Clostridium butyricum Ameliorates Atherosclerosis by Regulating Host Linoleic Acid Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Bacterial Preparation
2.3. Fecal DNA Extraction and qPCR Analysis
2.4. Assessment of Atherosclerotic Lesions in the Whole Aorta and Aortic Root
2.5. Sample Preparation and Untargeted Metabolomics Analysis
2.6. Immunofluorescence Analysis
2.7. Flow Cytometric Analysis
2.8. Data Statistical Analysis
3. Results
3.1. C. butyricum Inhibits Atherosclerosis in HFD-Fed Apoe−/− Mice
3.2. Impact of C. butyricum Administration on the Serum Metabolic Profiles in HFD-Fed Apoe−/− Mice
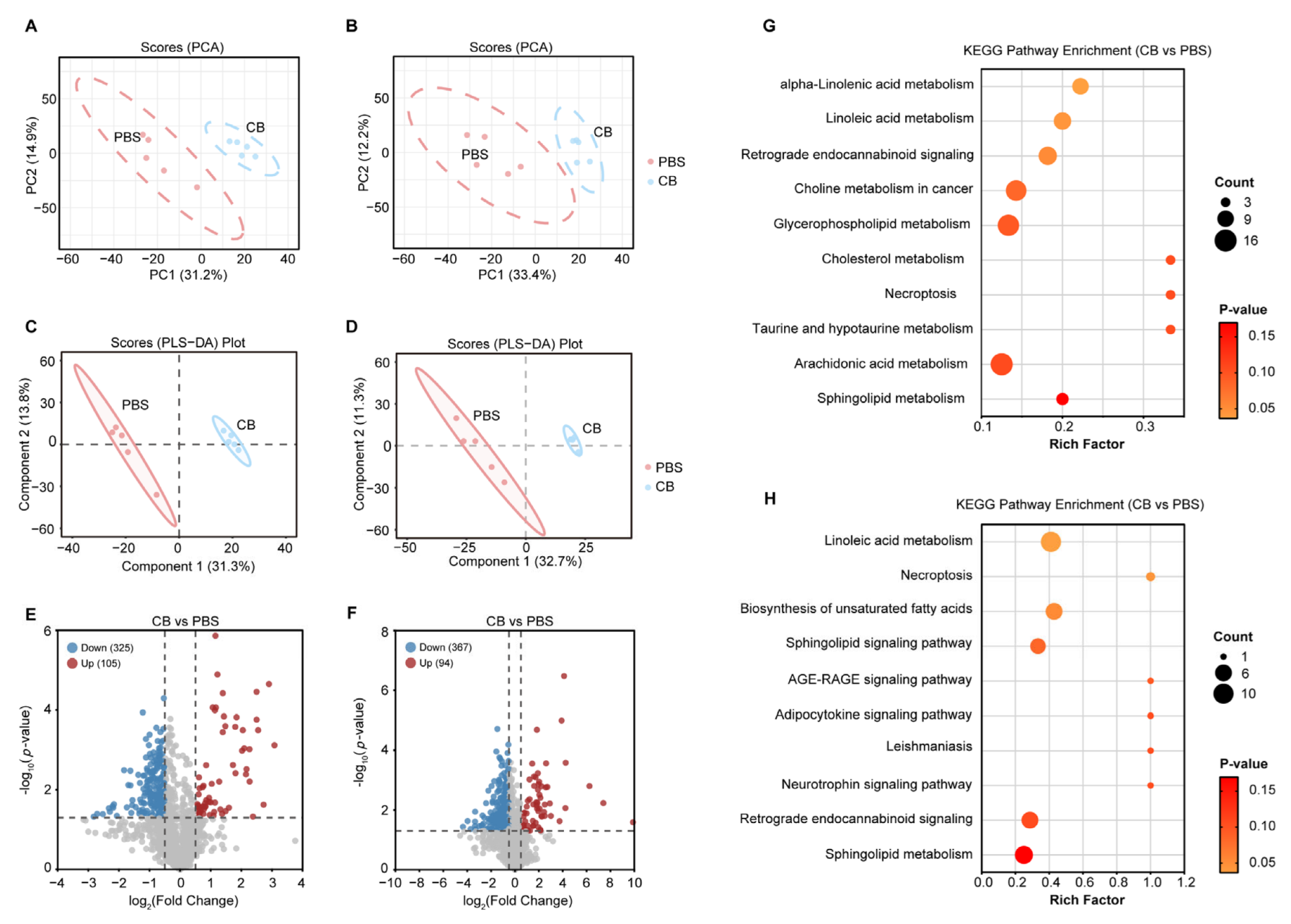
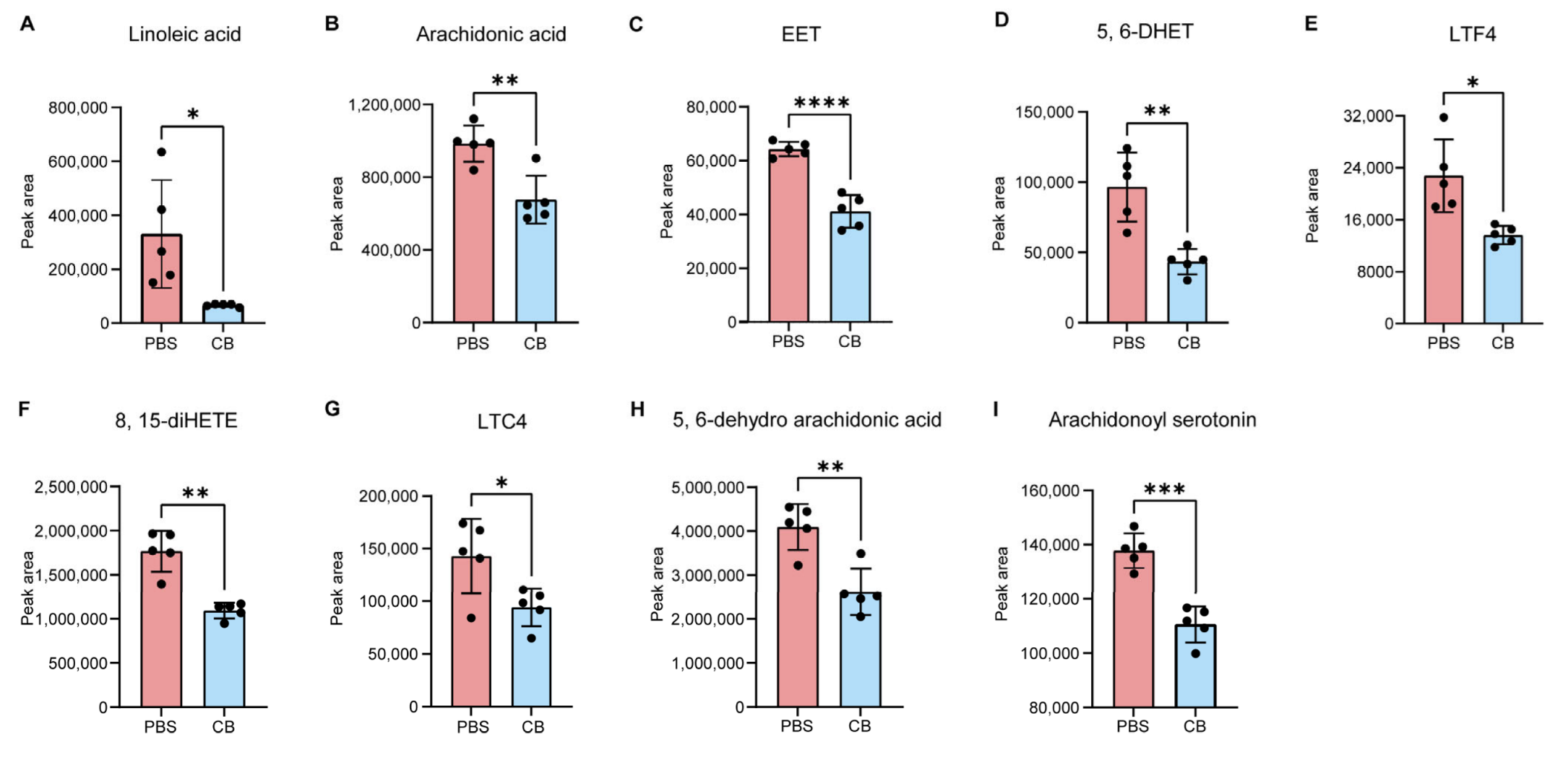
3.3. C. butyricum Modulates Host Linoleic Acid Metabolism
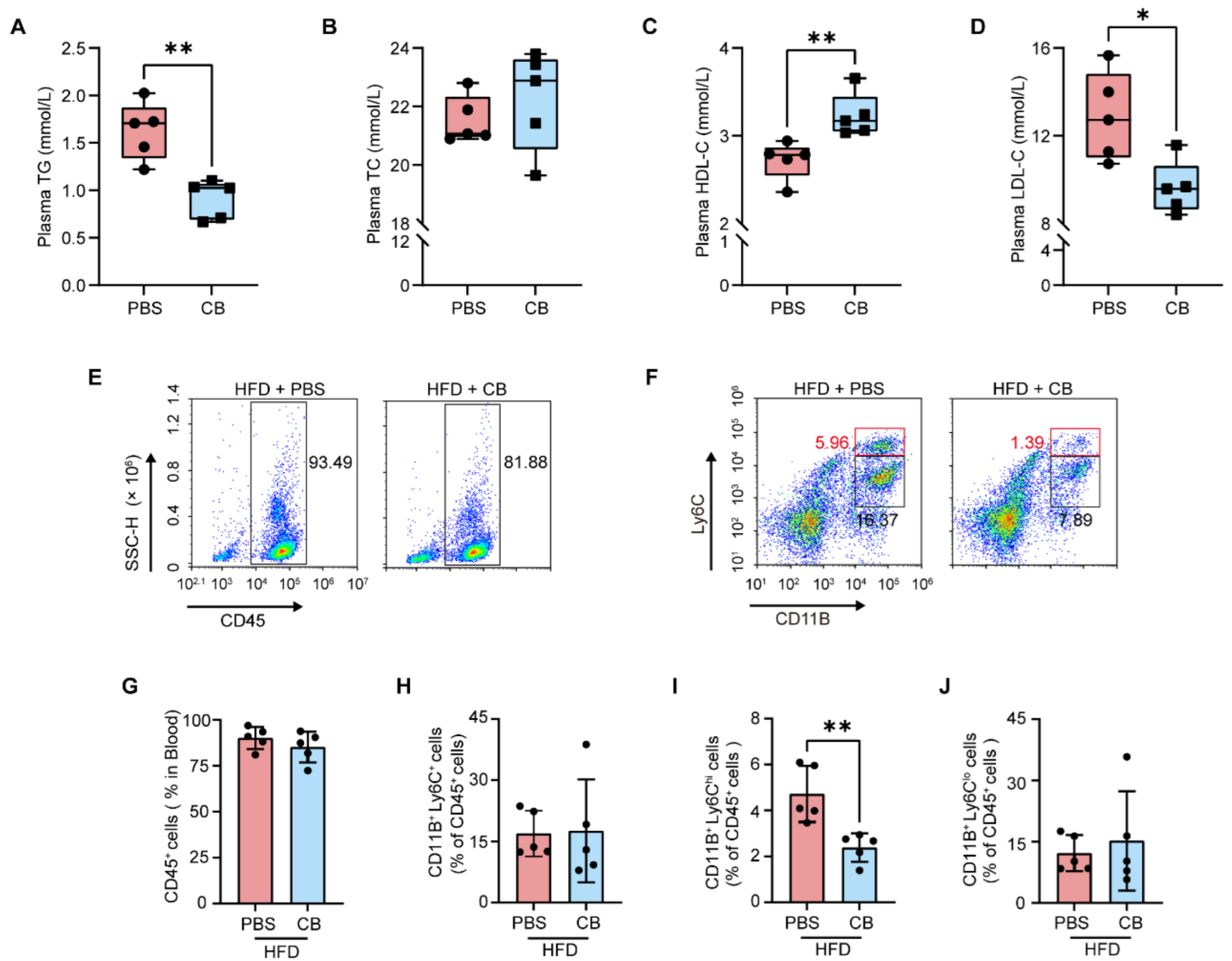
3.4. C. butyricum Reduces Circulating Pro-Inflammatory Monocytes by Improving HFD-Induced Dyslipidemia in Apoe−/− Mice
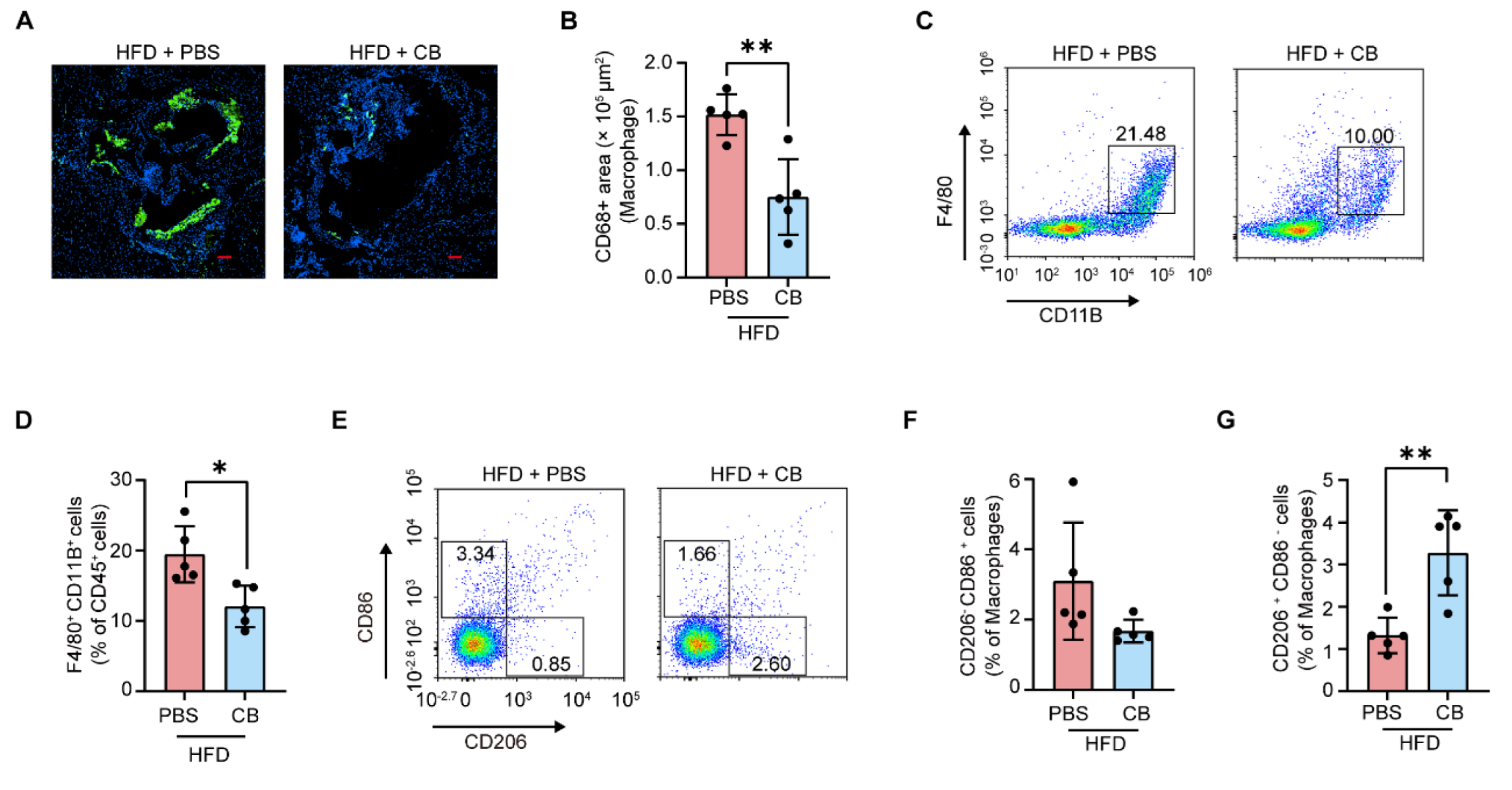
3.5. C. butyricum Attenuates Macrophage Infiltration in the Aortic Root and Promotes M2 Macrophage Polarization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Atherosclerosis |
| CB | Clostridium butyricum |
| ASCVD | Atherosclerotic cardiovascular disease |
| CVD | Cardiovascular disease |
| DSS | Dextran sodium sulfate |
| SCFAs | short-chain fatty acids |
| HFD | high-fat diet |
| Th17 | T helper cell 17 |
| Treg | Regulatory cell |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares-discriminant analysis |
| CFU | Colony-forming units |
| TG | Triglycerides |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| TC | Total cholesterol |
| qPCR | Quantitative PCR |
| PFA | Paraformaldehyde |
| OCT | optimal cutting temperature |
| LC-MS | Liquid chromatography-tandem mass spectrometry |
| KEGG | Kyoto encyclopedia of genes and genomes |
| PUFA | Polyunsaturated fatty acid |
| ARA | Arachidonic acid |
| COX | Cyclooxygenase |
| LOX | Lipoxygenase |
| CYP450 | Cytochrome P450 |
| EET | Epoxyeicosatrienoic acid |
| 5, 6-DHET | 5, 6-dihydroxyeicosatrienoic acid |
| LTF4 | Leukotriene F4 |
| LTC4 | Leukotriene C4 |
| 8,15-diHETE | 8,15-dihydroxyeicosatetraenoate |
| ox-LDL | Oxidized LDL |
| NGP | Next-generation probiotic |
| LPS | Lipopolysaccharide |
References
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, M.-X.; Xie, L.-S.; Wang, W.-Z.; Chen, B.-S.; Yun, C.-Y.; Sun, X.-W.; Luo, X.; Jiang, Y.; Wang, K.; et al. Gut commensal Christensenella minuta modulates host metabolism via acylated secondary bile acids. Nat. Microbiol. 2024, 9, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, W.; Qi, Z.; Gao, R.; Tong, J.; Gao, T.; Zhang, Y.; Sun, A.; Zhang, S.; Ge, J. Gut microbe-derived metabolite indole-3-carboxaldehyde alleviates atherosclerosis. Signal Transduct. Target. Ther. 2023, 8, 378. [Google Scholar] [CrossRef]
- Nie, Q.; Luo, X.; Wang, K.; Ding, Y.; Jia, S.; Zhao, Q.; Li, M.; Zhang, J.; Zhuo, Y.; Lin, J.; et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway. Cell 2024, 187, 2717–2734.e33. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Hu, X.; Ye, C.; Nie, Q.; Wang, K.; Yan, S.; Lin, J.; Xu, F.; Li, M.; et al. Candida albicans accelerates atherosclerosis by activating intestinal hypoxia-inducible factor2α signaling. Cell Host Microbe 2024, 32, 964–979.e7. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, X.; Hu, S.; Huang, Z.; Hu, M.; Jin, S.; Lu, B.; Sun, K.; Wang, Z.; Fu, J.; et al. Gut microbial DL-endopeptidase alleviates Crohn’s disease via the NOD2 pathway. Cell Host Microbe 2022, 30, 1435–1449.e9. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Zhang, Q.; Pradhan, M.; Mehrabian, M.; Lusis, A.J.; Bergström, G.; Bäckhed, F.; Rey, F.E. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053.e10. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Stražar, M.; Mohamed, A.M.; Pacheco, J.A.; Walker, R.L.; Lebar, T.; Zhao, S.; Lockart, J.; Dame, A.; Thurimella, K.; et al. Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria. Cell 2024, 187, 1834–1852.e19. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Hu, X.; Niu, H.; Tian, R.; Wang, H.; Pang, H.; Jiang, L.; Qiu, B.; Chen, X.; et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 2019, 7, 68. [Google Scholar] [CrossRef]
- Talmor-Barkan, Y.; Bar, N.; Shaul, A.A.; Shahaf, N.; Godneva, A.; Bussi, Y.; Lotan-Pompan, M.; Weinberger, A.; Shechter, A.; Chezar-Azerrad, C.; et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat. Med. 2022, 28, 295–302. [Google Scholar] [CrossRef]
- Yan, Y.; Lei, Y.; Qu, Y.; Fan, Z.; Zhang, T.; Xu, Y.; Du, Q.; Brugger, D.; Chen, Y.; Zhang, K.; et al. Bacteroides uniformis-induced perturbations in colonic microbiota and bile acid levels inhibit TH17 differentiation and ameliorate colitis developments. npj Biofilms Microbiomes 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Ambat, A.; Antony, L.; Maji, A.; Ghimire, S.; Mattiello, S.; Kashyap, P.C.; More, S.; Sebastian, V.; Scaria, J. Enhancing recovery from gut microbiome dysbiosis and alleviating DSS-induced colitis in mice with a consortium of rare short-chain fatty acid-producing bacteria. Gut Microbes 2024, 16, 2382324. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Nelson, J.W.; Eskew, J.R.; Ganesan, A.; Ajami, N.J.; Petrosino, J.F.; Bryan, R.M., Jr.; Durgan, D.J. Prebiotics, probiotics, and acetate supplementation prevent Hypertension in a model of obstructive sleep apnea. Hypertension 2018, 72, 1141–1150. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Paone, P.; Suriano, F.; Jian, C.; Korpela, K.; Delzenne, N.M.; Van Hul, M.; Salonen, A.; Cani, P.D. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes 2022, 14, 2152307. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut microbiota and cardiovascular disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Chajadine, M.; Laurans, L.; Radecke, T.; Mouttoulingam, N.; Al-Rifai, R.; Bacquer, E.; Delaroque, C.; Rytter, H.; Bredon, M.; Knosp, C.; et al. Harnessing intestinal tryptophan catabolism to relieve atherosclerosis in mice. Nat. Commun. 2024, 15, 6390. [Google Scholar] [CrossRef]
- Li, T.; Ma, X.; Wang, T.; Tian, W.; Liu, J.; Shen, W.; Liu, Y.; Li, Y.; Zhang, X.; Ma, J.; et al. Clostridium butyricum inhibits the inflammation in children with primary nephrotic syndrome by regulating Th17/Tregs balance via gut-kidney axis. BMC Microbiol. 2024, 24, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Qiu, S.; Zhang, L.; Li, Y.; Zhang, J.; Shen, D.; Zhao, P.; Yuan, L.; Zhao, L.; Duan, Y.; et al. Supplementation of Clostridium butyricum alleviates vascular inflammation in diabetic mice. Diabetes Metab. J. 2024, 48, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.L.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Weng, J.; Yan, J.; Zeng, Y.-H.; Hao, Q.-Y.; Sheng, H.-F.; Hua, Y.-Q.; Deng, Y.; Wen, Z.-P.; Wu, Z.-Y.; et al. Puerarin alleviates atherosclerosis via the inhibition of Prevotella copri and its trimethylamine production. Gut 2024, 73, 1934–1943. [Google Scholar]
- Wu, J.; Zhou, B.; Pang, X.; Song, X.; Gu, Y.; Xie, R.; Liu, T.; Xu, X.; Wang, B.; Cao, H. Clostridium butyricum, a butyrate-producing potential probiotic, alleviates experimental colitis through epidermal growth factor receptor activation. Food Funct. 2022, 13, 7046–7061. [Google Scholar] [CrossRef]
- Luo, X.; Han, Z.; Kong, Q.; Wang, Y.; Mou, H.; Duan, X. Clostridium butyricum prevents dysbiosis and the rise in blood pressure in spontaneously hypertensive rats. Int. J. Mol. Sci. 2023, 24, 4955. [Google Scholar] [CrossRef]
- Alexander, M.; Ang, Q.Y.; Nayak, R.R.; Bustion, A.E.; Sandy, M.; Zhang, B.; Upadhyay, V.; Pollard, K.S.; Lynch, S.V.; Turnbaugh, P.J. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe 2021, 30, 17–30.e9. [Google Scholar] [CrossRef]
- Kikuchi, E.; Miyamoto, Y.; Narushima, S. Design of species-specific primers to identify 13 species of Clostridium harbored in human intestinal tracts. Microbiol. Immunol. 2002, 46, 353–358. [Google Scholar] [CrossRef]
- Xie, S.; Li, J.; Lyu, F.; Xiong, Q.; Gu, P.; Chen, Y.; Chen, M.; Bao, J.; Zhang, X.; Wei, R.; et al. Novel tripeptide RKH derived from Akkermansia muciniphila protects against lethal sepsis. Gut 2023, 73, 78–91. [Google Scholar] [CrossRef]
- Choi, M.; Jang, H.-S.; Son, T.; Kim, D.; Youn, Y.-J.; Hwang, G.-B.; Choi, Y.P.; Jeong, Y.H. Effect sizes of cognitive and locomotive behavior tests in the 5XFAD-J mouse model of alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 15064. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, W.; Cheng, X.; Yang, H.; She, Z.-G.; Cai, J.; Li, H.; Zhang, X.-J. Emerging roles and therapeutic applications of arachidonic acid pathways in cardiometabolic diseases. Circ. Res. 2024, 135, 222–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Alsaad, A.M.S.; Zordoky, B.N.M.; Tse, M.M.Y.; El-Kadi, A.O.S. Role of cytochrome P450–mediated arachidonic acid metabolites in the pathogenesis of cardiac hypertrophy. Drug Metab. Rev. 2013, 45, 173–195. [Google Scholar] [CrossRef]
- Campbell, W.B.; Gebremedhin, D.; Pratt, P.F.; Harder, D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996, 78, 415–423. [Google Scholar] [CrossRef]
- Zordoky, B.N.; El-Kadi, A.O. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol. Ther. 2010, 125, 446–463. [Google Scholar] [CrossRef]
- Picklo, M.J., Sr.; Newman, J.W. Antioxidant supplementation and obesity have independent effects on hepatic oxylipin profiles in insulin-resistant, obesity-prone rats. Free Radic Biol Med. 2015, 89, 182–191. [Google Scholar] [CrossRef]
- Levi-Rosenzvig, R.; Beyer, A.M.; Hockenberry, J.; Ben-Shushan, R.S.; Chuyun, D.; Atiya, S.; Tamir, S.; Gutterman, D.D.; Szuchman-Sapir, A. 5,6-δ-DHTL, a stable metabolite of arachidonic acid, is a potential EDHF that mediates microvascular dilation. Free. Radic. Biol. Med. 2017, 103, 87–94. [Google Scholar] [CrossRef]
- Arnold, W.R.; Carnevale, L.N.; Xie, Z.; Baylon, J.L.; Tajkhorshid, E.; Hu, H.; Das, A. Anti-inflammatory dopamine- and serotonin-based endocannabinoid epoxides reciprocally regulate cannabinoid receptors and the TRPV1 channel. Nat. Commun. 2021, 12, 926. [Google Scholar] [CrossRef]
- Bjorkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Yu, H.; Qin, X.; Yu, Z.; Chen, Y.; Tang, L.; Shan, W. Effects of high-fat diet on the formation of depressive-like behavior in mice. Food Funct. 2021, 12, 6416–6431. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Akhtari, M.; Tolani, S.; Pagler, T.; Bijl, N.; Kuo, C.-L.; Wang, M.; Sanson, M.; Abramowicz, S.; Welch, C.; et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Investig. 2011, 121, 4138–4149. [Google Scholar] [CrossRef] [PubMed]
- Potteaux, S.; Gautier, E.L.; Hutchison, S.B.; Van Rooijen, N.; Rader, D.J.; Thomas, M.J.; Sorci-Thomas, M.G.; Randolph, G.J. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J. Clin. Investig. 2011, 121, 2025–2036. [Google Scholar] [CrossRef] [PubMed]
- A Stiekema, L.C.; Willemsen, L.; Kaiser, Y.; Prange, K.H.M.; Wareham, N.J.; Boekholdt, S.M.; Kuijk, C.; de Winther, M.P.J.; Voermans, C.; Nahrendorf, M.; et al. Impact of cholesterol on proinflammatory monocyte production by the bone marrow. Eur. Hear. J. 2021, 42, 4309–4320. [Google Scholar] [CrossRef]
- Yao, M.; Kitamura, F.; Han, Y.; Du, H.; McClements, D.J.; Xiao, H. Adverse effects of linoleic acid: Influence of lipid oxidation on lymphatic transport of citrus flavonoid and enterocyte morphology. Food Chem. 2022, 369, 130968. [Google Scholar] [CrossRef]
- Spiteller, D.; Spiteller, G. Oxidation of linoleic acid in low-density lipoprotein: An important event in atherogenesis. Angew. Chem. Int. Ed. Engl. 2000, 39, 585–589. [Google Scholar] [CrossRef]
- Hennig, B.; Shasby, D.M.; Spector, A.A. Exposure to fatty acid increases human low density lipoprotein transfer across cultured endothelial monolayers. Circ. Res. 1985, 57, 776–780. [Google Scholar] [CrossRef]
- Spiteller, G. Peroxidation of linoleic acid and its relation to aging and age dependent diseases. Mech. Ageing Dev. 2001, 122, 617–657. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Valencia, R.; Kranrod, J.W.; Fang, L.; Soliman, A.M.; Azer, B.; Clemente-Casares, X.; Seubert, J.M. Linoleic acid-derived diol 12,13-DiHOME enhances NLRP3 inflammasome activation in macrophages. FASEB J. 2024, 38, e23748. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 272. [Google Scholar] [CrossRef]
- Lovren, F.; Teoh, H.; Verma, S. Obesity and atherosclerosis: Mechanistic insights. Can. J. Cardiol. 2015, 31, 177–183. [Google Scholar] [CrossRef]
- Schleh, M.W.; Caslin, H.L.; Garcia, J.N.; Mashayekhi, M.; Srivastava, G.; Bradley, A.B.; Hasty, A.H. Metaflammation in obesity and its therapeutic targeting. Sci. Transl. Med. 2023, 15, eadf9382. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kameyama, K.; Miyauchi, E.; Nakanishi, Y.; Kanaya, T.; Fujii, T.; Kato, T.; Sasaki, T.; Tachibana, N.; Negishi, H.; et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023, 35, 361–375.e9. [Google Scholar] [CrossRef]
- Hsieh, J.; Koseki, M.; Molusky, M.M.; Yakushiji, E.; Ichi, I.; Westerterp, M.; Iqbal, J.; Chan, R.B.; Abramowicz, S.; Tascau, L.; et al. TTC39B deficiency stabilizes LXR reducing both atherosclerosis and steatohepatitis. Nature 2016, 535, 303–307. [Google Scholar] [CrossRef]
- Meng, Z.-X.; Wang, L.; Chang, L.; Sun, J.; Bao, J.; Li, Y.; Chen, Y.E.; Lin, J.D. A diet-sensitive BAF60a-mediated pathway links hepatic bile acid metabolism to cholesterol absorption and atherosclerosis. Cell Rep. 2015, 13, 1658–1669. [Google Scholar] [CrossRef]
- Tao, G.; Wang, H.; Shen, Y.; Zhai, L.; Liu, B.; Wang, B.; Chen, W.; Xing, S.; Chen, Y.; Gu, H.-M.; et al. Surf4 (Surfeit locus protein 4) deficiency reduces intestinal lipid absorption and secretion and decreases metabolism in mice. Arter. Thromb. Vasc. Biol. 2023, 43, 562–580. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Jadhav, K.; Pan, X.; Zhu, Y.; Hu, S.; Chen, S.; Chen, L.; Tang, Y.; Wang, H.H.; et al. Hepatocyte ATF3 protects against atherosclerosis by regulating HDL and bile acid metabolism. Nat. Metab. 2021, 3, 59–74. [Google Scholar] [CrossRef]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.-I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef]
- Zheng, M.; Ye, H.; Yang, X.; Shen, L.; Dang, X.; Liu, X.; Gong, Y.; Wu, Q.; Wang, L.; Ge, X.; et al. Probiotic Clostridium butyricum ameliorates cognitive impairment in obesity via the microbiota-gut-brain axis. Brain Behav. Immun. 2023, 115, 565–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, Z.; Wang, Q.; Wu, C.; Sun, Y.; Wang, Z.; Xu, X.; Xue, W.; Cao, Z.; Zhang, M.; et al. Bacteroides methylmalonyl-CoA mutase produces propionate that promotes intestinal goblet cell differentiation and homeostasis. Cell Host Microbe 2023, 32, 63–78.e7. [Google Scholar] [CrossRef]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jiang, Y.; Wu, L.; Fu, J.; Du, J.; Luo, Z.; Guo, L.; Xu, J.; Liu, Y. Porphyromonas gingivalis aggravates colitis via a gut microbiota-linoleic acid metabolism-Th17/Treg cell balance axis. Nat. Commun. 2024, 15, 1617. [Google Scholar] [CrossRef]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Fan, P.; Mou, X.; Zhao, W. Clostridium butyricum Ameliorates Atherosclerosis by Regulating Host Linoleic Acid Metabolism. Microorganisms 2025, 13, 1220. https://doi.org/10.3390/microorganisms13061220
Yin C, Fan P, Mou X, Zhao W. Clostridium butyricum Ameliorates Atherosclerosis by Regulating Host Linoleic Acid Metabolism. Microorganisms. 2025; 13(6):1220. https://doi.org/10.3390/microorganisms13061220
Chicago/Turabian StyleYin, Chao, Peizhi Fan, Xiangyu Mou, and Wenjing Zhao. 2025. "Clostridium butyricum Ameliorates Atherosclerosis by Regulating Host Linoleic Acid Metabolism" Microorganisms 13, no. 6: 1220. https://doi.org/10.3390/microorganisms13061220
APA StyleYin, C., Fan, P., Mou, X., & Zhao, W. (2025). Clostridium butyricum Ameliorates Atherosclerosis by Regulating Host Linoleic Acid Metabolism. Microorganisms, 13(6), 1220. https://doi.org/10.3390/microorganisms13061220





