon pea-maize intercropping system. Rhizosphere 2021, 18, 100331. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Schmidt, R.; Ulanova, D.; Wick, L.Y.; Bode, H.B.; Garbeva, P. Microbe-driven chemical ecology: Past, present and future. ISME J. 2019, 13, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, X.; Tan, W.; Ling, Q.; Pei, F.; Luo, S.; Qin, P.; Yuan, H.; Huang, L.; Yu, F. Metagenomics combined with metabolomics reveals the effect of Enterobacter sp. inoculation on the rhizosphere microenvironment of Bidens pilosa L. in heavy metal contaminated soil. J. Hazard. Mater. 2023, 458, 132033. [Google Scholar] [CrossRef]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef]
- Ling, N.; Raza, W.; Ma, J.; Huang, Q.; Shen, Q. Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur. J. Soil Biol. 2011, 47, 374–379. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef]
- Wen, T.; Zhao, M.; Yuan, J.; Kowalchuk, G.A.; Shen, Q. Root exudates mediate plant defense against foliar pathogens by recruiting beneficial microbes. Soil Ecol. Lett. 2021, 3, 42–51. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Q.; Zhang, Z.; Li, Y.; Xu, N.; Qu, Q.; Lu, T.; Pan, X.; Qian, H. Enantioselective effects of imazethapyr on Arabidopsis thaliana root exudates and rhizosphere microbes. Sci. Total Environ. 2020, 716, 137121. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Li, Y.; Zhang, Z.; Cui, H.; Zhao, Q.; Liu, W.; Lu, T.; Qian, H. Effects of S-metolachlor on wheat (Triticum aestivum L.) seedling root exudates and the rhizosphere microbiome. J. Hazard. Mater. 2021, 411, 125137. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G. How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef]
- Ghitti, E.; Rolli, E.; Vergani, L.; Borin, S. Flavonoids influence key rhizocompetence traits for early root colonization and PCB degradation potential of Paraburkholderia xenovorans LB400. Front. Plant Sci. 2024, 15, 1325048. [Google Scholar] [CrossRef]
- Yaryura, P.; León, M.; Correa, O.; Kerber, N.; Pucheu, N.; García, A. Assessment of the role of chemotaxis and biofilm formation as requirements for colonization of roots and seeds of soybean plants by Bacillus amyloliquefaciens BNM339. Curr. Microbiol. 2008, 56, 625–632. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, R.; Peng, W.; Yang, Y.; Ma, X.; Zhang, W.; Ji, A.; Liu, L.; Liu, P.; Yan, L. Tea plants with gray blight have altered root exudates that recruit a beneficial rhizosphere microbiome to prime immunity against aboveground pathogen infection. Front. Microbiol. 2021, 12, 774438. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; u Rahman, M.K.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef]
- Song, H.; Wu, G.; Wang, H.; Huang, R.; Gong, X.; Wang, H. Rhizosphere inoculation of PGPR strain Bacillus cereus BC56 enhances salt tolerance of cucumber. Plant Growth Regul. 2024, 103, 509–523. [Google Scholar] [CrossRef]
- Khan, W.; Zhu, Y.; Khan, A.; Zhao, L.; Yang, Y.-M.; Wang, N.; Hao, M.; Ma, Y.; Nepal, J.; Ullah, F. Above-and below-ground feedback loop of maize is jointly enhanced by plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi in drier soil. Sci. Total Environ. 2024, 917, 170417. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Synergistic effects of biofilm-producing PGPR strains on wheat plant colonization, growth and soil resilience under drought stress. Saudi J. Biol. Sci. 2023, 30, 103664. [Google Scholar] [CrossRef] [PubMed]
- Khandagale, P.; Kansara, S.; Padsala, J.; Patel, P. Plant Growth Promoting Rhizobacteria for Sustainable Production of Sugarcane and Rice. Int. J. Plant Soil Sci. 2024, 36, 298–305. [Google Scholar] [CrossRef]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J. Genet. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Fernandez-Valverde, S.L.; Romero, J.C.M.; Martínez-Romero, E. Metatranscriptomics and nitrogen fixation from the rhizoplane of maize plantlets inoculated with a group of PGPRs. Syst. Appl. Microbiol. 2019, 42, 517–525. [Google Scholar] [CrossRef]
- Alam, M.S.; Cui, Z.-j.; Yamagishi, T.; Ishii, R. Grain yield and related physiological characteristics of rice plants (Oryza sativa L.) inoculated with free-living rhizobacteria. Plant Prod. Sci. 2001, 4, 126–130. [Google Scholar] [CrossRef]
- Zhang, T.; Li, F.Y.; Wang, H.; Wu, L.; Shi, C.; Li, Y.; Hu, J. Effects of defoliation timing on plant nutrient resorption and hay production in a semi-arid steppe. J. Plant Ecol. 2021, 14, 44–57. [Google Scholar] [CrossRef]
- Garrido-Oter, R.; Nakano, R.T.; Dombrowski, N.; Ma, K.-W.; McHardy, A.C.; Schulze-Lefert, P. Modular traits of the Rhizobiales root microbiota and their evolutionary relationship with symbiotic rhizobia. Cell Host Microbe 2018, 24, 155–167.e5. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Xing, Y.; Dao, J.; Zhao, D.; Li, Y.; Li, W.; Wang, Z. A meta-analysis on morphological, physiological and biochemical responses of plants with PGPR inoculation under drought stress. Plant Cell Environ. 2023, 46, 199–214. [Google Scholar] [CrossRef]
- Goswami, M.; Suresh, D. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, J.; Wu, J.; Wang, K.; Wu, S.; Liu, H.; Jiang, M. Transcriptome analysis of the growth-promoting effect of volatile organic compounds produced by Microbacterium aurantiacum GX14001 on tobacco (Nicotiana benthamiana). BMC Plant Biol. 2022, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, Y.; Na, X.; Wang, M.; Qu, Y.; Ge, L.; Wang, Y.; Gao, L.; Bai, W.; Bi, Y. Integrative transcriptome and metabolome revealed the molecular mechanism of Bacillus megaterium BT22-mediated growth promotion in Arabidopsis thaliana. J. Plant Physiol. 2023, 285, 153995. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Sabri, A.N.; Hasnain, S. Rhizobacterial potential to alter auxin content and growth of Vigna radiata (L.). World J. Microbiol. Biotechnol. 2010, 26, 1379–1384. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, M.; Zhang, S.; Wang, Z.; Meng, M.; Sun, F.; Zhang, C.; Xi, Y. MicroRNA and regulation of auxin and cytokinin signalling during post-mowing regeneration of winter wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2020, 155, 769–779. [Google Scholar] [CrossRef]
- Tang, D.; Quan, C.; Lin, Y.; Wei, K.; Qin, S.; Liang, Y.; Wei, F.; Miao, J. Physio-morphological, biochemical and transcriptomic analyses provide insights into drought stress responses in Mesona chinensis Benth. Front. Plant Sci. 2022, 13, 809723. [Google Scholar] [CrossRef]
- Figueiredo, M.d.V.B.; Seldin, L.; de Araujo, F.F.; Mariano, R.d.L.R. Plant growth promoting rhizobacteria: Fundamentals and applications. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–43. [Google Scholar]
- Lan, G.; Jiao, C.; Wang, G.; Sun, Y.; Sun, Y. Effects of dopamine on growth, carbon metabolism, and nitrogen metabolism in cucumber under nitrate stress. Sci. Hortic. 2020, 260, 108790. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Chen, H.; Jin, J.; Zhang, P.; Shen, L.; Hu, S.; Liu, H. Metabolomic analysis reveals the impact of ketoprofen on carbon and nitrogen metabolism in rice (Oryza sativa L.) seedling leaves. Environ. Sci. Pollut. Res. 2023, 30, 21825–21837. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef]
- Campos, M.L.; Yoshida, Y.; Major, I.T.; de Oliveira Ferreira, D.; Weraduwage, S.M.; Froehlich, J.E.; Johnson, B.F.; Kramer, D.M.; Jander, G.; Sharkey, T.D. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 2016, 7, 12570. [Google Scholar] [CrossRef]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Marcotuli, I.; Gadaleta, A.; de Pinto, M.C. The role of glutamine synthetase (GS) and glutamate synthase (GOGAT) in the Improvement of nitrogen use efficiency in cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fernie, A.R. The role of TCA cycle enzymes in plants. Adv. Biol. 2023, 7, 2200238. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hou, H.; Liu, Y.; Yin, S.; Bian, S.; Liang, S.; Wan, C.; Yuan, S.; Xiao, K.; Liu, B. Microplastics affect rice (Oryza sativa L.) quality by interfering metabolite accumulation and energy expenditure pathways: A field study. J. Hazard. Mater. 2022, 422, 126834. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Peng, X.; Yang, M.; Zhang, W.; Dazzo, F.B.; Uphoff, N.; Jing, Y.; Shen, S. Rhizobia promote the growth of rice shoots by targeting cell signaling, division and expansion. Plant Mol. Biol. 2018, 97, 507–523. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, T.; Zhang, J.; Zhang, S.; Liang, S.; Zhu, C.; Ren, W.; Liang, J. Alleviating Overgrazing Stress and Promoting Grassland Plant Regeneration via Root Exudate-Mediated Recruitment of Beneficial Bacteria. Microorganisms 2025, 13, 1225. https://doi.org/10.3390/microorganisms13061225
Yuan T, Zhang J, Zhang S, Liang S, Zhu C, Ren W, Liang J. Alleviating Overgrazing Stress and Promoting Grassland Plant Regeneration via Root Exudate-Mediated Recruitment of Beneficial Bacteria. Microorganisms. 2025; 13(6):1225. https://doi.org/10.3390/microorganisms13061225
Chicago/Turabian StyleYuan, Ting, Jiatao Zhang, Shaohong Zhang, Shuang Liang, Changhong Zhu, Weibo Ren, and Jialu Liang. 2025. "Alleviating Overgrazing Stress and Promoting Grassland Plant Regeneration via Root Exudate-Mediated Recruitment of Beneficial Bacteria" Microorganisms 13, no. 6: 1225. https://doi.org/10.3390/microorganisms13061225
APA StyleYuan, T., Zhang, J., Zhang, S., Liang, S., Zhu, C., Ren, W., & Liang, J. (2025). Alleviating Overgrazing Stress and Promoting Grassland Plant Regeneration via Root Exudate-Mediated Recruitment of Beneficial Bacteria. Microorganisms, 13(6), 1225. https://doi.org/10.3390/microorganisms13061225






