The Impacts of Farming Activities on the Coevolutionary Structure of Plant Rhizosphere Soil Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
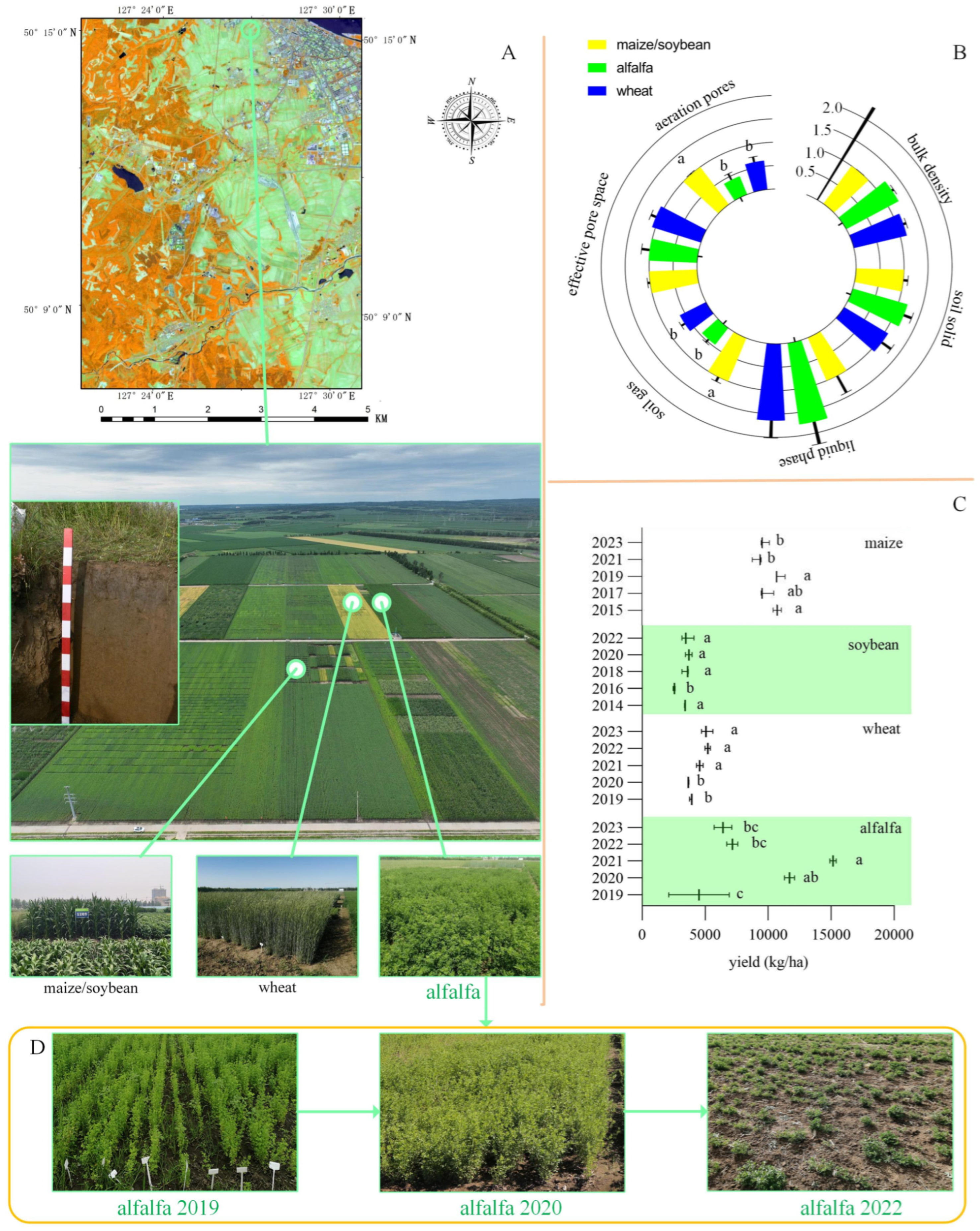
2.1. Experimental Area and Location
2.2. Experimental Design and Sample Collection
2.3. Determination of Soil Physical and Chemical Properties
2.4. Soil Microbial Measurement
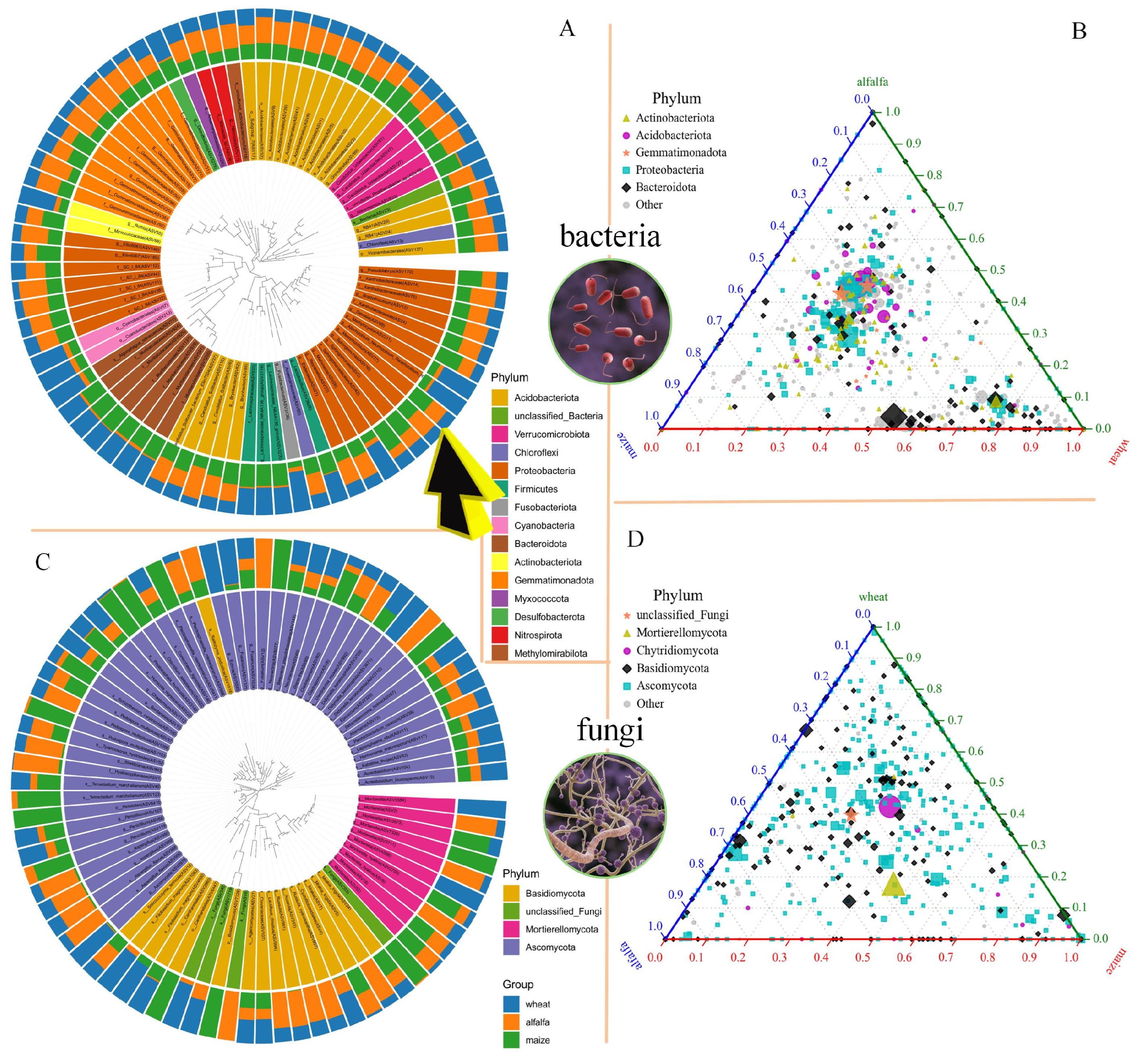
2.4.1. Species Taxonomic Analysis
2.4.2. Species Diversity Analysis
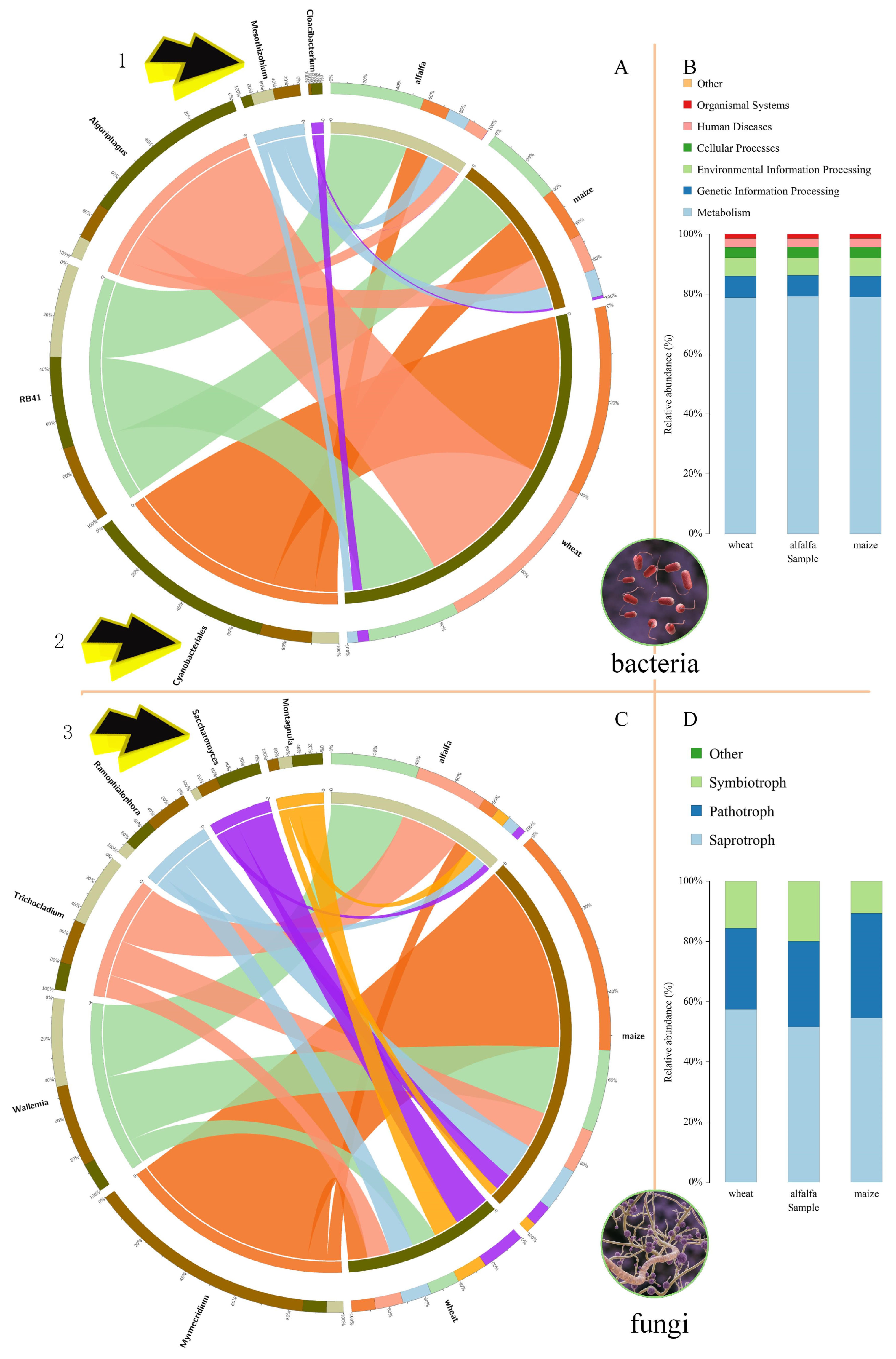
2.4.3. Plant and Rhizosphere Microbial Coevolution
2.5. Environmental Factors and Microbial Correlation Analysis
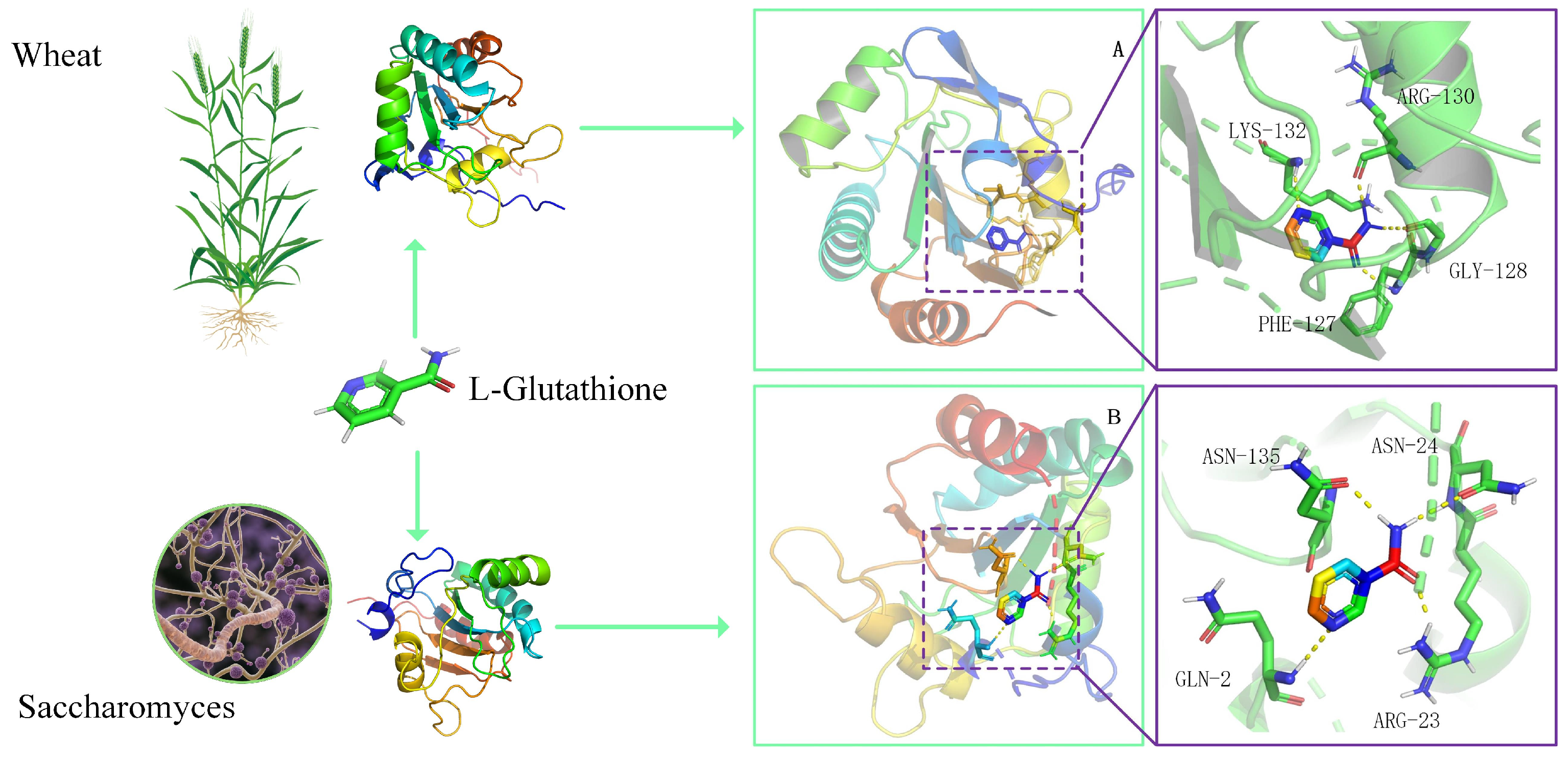
2.6. Peroxidase Protein 3D Structure Docking
3. Results
3.1. Changes in Soil Physical Properties and Crop Yields
3.2. Coevolutionary Structure of Plant Rhizosphere Soil Microbial Communities
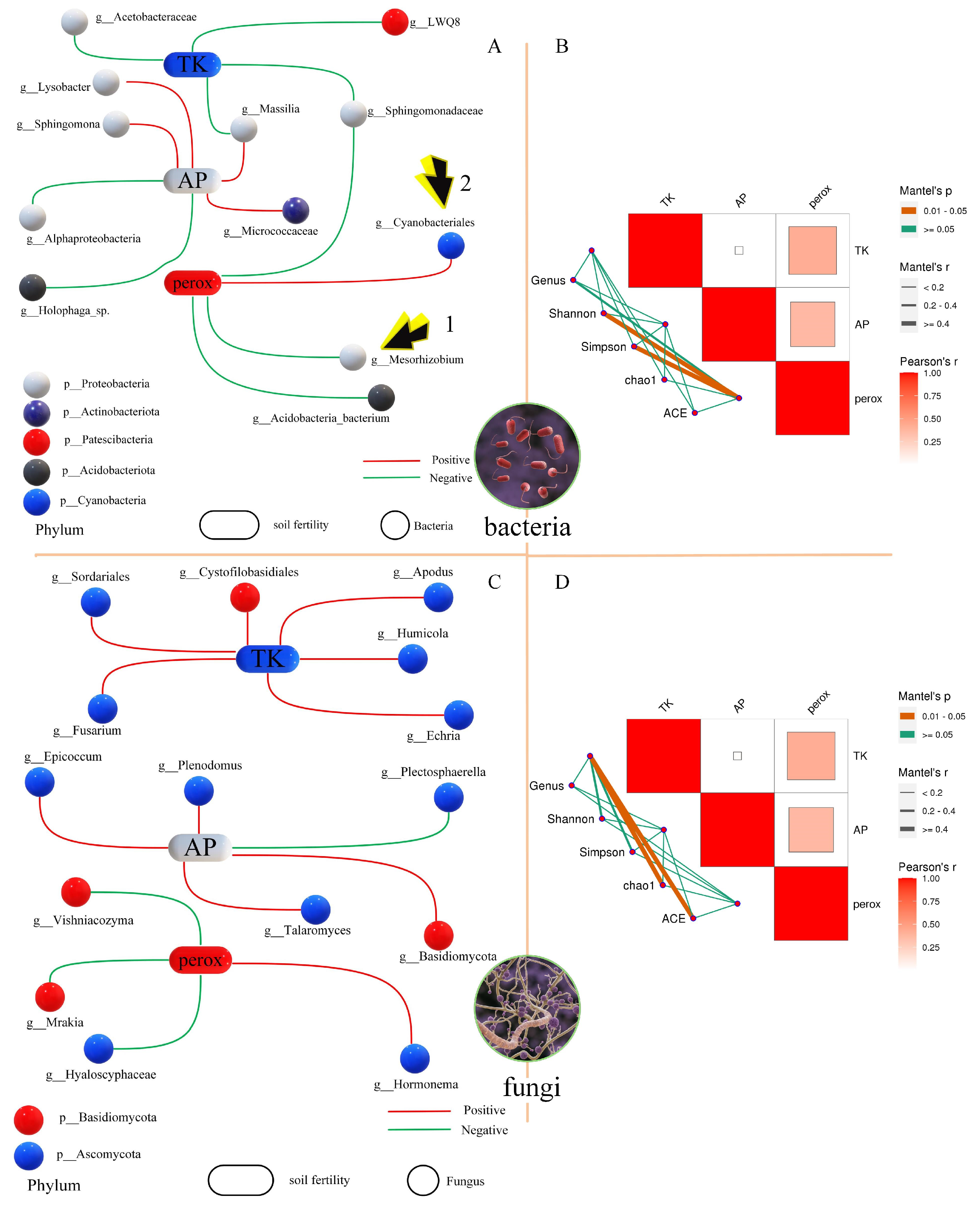
3.2.1. Taxonomic Analysis of Species
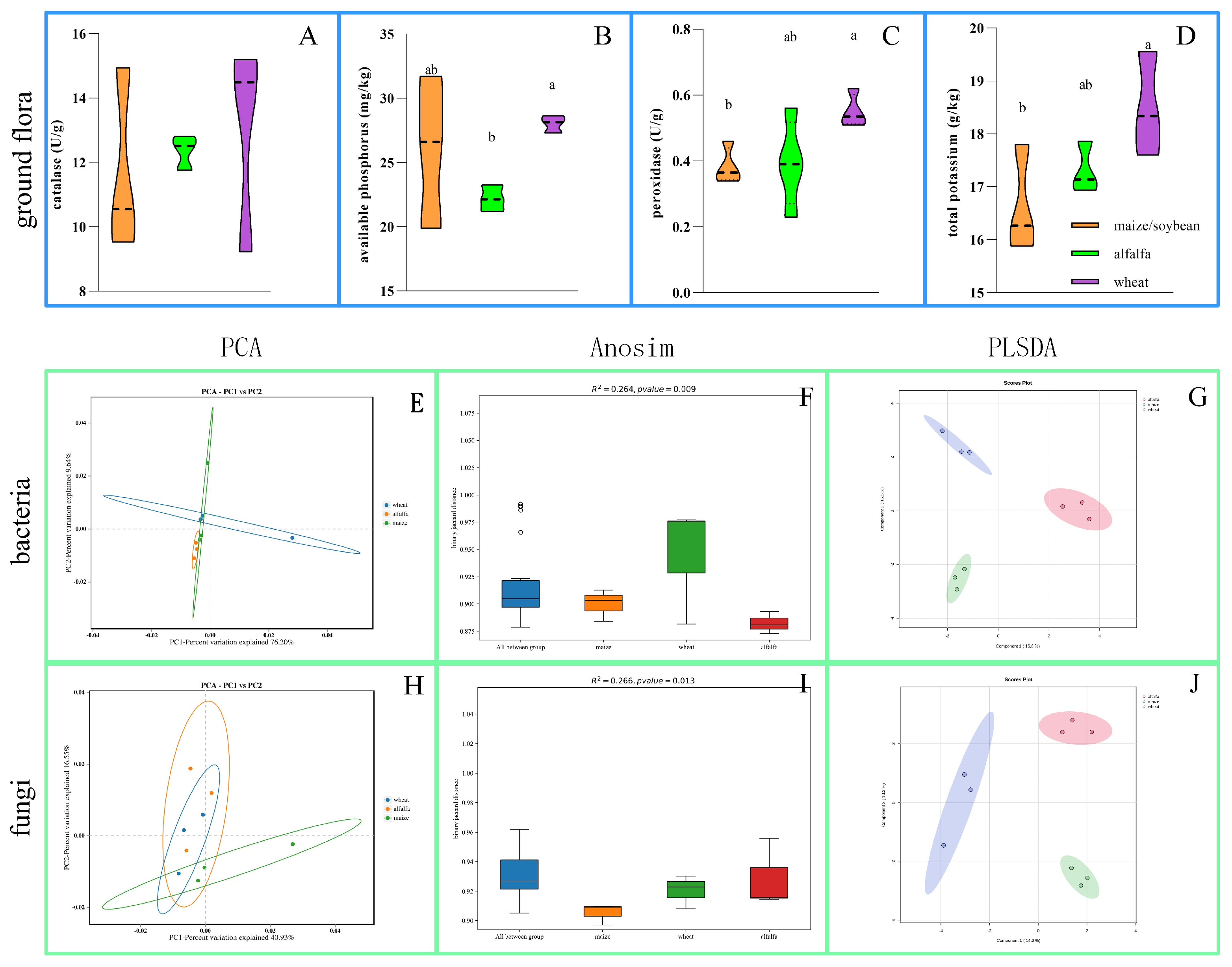
3.2.2. Soil Chemical Changes and Species Diversity Analysis
3.2.3. Plant and Microbial Coevolution
3.3. Correlation Analysis of Environmental Factors and Microorganisms
3.4. Peroxidase Protein 3D Structure Docking with L-Glutathione
4. Discussion
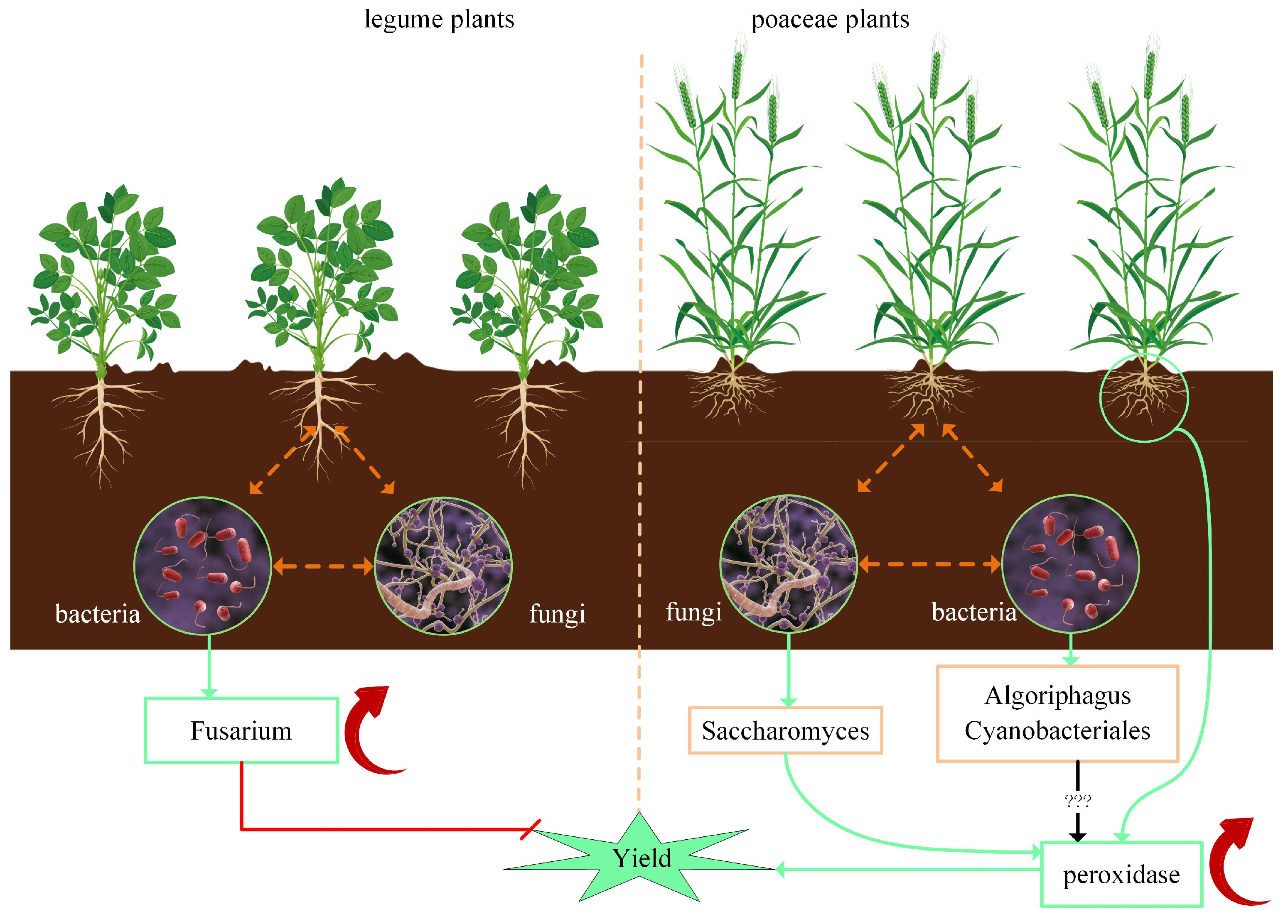
4.1. Correlation of Rhizobia and Peroxidase in Soil
4.2. Characteristics of Rhizosphere Microorganisms of Leguminous and Poaceae Plants
4.3. Plants and Rhizosphere Microbial Fertilizers
4.4. Coevolution of Plants and Rhizosphere Microorganisms
4.5. Human Activities Determine the Rhizobiont
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| OTUs | Operational taxonomic units |
| PLSDA | Partial Least Squares Discrimination Analysis |
| GMQE | Global match quality estimate value |
| AMF | Arbuscular Mycorrhizal Fungi |
References
- Riris, P.; Silva, F.; Crema, E.; Palmisano, A.; Robinson, E.; Siegel, P.E.; French, J.C.; Jorgensen, E.K.; Maezumi, S.Y.; Solheim, S.; et al. Frequent disturbances enhanced the resilience of past human populations. Nature 2024, 629, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.K.; Hemp, A.; Appelhans, T.; Becker, J.N.; Behler, C.; Classen, A.; Detsch, F.; Ensslin, A.; Ferger, S.W.; Frederiksen, S.B.; et al. Climate-land-use interactions shape tropical mountain biodiversity and ecosystem functions. Nature 2019, 568, 88–92. [Google Scholar] [CrossRef]
- Hong, C.; Burney, J.A.; Pongratz, J.; Nabel, J.; Mueller, N.D.; Jackson, R.B.; Davis, S.J. Global and regional drivers of land-use emissions in 1961–2017. Nature 2021, 589, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Chen, F.; Qin, W.; Yang, H.; Zhao, S.; Xia, J.; Du, X.; Zhu, Y.; Wu, L.; et al. Maize smart-canopy architecture enhances yield at high densities. Nature 2024, 632, 576–584. [Google Scholar] [CrossRef]
- Zhu, Y.; Qu, Z.; Zhao, J.; Wang, J.; Wei, D.; Meng, Q. Can high-yielding maize system decrease greenhouse gas emissions largely while simultaneously enhancing economic and ecosystem benefits through the “Rhizobiont” concept? Evidence from field. Sci. Total Environ. 2024, 914, 169889. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.W.; Li, J.; Koch, B.J.; Blazewicz, S.J.; Dijkstra, P.; Hayer, M.; Hofmockel, K.S.; Liu, X.A.; Mau, R.L.; Morrissey, E.M.; et al. Nutrients cause consolidation of soil carbon flux to small proportion of bacterial community. Nat. Commun. 2021, 12, 3381. [Google Scholar] [CrossRef]
- Bian, Q.; Cheng, K.; Chen, L.; Jiang, Y.; Li, D.; Xie, Z.; Wang, X.; Sun, B. Organic amendments increased Chinese milk vetch symbiotic nitrogen fixation by enriching Mesorhizobium in rhizosphere. Environ. Res. 2024, 252, 118923. [Google Scholar] [CrossRef]
- Nahusenay, G.; Wolde, G.; Tena, W.; Tamiru, T. Chickpea (Cicer arietinum L.) growth, nodulation, and yield as affected by varieties, Mesorhizobium strains, and NPSB fertilizer in Southern Ethiopia. Front. Plant Sci. 2024, 15, 1372082. [Google Scholar] [CrossRef]
- Li, Y.; Guo, T.; Sun, L.; Wang, E.T.; Young, J.P.W.; Tian, C.F. Phylogenomic analyses and reclassification of the Mesorhizobium complex: Proposal for 9 novel genera and reclassification of 15 species. BMC Genom. 2024, 25, 419. [Google Scholar] [CrossRef]
- Das, K.; Rajawat, M.V.S.; Saxena, A.K.; Prasanna, R. Development of Mesorhizobium ciceri-Based Biofilms and Analyses of Their Antifungal and Plant Growth Promoting Activity in Chickpea Challenged by Fusarium Wilt. Indian J. Microbiol. 2017, 57, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Mayhood, P.; Mirza, B.S. Soybean Root Nodule and Rhizosphere Microbiome: Distribution of Rhizobial and Nonrhizobial Endophytes. Appl. Environ. Microbiol. 2021, 87. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pan, F.; Han, X.; Song, F.; Zhang, Z.; Yan, J.; Xu, Y. Response of Soil Fungal Community Structure to Long-Term Continuous Soybean Cropping. Front. Microbiol. 2018, 9, 3316. [Google Scholar] [CrossRef]
- Benidire, L.; Madline, A.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 2021, 262, 127803. [Google Scholar] [CrossRef] [PubMed]
- Hohenfeld, C.S.; de Oliveira, S.A.S.; Ferreira, C.F.; Mello, V.H.; Margarido, G.R.A.; Passos, A.R.; de Oliveira, E.J. Comparative analysis of infected cassava root transcriptomics reveals candidate genes for root rot disease resistance. Sci. Rep. 2024, 14, 10587. [Google Scholar] [CrossRef]
- Okeke, P.; Onyeocha, I.; Ezeji, U.; Chukwudi, P.; Engwa, G. Biodegradation of hydrocarbons in a crude-oil polluted soil using peroxidase from fungal di-culture of Rhizopus and Saccharomyces spp. J. Biotech. Res. 2020, 11, 187–196. [Google Scholar]
- El-Sayed, S. Collaborative Potentialities of Trichoderma spp. and Saccharomyces cerevisiae Against Damping-off and Root Rot Diseases of Faba Bean. Egypt. J. Phytopathol. 2022, 50, 65–78. [Google Scholar] [CrossRef]
- Aiyer, H.S.; McKenzie-Gopsill, A.; Mills, A.; Foster, A.J. Select Cover Crop Residue and Soil Microbiomes Contribute to Suppression of Fusarium Root and Crown Rot in Barley and Soybean. Microorganisms 2024, 12, 404. [Google Scholar] [CrossRef]
- Rafi, N.; Dominguez, M.; Okello, P.N.; Mathew, F.M. No Common Candidate Genes for Resistance to Fusarium graminearum, F. proliferatum, F. sporotrichioides, and F. subglutanins in Soybean (Glycine max L.) Accessions from Maturity Groups 0 and I: Findings from Genome-Wide Association Mapping. Plant Dis. 2024, 108. [Google Scholar] [CrossRef]
- Shi, X.; Yang, G.; Yu, D.; Xu, S.; Warner, E.D.; Petersen, G.W.; Sun, W.; Zhao, Y.; Easterling, W.E.; Wang, H. A WebGIS system for relating genetic soil classification of China to soil taxonomy. Comput. Geosci. 2010, 36, 768–775. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Li, J.; Li, P.; Zuo, X.; Chang, B.; Liu, Y.; Zhang, N.; Yu, H.; Miao, L.; et al. Temporal variation in soil carbon in various paddy soil types in a cold temperate continental monsoon climate. Soil. Use Manag. 2023, 39, 122–133. [Google Scholar] [CrossRef]
- NY/T 1121.1-2006; Soil Testing. Part 1: Soil Sampling, Processing and Reposition. National Standards of the People’s Republic of China: Beijing, China, 2006.
- Lin, D. Guidance of Soil Science Experiment; Chinese Forestry Publishing House: Beijing, China, 2004; pp. 97–137. [Google Scholar]
- NY/T 1121.24-2012; Soil Testing. Part 24: Determination of Total Nitrogen in Soil. Automatic Kjeldahl Apparatus Method. National Standards of the People’s Republic of China: Beijing, China, 2012.
- Chen, Y.; Shan, X.; Jiang, H.; Sun, L.; Guo, Z. Regulation of litter size in sheep (Ovis aries) by the GDF9 and BMP15 genes. Ann. Agric. Sci. 2023, 68, 148–158. [Google Scholar] [CrossRef]
- de Carvalho Neta, S.J.; Araujo, V.; Fracetto, F.J.C.; da Silva, C.C.G.; de Souza, E.R.; Silva, W.R.; Lumini, E.; Fracetto, G.G.M. Growth-promoting bacteria and arbuscular mycorrhizal fungus enhance maize tolerance to saline stress. Microbiol. Res. 2024, 284, 127708. [Google Scholar] [CrossRef]
- Li, M.Y.; Wang, W.; Mo, F.; Ren, A.T.; Wang, Z.Y.; Zhu, Y.; Xiong, Y.C. Seven-year long-term inoculation with Funneliformis mosseae increases maize yield and soil carbon storage evidenced by in situ (13)C-labeling in a dryland. Sci. Total Environ. 2024, 944, 173975. [Google Scholar] [CrossRef]
- Becerra, A.G.; Menoyo, E.; Faggioli, V.; Cabello, M.; Salazar, M.J. Mycorrhizal fungal communities associated with three metal accumulator plants growing in an abandoned Pb smelting factory. Braz. J. Microbiol. 2023, 54, 2979–2990. [Google Scholar] [CrossRef]
- Jia, Q.; Sun, J.; Gan, Q.; Shi, N.N.; Fu, S. Zea mays cultivation, biochar, and arbuscular mycorrhizal fungal inoculation influenced lead immobilization. Microbiol. Spectr. 2024, 12, e0342723. [Google Scholar] [CrossRef]
- Barajas Gonzalez, J.A.; Carrillo-Gonzalez, R.; Gonzalez-Chavez, M.; Chimal Sanchez, E.; Tapia Maruri, D. Selection of Salinity-Adapted Endorhizal Fungal Consortia from Two Inoculum Sources and Six Halophyte Plants. J. Fungi 2023, 9, 893. [Google Scholar] [CrossRef]
- Huang, P.; Huang, S.; Ma, Y.; Danish, S.; Hareem, M.; Syed, A.; Elgorban, A.M.; Eswaramoorthy, R.; Wong, L.S. Alleviation of salinity stress by EDTA chelated-biochar and arbuscular mycorrhizal fungi on maize via modulation of antioxidants activity and biochemical attributes. BMC Plant Biol. 2024, 24, 63. [Google Scholar] [CrossRef] [PubMed]
- Yagmur, A.; Demir, S.; Canpolat, S.; Rezaee Danesh, Y.; Farda, B.; Djebaili, R.; Pace, L.; Pellegrini, M. Onion Fusarium Basal Rot Disease Control by Arbuscular Mycorrhizal Fungi and Trichoderma harzianum. Plants 2024, 13, 386. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, J.; Ye, Z.; Qiu, W.; Liu, J.; Cao, X.; Ge, J.; Ping, W. Synergistic benefits of Funneliformis mosseae and Bacillus paramycoides: Enhancing soil health and soybean tolerance to root rot disease. Environ. Res. 2023, 238, 117219. [Google Scholar] [CrossRef]
- Khan, W.; Zhu, Y.; Khan, A.; Zhao, L.; Yang, Y.M.; Wang, N.; Hao, M.; Ma, Y.; Nepal, J.; Ullah, F.; et al. Above-and below-ground feedback loop of maize is jointly enhanced by plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi in drier soil. Sci. Total Environ. 2024, 917, 170417. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, H.; Wang, Y.; Wang, M.; Xie, X.; Chang, H.; Wang, L.; Qu, J.; Sun, K.; He, W.; et al. Mycorrhizal symbiosis modulates the rhizosphere microbiota to promote rhizobia-legume symbiosis. Mol. Plant 2021, 14, 503–516. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, Y.; Chang, H.; Wang, C.; Yang, J.; Shi, J.; Gao, J.; Yang, W.; Lan, L.; Wang, Y.; et al. An SHR-SCR module specifies legume cortical cell fate to enable nodulation. Nature 2021, 589, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wang, J.; Ma, C.; Jong, C.; Jin, M.; Cha, J.; Wang, J.; Peng, Y.; Ni, H.; Li, H.; et al. GmTCP and GmNLP Underlying Nodulation Character in Soybean Depending on Nitrogen. Int. J. Mol. Sci. 2023, 24, 7750. [Google Scholar] [CrossRef]
- Khan, A.; Wadood, S.F.; Chen, M.; Wang, Y.; Xie, Z.P.; Staehelin, C. Effector-triggered inhibition of nodulation: A rhizobial effector protease targets soybean kinase GmPBS1-1. Plant Physiol. 2022, 189, 2382–2395. [Google Scholar] [CrossRef]
- He, D.; Yao, X.; Zhang, P.; Liu, W.; Huang, J.; Sun, H.; Wang, N.; Zhang, X.; Wang, H.; Zhang, H.; et al. Effects of continuous cropping on fungal community diversity and soil metabolites in soybean roots. Microbiol. Spectr. 2023, 11, e0178623. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, A.; Read, Q.D.; Sykes, V.; Kelly, H.; Kharel, T.; Bellaloui, N. Cover Crop and Crop Rotation Effects on Tissue and Soil Population Dynamics of Macrophomina phaseolina and Yield Under No-Till System. Plant Dis. 2024, 108, 302–310. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Gu, W.; Feng, Z.; Xiu, L.; Zhang, W.; Chen, W. Long term co-application of biochar and fertilizer could increase soybean yield under continuous cropping: Insights from photosynthetic physiology. J. Sci. Food Agric. 2024, 104, 3113–3122. [Google Scholar] [CrossRef]
- Perez-Brandan, C.; Huidobro, J.; Grumberg, B.; Scandiani, M.M.; Luque, A.G.; Meriles, J.M.; Vargas-Gil, S. Soybean fungal soil-borne diseases: A parameter for measuring the effect of agricultural intensification on soil health. Can. J. Microbiol. 2014, 60, 73–84. [Google Scholar] [CrossRef]
- Kang, I.J.; Lee, M.; Han, S.Y.; Kim, Y.H.; Lee, S. First report of soybean root rot caused by Fusarium proliferatum in the Republic of Korea. Plant Dis. 2024. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, B.; Zheng, L.; Tan, P.; Wang, Y.; Leng, H.; de Souza, N.; Liu, Z.; Hong, L.; Xiao, X. Proteome-wide 3D structure prediction provides insights into the ancestral metabolism of ancient archaea and bacteria. Nat. Commun. 2022, 13, 7861. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Shu, Z.; Ma, C.; Yan, P.; Huang, L.; He, R.; Ma, H. Isolation of a surfactin-producing strain of Bacillus subtilis and evaluation of the probiotic potential and antioxidant activity of surfactin from fermented soybean meal. J. Sci. Food Agric. 2024, 104, 8469–8479. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Fatema; Hoque, M.N.; Gupta, D.R.; Mahmud, N.U.; Sakif, T.I.; Sharpe, A.G. Improvement of growth, yield and associated bacteriome of rice by the application of probiotic Paraburkholderia and Delftia. Front. Microbiol. 2023, 14, 1212505. [Google Scholar] [CrossRef]
- Abuhena, M.; Al-Rashid, J.; Azim, M.F.; Khan, M.N.M.; Kabir, M.G.; Barman, N.C.; Rasul, N.M.; Akter, S.; Huq, M.A. Optimization of industrial (3000 L) production of Bacillus subtilis CW-S and its novel application for minituber and industrial-grade potato cultivation. Sci. Rep. 2022, 12, 11153. [Google Scholar] [CrossRef]
- Anusauskas, J.; Steponavicius, D.; Romaneckas, K.; Lekaviciene, K.; Zaleckas, E.; Sendzikiene, E. The Influence of Bacteria-Inoculated Mineral Fertilizer on the Productivity and Profitability of Spring Barley Cultivation. Plants 2023, 12, 1227. [Google Scholar] [CrossRef]
- Shukla, A.; Gupta, A.; Srivastava, S. Bacterial consortium (Priestia endophytica NDAS01F, Bacillus licheniformis NDSA24R, and Priestia flexa NDAS28R) and thiourea mediated amelioration of arsenic stress and growth improvement of Oryza sativa L. Plant Physiol. Biochem. 2023, 195, 14–24. [Google Scholar] [CrossRef]
- Rios-Ruiz, W.F.; Tuanama-Reategui, C.; Huaman-Cordova, G.; Valdez-Nunez, R.A. Co-Inoculation of Endophytes Bacillus siamensis TUR07-02b and Priestia megaterium SMBH14-02 Promotes Growth in Rice with Low Doses of Nitrogen Fertilizer. Plants 2023, 12, 524. [Google Scholar] [CrossRef]
- Romero-Munar, A.; Aroca, R. A non-K(+)-solubilizing PGPB (Bacillus megaterium) increased K(+) deprivation tolerance in Oryza sativa seedlings by up-regulating root K(+) transporters. Plant Physiol. Biochem. 2023, 196, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Feng, A.; Wang, C.; Zhu, X.; Su, J.; Xu, Z.; Yang, J.; Wang, W.; Chen, K.; Chen, B.; et al. Bacillus amyloliquefaciens LM-1 Affects Multiple Cell Biological Processes in Magnaporthe oryzae to Suppress Rice Blast. Microorganisms 2024, 12, 1246. [Google Scholar] [CrossRef]
- Zveushe, O.K.; de Dios, V.R.; Zhang, H.; Zeng, F.; Liu, S.; Shen, S.; Kang, Q.; Zhang, Y.; Huang, M.; Sarfaraz, A.; et al. Effects of Co-Inoculating Saccharomyces spp. with Bradyrhizobium japonicum on Atmospheric Nitrogen Fixation in Soybeans (Glycine max (L.)). Plants 2023, 12, 681. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, M.; Yang, R.; Sun, J.; Yu, Z.; Bai, C.; Xue, Y. Effect of Regulation of Whole-Plant Corn Silage Inoculated with Lactobacillus buchneri or Bacillus licheniformis Regarding the Dynamics of Bacterial and Fungal Communities on Aerobic Stability. Plants 2024, 13, 1471. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, N.; Xia, L.; Li, Q.; Shao, J.; Shen, Q.; Zhang, R. ResDE Two-Component Regulatory System Mediates Oxygen Limitation-Induced Biofilm Formation by Bacillus amyloliquefaciens SQR9. Appl. Environ. Microbiol. 2018, 84, e02744-17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Y.; Zhang, N.; Xun, W.; Feng, H.; Miao, Y.; Shao, J.; Shen, Q.; Zhang, R. Chemical communication in plant-microbe beneficial interactions: A toolbox for precise management of beneficial microbes. Curr. Opin. Microbiol. 2023, 72, 102269. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Wu, G.; Zhang, N.; Shen, Q.; Zhang, R. Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 Induces Plant Salt Tolerance through Spermidine Production. Mol. Plant Microbe Interact. 2017, 30, 423–432. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Xu, Y.; Zhang, G.; Shen, Q.; Zhang, R. Exploring Elicitors of the Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 to Induce Plant Systemic Resistance and Their Interactions with Plant Signaling Pathways. Mol. Plant Microbe Interact. 2018, 31, 560–567. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, D.; Wang, D.; Miao, Y.; Shao, J.; Zhou, X.; Xu, Z.; Li, Q.; Feng, H.; Li, S.; et al. Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genom. 2015, 16, 685. [Google Scholar] [CrossRef]
- Hata, S.; Tsuda, R.; Kojima, S.; Tanaka, A.; Kouchi, H. Both incompatible and compatible rhizobia inhabit the intercellular spaces of leguminous root nodules. Plant Signal Behav. 2023, 18, 2245995. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.; Park, Y.; Jung, J.; Park, W. Lineage-specific evolution of Aquibium, a close relative of Mesorhizobium, during habitat adaptation. Appl. Environ. Microbiol. 2024, 90, e0209123. [Google Scholar] [CrossRef]
- Fudjoe, S.K.; Li, L.; Anwar, S.; Shi, S.; Xie, J.; Wang, L.; Xie, L.; Yongjie, Z. Nitrogen fertilization promoted microbial growth and N2O emissions by increasing the abundance of nirS and nosZ denitrifiers in semiarid maize field. Front. Microbiol. 2023, 14, 1265562. [Google Scholar] [CrossRef]
- Hestrin, R.; Kan, M.; Lafler, M.; Wollard, J.; Kimbrel, J.A.; Ray, P.; Blazewicz, S.J.; Stuart, R.; Craven, K.; Firestone, M.; et al. Plant-associated fungi support bacterial resilience following water limitation. ISME J. 2022, 16, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Getzke, F.; Thiergart, T.; Hacquard, S. Contribution of bacterial-fungal balance to plant and animal health. Curr. Opin. Microbiol. 2019, 49, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Perez-de-Luque, A.; Tille, S.; Johnson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More Than the Sum of Its Parts: Microbiome Biodiversity as a Driver of Plant Growth and Soil Health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Cao, T.; Fang, Y.; Chen, Y.; Kong, X.; Yang, J.; Alharbi, H.; Kuzyakov, Y.; Tian, X. Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere. Geoderma 2022, 410, 115662. [Google Scholar] [CrossRef]
- Jing, J.; Cong, W.F.; Bezemer, T.M. Legacies at work: Plant-soil-microbiome interactions underpinning agricultural sustainability. Trends Plant Sci. 2022, 27, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, X.; Wang, E. Mycorrhizal Symbiosis in Plant Growth and Stress Adaptation: From Genes to Ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef]
- Shi, Y.C.; Zheng, Y.J.; Lin, Y.C.; Huang, C.H.; Shen, T.L.; Hsu, Y.C.; Lee, B.H. Investigation of the Microbial Diversity in the Oryza sativa Cultivation Environment and Artificial Transplantation of Microorganisms to Improve Sustainable Mycobiota. J. Fungi 2024, 10, 412. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
| Crop | Urea (kg/h × m2) | Diammonium Phosphate (kg/h × m2) | Potassium Sulfate (kg/h × m2) |
|---|---|---|---|
| Soybean | 25 | 150 | 65 |
| Maize | 250 | 150 | 75 |
| Wheat | 100 | 163 | 75 |
| Alfalfa | 50 | 200 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Jiang, Y.; Mi, G.; Liu, X.; Zou, J.; Li, J.; Guo, Z. The Impacts of Farming Activities on the Coevolutionary Structure of Plant Rhizosphere Soil Microbial Communities. Microorganisms 2025, 13, 1216. https://doi.org/10.3390/microorganisms13061216
Wang Q, Jiang Y, Mi G, Liu X, Zou J, Li J, Guo Z. The Impacts of Farming Activities on the Coevolutionary Structure of Plant Rhizosphere Soil Microbial Communities. Microorganisms. 2025; 13(6):1216. https://doi.org/10.3390/microorganisms13061216
Chicago/Turabian StyleWang, Qiuju, Yu Jiang, Gang Mi, Xin Liu, Jiahe Zou, Jingyang Li, and Zhenhua Guo. 2025. "The Impacts of Farming Activities on the Coevolutionary Structure of Plant Rhizosphere Soil Microbial Communities" Microorganisms 13, no. 6: 1216. https://doi.org/10.3390/microorganisms13061216
APA StyleWang, Q., Jiang, Y., Mi, G., Liu, X., Zou, J., Li, J., & Guo, Z. (2025). The Impacts of Farming Activities on the Coevolutionary Structure of Plant Rhizosphere Soil Microbial Communities. Microorganisms, 13(6), 1216. https://doi.org/10.3390/microorganisms13061216







