Isolation and Characterization of Microorganisms from Buckwheat Farmland for the Bioconversion of Quercetin
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Bacterial Isolation
2.2. Bacterial Identification
2.3. Screening of Flavonoid-Converting Bacteria
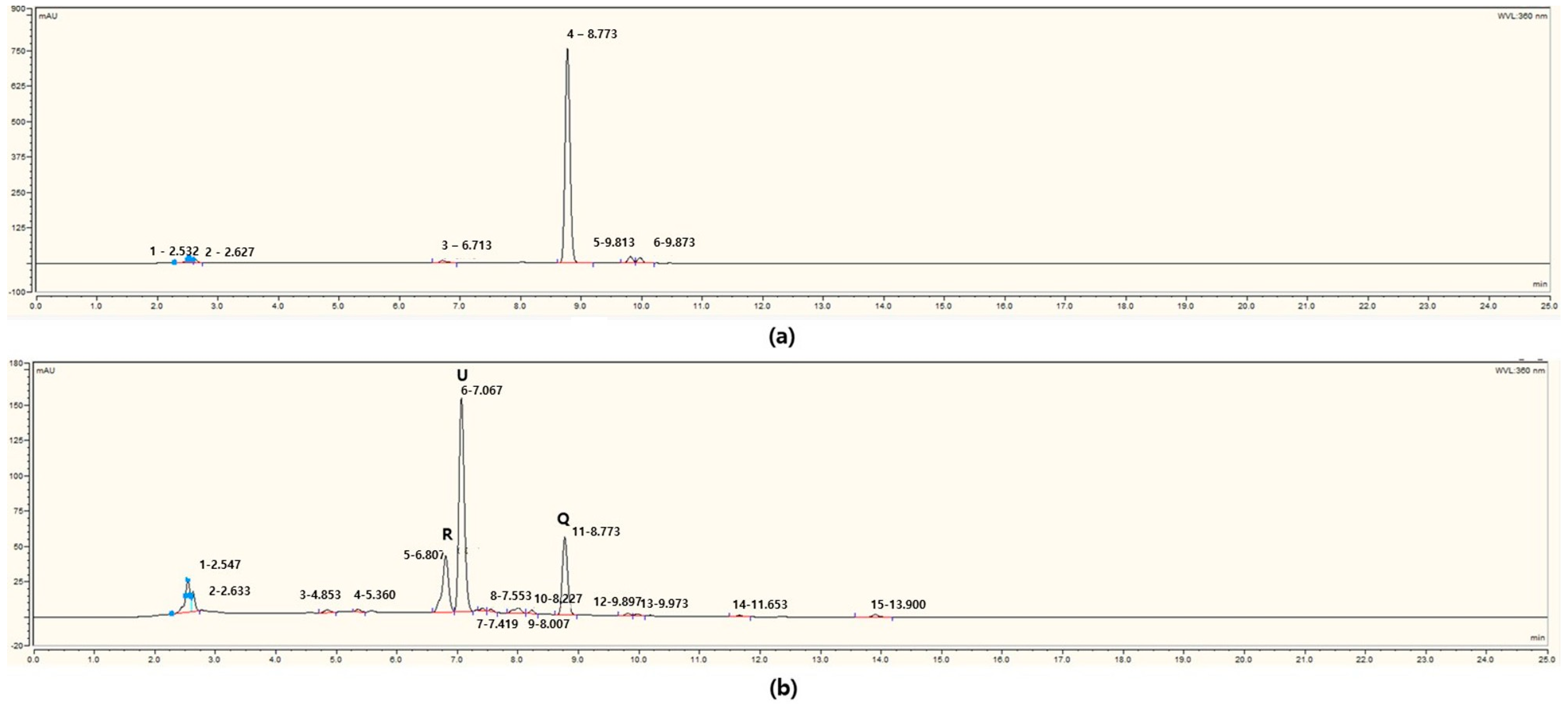
2.4. Rutin and Quercetin Quantification Using HPLC
2.5. Unknown Compound Analysis Using HPLC-TOF/MS
3. Results and Discussion
3.1. Isolation of Bacteria
3.2. Quercetin Bioconversion
3.3. Changes in Flavonoid Content Before and After Bioconversion Using 3P-1
3.4. HPLC/MS Analysis of Fermented Flavonoids Using Isolated Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| -OH | Hydroxyl |
| HPLC | High-performance liquid chromatography |
| LC/Q-TOF | Liquid chromatography/quantitative time-of-flight mass spectrometry |
| MS | Mass spectrometry |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| TOF/MS | Time-of-flight mass spectrometry |
References
- Cook, N.C.; Samman, S. Flavonoids? Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Eur. Ceram. Soc. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Jing, R.; Li, H.Q.; Hu, C.L.; Jiang, Y.P.; Qin, L.P.; Zheng, C.J. Phytochemical and pharmacological profiles of three Fagopyrum buckwheats. Int. J. Mol. Sci. 2016, 17, 589. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Nathiya, S.; Durga, M.; Devasena, T. Quercetin, encapsulated quercetin and its application—A review. Analgesia 2014, 10, 20–26. [Google Scholar]
- Di Gioia, D.; Strahsburger, E.; Lopez de Lacey, A.M.; Bregola, V.; Marotti, I.; Aloisio, I.; Biavati, B.; Dinelli, G. Flavonoid bioconversion in Bifidobacterium pseudocatenulatum B7003: A potential probiotic strain for functional food development. J. Funct. Foods 2014, 7, 671–679. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, Q.; Wen, L.; Yang, B.; Jiang, G.; Gao, H.; Chen, H.; Jiang, Y. Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem. 2014, 145, 220–227. [Google Scholar] [CrossRef]
- Perkins, C.; Siddiqui, S.; Puri, M.; Demain, A.L. Biotechnological applications of microbial bioconversions. Crit. Rev. Biotechnol. 2016, 36, 1050–1065. [Google Scholar] [CrossRef]
- Missoum, A. Methods for isolation and identification of microorganisms. In Microbial Systematics; CRC Press: Boca Raton, FL, USA, 2020; pp. 28–50. [Google Scholar]
- Ponnusamy, L.; Xu, N.; Nojima, S.; Wesson, D.M.; Schal, C.; Apperson, C.S. Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 9262–9267. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Atala, E.; Fuentes, J.; Wehrhahn, M.J.; Speisky, H. Quercetin and related flavonoids conserve their antioxidant properties despite undergoing chemical or enzymatic oxidation. Food Chem. 2017, 234, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Espinal, A.; Yañez, O.; Osorio, E.; Areche, C.; García-Beltrán, O.; Ruiz, L.M.; Cassels, B.K.; Tiznado, W. Theoretical study of the antioxidant activity of quercetin oxidation products. Front. Chem. 2019, 7, 818. [Google Scholar] [CrossRef]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef]
- Cho, K.M.; Hong, S.Y.; Math, R.K.; Lee, J.H.; Kambiranda, D.M.; Kim, J.M.; Islam, S.M.A.; Yun, M.G.; Cho, J.J.; Lim, W.J.; et al. Biotransformation of phenolics (isoflavones, flavanols and phenolic acids) during the fermentation of cheonggukjang by Bacillus pumilus HY1. Food Chem. 2009, 114, 413–419. [Google Scholar] [CrossRef]
- Chung, I.M.; Seo, S.H.; Ahn, J.K.; Kim, S.H. Effect of processing, fermentation, and aging treatment to content and profile of phenolic compounds in soybean seed, soy curd and soy paste. Food Chem. 2011, 127, 960–967. [Google Scholar] [CrossRef]
- Rao, K.V.; Weisner, N.T. Microbial transformation of quercetin by Bacillus cereus. Appl. Environ. Microbiol. 1981, 42, 450–452. [Google Scholar] [CrossRef]
- Berby, B.; Bichara, C.; Rives-Feraille, A.; Jumeau, F.; Pizio, P.D.; Sétif, V.; Sibert, L.; Dumont, L.; Rondanino, C.; Rives, N. Oxidative stress is associated with telomere interaction impairment and chromatin condensation defects in spermatozoa of infertile males. Antioxidants 2021, 10, 593. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.; Morris, M.E. Pharmacokinetics and bioavailability of quercetin and its glycosides. Pharm. Res. 2021, 38, 1061–1070. [Google Scholar]
- Agrawal, P.K.; Blunden, G.; Jacob, C. Antiviral significance of isoquercetin (quercetin-3-o-glucoside) with special reference to its anti-coronaviral potential. Nat. Prod. Commun. 2024, 19, 1934578X231219560. [Google Scholar] [CrossRef]
- Lucci, N.; Mazzafera, P. Rutin synthase in fava d’anta: Purification and influence of stressors. Can. J. Plant Sci. 2009, 89, 895–902. [Google Scholar] [CrossRef]
- Miadoková, E. Isoflavonoids–an overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211. [Google Scholar] [CrossRef]
- Kang, J.Y.; Park, W.J.; Yoon, Y.; Kim, B.G. Production of isoquercitrin from quercetin by biotransformation using Bacillus sp. CSQ10 isolated from Camellia sinensis cultivation soils. Appl. Biol. Chem. 2022, 65, 59. [Google Scholar] [CrossRef]
- Han, J.; Ma, J.; He, R.; Yang, F.; Meng, J.; Liu, J.; Shi, F.; Duan, J.; Chen, L.; Zhang, S. Efficient directional biosynthesis of isoquercitrin from quercetin by Bacillus subtilis CD-2 and its anti-inflammatory activity. Nat. Prod. Res. 2024, 1–5. [Google Scholar] [CrossRef]
- PubChem. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004. PubChem Compound Summary for CID 5280343, Quercetin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Quercetin (accessed on 13 April 2025).
- PubChem. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004. PubChem Compound Summary for CID 5280804, Isoquercetin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Isoquercetin (accessed on 13 April 2025).
- PubChem. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004. PubChem Compound Summary for CID 5280805, Rutin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Rutin (accessed on 13 April 2025).


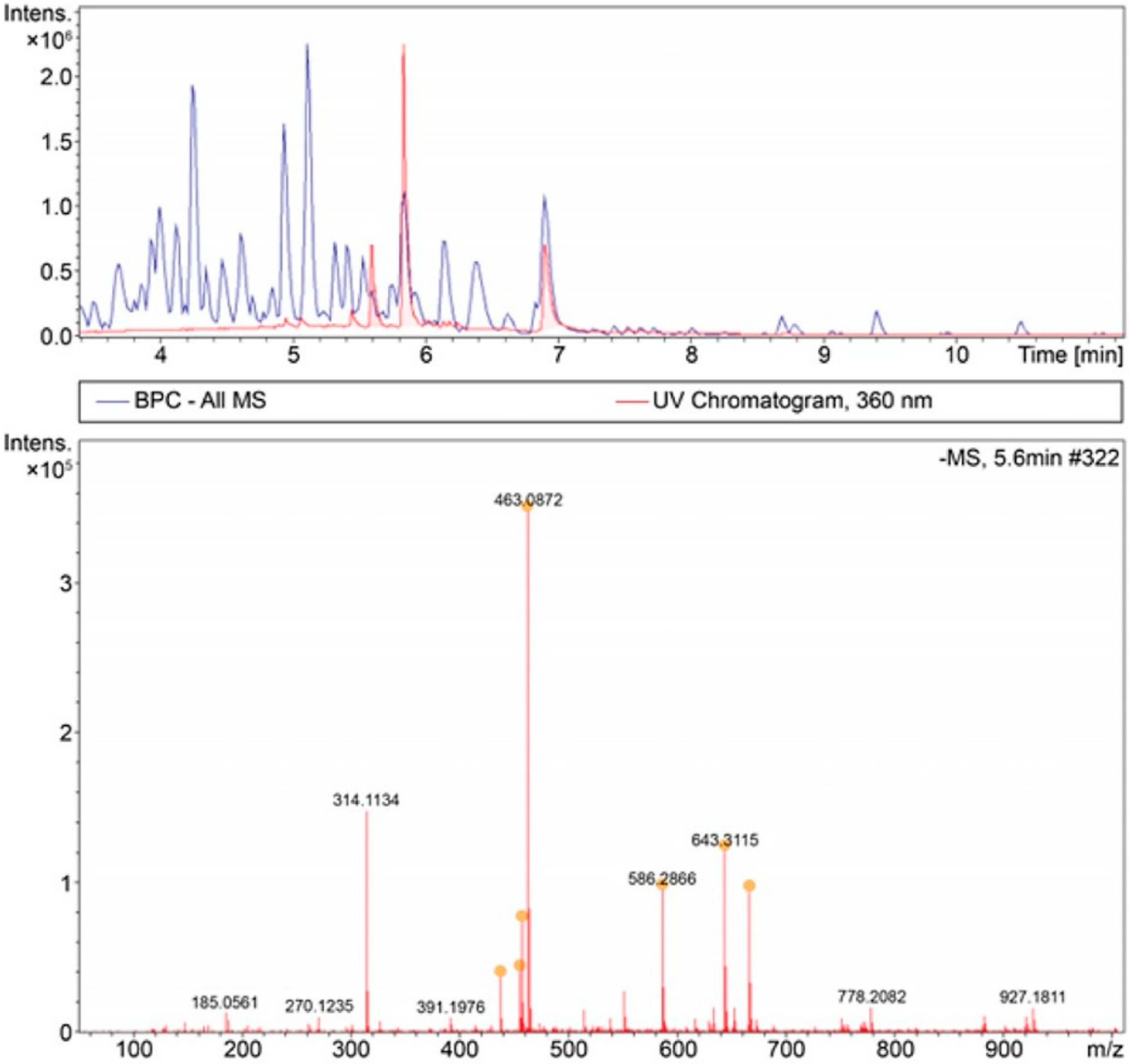


| Retention Time | Putative Molecular Weight (m/z) | Putative Chemical Compositions | Putative Molecular Weight Based on Ion Formula |
|---|---|---|---|
| 5.6 | 437.275 | C21H41O9 | 437.2756 |
| 455.2130 | C17H23N14O2 | 455.2134 | |
| C16H27N10O6 | 455.2121 | ||
| C19H35O12 | 455.2134 | ||
| 457.2440 | C23H37O9 | 457.2443 | |
| 463.0872 | C21H19O12 | 463.0882 | |
| C18H11N10O6 | 463.0869 | ||
| C19H7N14O2 | 463.0882 | ||
| 586.2866 | C28H44NO12 | 586.2869 | |
| C25H36N11O6 | 586.2856 | ||
| C26H32N15O2 | 586.2869 | ||
| 643.3115 | C32H39N10O5 | 643.3110 | |
| C33H35N14O | 643.3124 | ||
| 666.3451 | C27H40N17O4 | 666.3455 | |
| C26H44N13O8 | 666.3441 | ||
| 5.8 | 463.0872 | C21H19O12 | 463.0882 |
| C18H11N10O6 | 463.0869 | ||
| C19H7N14O2 | 463.0882 | ||
| 563.1039 | C22H15N10O9 | 563.1029 | |
| C23H11N14O5 | 563.1042 | ||
| C25H23O15 | 563.1042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Yang, J.; Cho, B.-S.; Han, J.; Yang, J.-Y. Isolation and Characterization of Microorganisms from Buckwheat Farmland for the Bioconversion of Quercetin. Microorganisms 2025, 13, 1224. https://doi.org/10.3390/microorganisms13061224
Shin J, Yang J, Cho B-S, Han J, Yang J-Y. Isolation and Characterization of Microorganisms from Buckwheat Farmland for the Bioconversion of Quercetin. Microorganisms. 2025; 13(6):1224. https://doi.org/10.3390/microorganisms13061224
Chicago/Turabian StyleShin, Jiyoung, Junho Yang, Beom-Su Cho, Jisoo Han, and Ji-Young Yang. 2025. "Isolation and Characterization of Microorganisms from Buckwheat Farmland for the Bioconversion of Quercetin" Microorganisms 13, no. 6: 1224. https://doi.org/10.3390/microorganisms13061224
APA StyleShin, J., Yang, J., Cho, B.-S., Han, J., & Yang, J.-Y. (2025). Isolation and Characterization of Microorganisms from Buckwheat Farmland for the Bioconversion of Quercetin. Microorganisms, 13(6), 1224. https://doi.org/10.3390/microorganisms13061224






