Porcine Teschovirus 2 3Cpro Evades Host Antiviral Innate Immunity by Inhibiting the IFN-β Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viral Strains, and Plasmids
2.2. Antibodies
2.3. Luciferase Reporter Assay
2.4. RNA Extraction and Plasmid Construction
2.5. Real-Time PCR Assay
2.6. Coimmunoprecipitation (Co-IP), Nuclear and Cytoplasmic Protein Extraction, and Western Blotting
2.7. Gene Site-Directed Mutagenesis Experiment
2.8. Indirect Immunofluorescence Assay (IFA)
2.9. VSV Infection Experiment
2.10. Statistics
3. Results
3.1. PTV Infection Inhibits IFN-β Promoter Activation
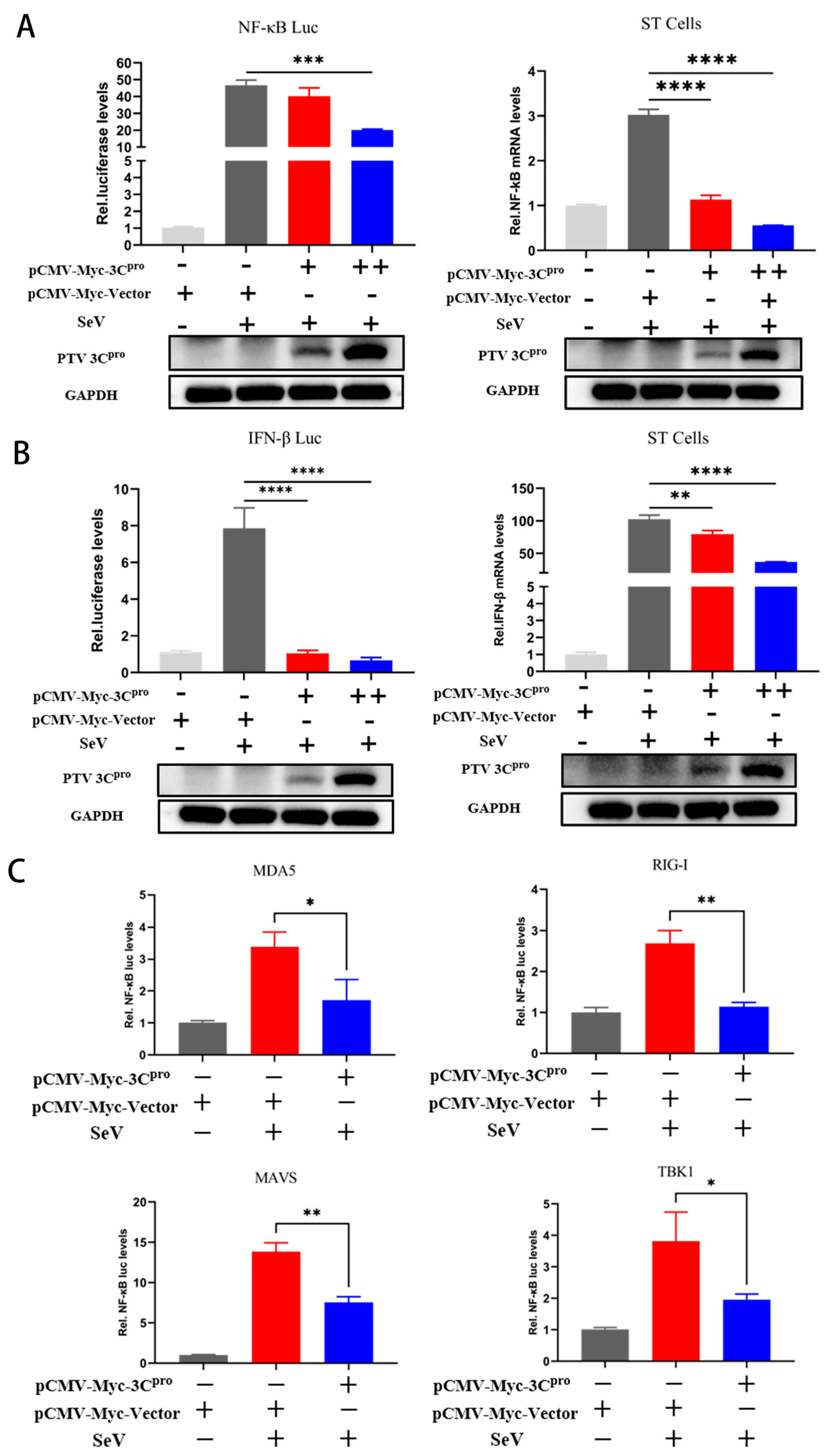
3.2. PTV 3Cpro Decreases NF-κB and IFN-β mRNA Levels and Inhibits Promoter Activity
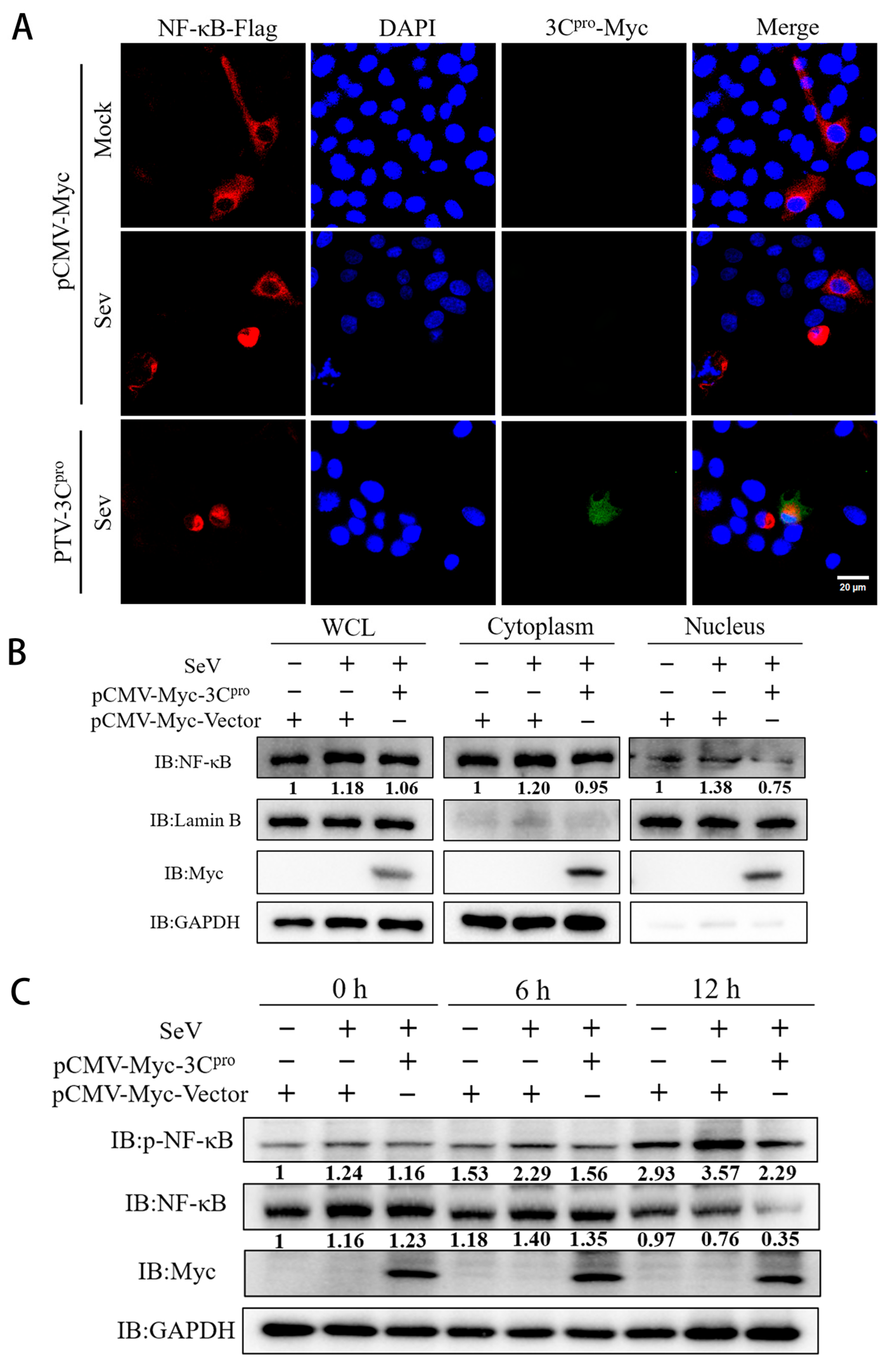
3.3. PTV 3Cpro Inhibits the Phosphorylation and Translocation of NF-κB
3.4. Mechanism of 3Cpro-Mediated Degradation of NF-κB
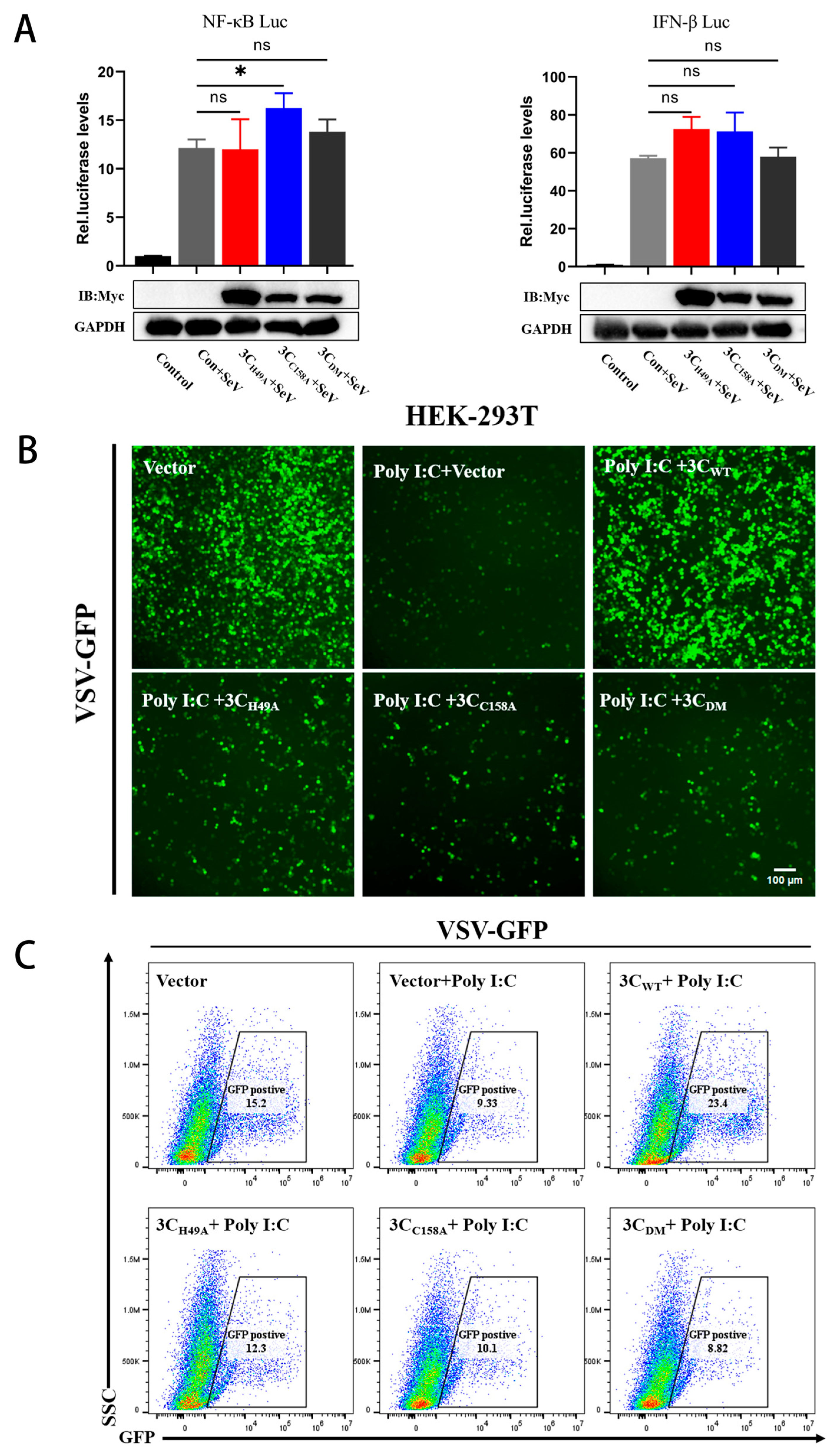
3.5. The PTV 3Cpro Mutant Loses the Ability to Evade Innate Immunity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiu, S.C.; Hu, S.C.; Chang, C.C.; Chang, C.-Y.; Huang, C.-C.; Pang, V.F.; Wang, F.-I. The role of porcine teschovirus in causing diseases in endemically infected pigs. Vet. Microbiol. 2012, 161, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Agrawal, A.; Pathak, M.; Singh, A.; Varshney, R.; Rana, J.; Saikumar, G. Detection of porcine enteric picornaviruses from faecal samples of Indian pigs. Virusdisease 2022, 33, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Villanova, F.; Cui, S.; Ai, X.; Leal, É. Analysis of full-length genomes of porcine teschovirus (PTV) and the effect of purifying selection on phylogenetic trees. Arch. Virol. 2016, 161, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lu, Y.; Zhang, L.; Li, X. Identification of novel genotypes belonging to the species Teschovirus A from indigenous pigs in western Jiangxi, China. Arch. Virol. 2020, 165, 993–1001. [Google Scholar] [CrossRef]
- Trefny, L. Massive illness of swine in Teschen area. Zveroleki Obz. 1930, 23, 235–236. [Google Scholar]
- Izawa, H.; Bankowski, R.A.; Howarth, J.A. Porcine enteroviruses. I. Properties of three isolates from swine with diarrhea and one from apparently normal swine. Am. J. Vet. Res. 1962, 23, 1131–1141. [Google Scholar]
- Yamada, M.; Nakamura, K.; Kaku, Y.; Yoshii, M.; Haritani, M.; Kozakura, R.; Ikegami, R. Enterovirus encephalomyelitis in pigs in Japan caused by porcine teschovirus. Vet. Rec. 2004, 155, 304–306. [Google Scholar] [CrossRef]
- Ray, P.K.; Desingu, P.A.; Anoopraj, R.; Singh, R.K.; Saikumar, G. Identification and genotypic characterization of porcine teschovirus from selected pig populations in India. Trop. Anim. Health Prod. 2020, 52, 1161–1166. [Google Scholar] [CrossRef]
- Feng, L.; Shi, H.Y.; Liu, S.W.; Wu, B.P.; Chen, J.F.; Sun, D.B.; Tong, Y.E.; Fu, M.S.; Wang, Y.F.; Tong, G.Z. Isolation and molecular characterization of a porcine teschovirus 1 isolate from China. Acta Virol. 2007, 51, 7–11. [Google Scholar]
- Salles, M.W.; Scholes, S.F.; Dauber, M.; Strebelow, G.; Wojnarowicz, C.; Hassard, L.; Acton, A.C.; Bollinger, T.K. Porcine teschovirus polioencephalomyelitis in western Canada. J. Vet. Diagn. Investig. 2011, 23, 367–373. [Google Scholar] [CrossRef]
- Bangari, D.S.; Pogranichniy, R.M.; Gillespie, T.; Stevenson, G.W. Genotyping of Porcine teschovirus from nervous tissue of pigs with and without polioencephalomyelitis in Indiana. J. Vet. Diagn. Investig. 2010, 22, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.Y.; Millien, M.; Jacques-Simon, R.; Flanagan, J.K.; Bracht, A.J.; Carrillo, C.; Barrette, R.W.; Fabian, A.; Mohamed, F.; Moran, K.; et al. Diagnosis of Porcine teschovirus encephalomyelitis in the Republic of Haiti. J. Vet. Diagn. Investig. 2012, 24, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Sarvari, G.; Boehr, D.D. Picornavirus 3C Proteins Intervene in Host Cell Processes through Proteolysis and Interactions with RNA. Viruses 2023, 15, 2413. [Google Scholar] [CrossRef]
- Ng, C.S.; Stobart, C.C.; Luo, H. Innate immune evasion mediated by picornaviral 3C protease: Possible lessons for coronaviral 3C-like protease? Rev. Med. Virol. 2021, 31, 1–22. [Google Scholar] [CrossRef]
- Fernandes, M.H.V.; Maggioli, M.F.; Otta, J.; Joshi, L.R.; Lawson, S.; Diel, D.G. Senecavirus A 3C Protease Mediates Host Cell Apoptosis Late in Infection. Front. Immunol. 2019, 10, 363. [Google Scholar] [CrossRef]
- Wen, W.; Yin, M.; Zhang, H.; Liu, T.; Chen, H.; Qian, P.; Hu, J.; Li, X. Seneca Valley virus 2C and 3C inhibit type I interferon production by inducing the degradation of RIG-I. Virology 2019, 535, 122–129. [Google Scholar] [CrossRef]
- Lei, X.; Sun, Z.; Liu, X.; Jin, Q.; He, B.; Wang, J. Cleavage of the adaptor protein TRIF by enterovirus 71 3C inhibits antiviral responses mediated by Toll-like receptor 3. J. Virol. 2011, 85, 8811–8818. [Google Scholar] [CrossRef]
- Huang, L.; Xiong, T.; Yu, H.; Zhang, Q.; Zhang, K.; Li, C.; Hu, L.; Zhang, Y.; Zhang, L.; Liu, Q.; et al. Encephalomyocarditis virus 3C protease attenuates type I interferon production through disrupting the TANK-TBK1-IKKε-IRF3 complex. Biochem. J. 2017, 474, 2051–2065. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhao, B.; Zhao, C.; Lei, X.; Huang, H.; Li, C.; Zheng, M.; Lan, T.; Sun, W.; et al. The VP1 Protein of Porcine Teschovirus Inhibits the Innate Immune Response to Viral Infection by Blocking MDA5 Activation. Transbound. Emerg. Dis. 2024, 2024, 6649669. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, Y.; Shi, X.; Xin, Y.; Shan, Y.; Chen, C.; Cao, T.; Fang, W.; Li, X. Porcine teschovirus 2 induces an incomplete autophagic response in PK-15 cells. Arch. Virol. 2018, 163, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Vreman, S.; Caliskan, N.; Harders, F.; Boonstra, J.; Peperkamp, K.; Ho, C.K.Y.; Kuller, W.; Kortekaas, J. Two novel porcine teschovirus strains as the causative agents of encephalomyelitis in the Netherlands. BMC Vet. Res. 2020, 16, 51. [Google Scholar] [CrossRef]
- Hammerschmitt, M.E.; de Almeida, P.R.; de Cecco, B.S.; Lorenzett, M.P.; Schwertz, C.I.; da Cruz, R.A.S.; Caprioli, R.A.; Schuh, D.T.; Demoliner, M.; Eisen, A.K.A.; et al. Swine polioencephalomyelitis in Brazil: Identification of Teschovirus A, Sapelovirus A, and Enterovirus G in a farm from Southern Brazil. Braz. J. Microbiol. 2021, 52, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.; Junker, S.; Gründl, J.; Fröhlich, S.; Beisl, M.; Zöls, S.; Ritzmann, M.; Eddicks, M.; Palzer, A.; Sehl, J.; et al. Hinterhandlähmungen bei Mastschweinen im Zusammenhang mit einem neuen Stamm des porzinen Teschovirus A11 [Hind limb paralysis in fattening pigs due to a new strain of porcine Teschovirus A11]. Tierarztl. Prax. Ausg. G. Grosstiere Nutztiere. 2022, 50, 59–67. (In German) [Google Scholar] [PubMed]
- Wang, J.T.; Dunne, H.W.; Griel, L.C.; Hokanson, J.F.; Murphy, D.M. Mortality, antibody development, and viral persistence in porcine fetuses incubated in utero with SMEDI (entero-) virus. Am. J. Vet. Res. 1973, 34, 785–791. [Google Scholar] [CrossRef]
- Huang, J.; Gentry, R.F.; Zarkower, A. Experimental infection of pregnant sows with porcine enteroviruses. Am. J. Vet. Res. 1980, 41, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, M.; Wu, M.; Ghonaim, A.; Fan, S.; He, Q. Isolation and genetic characteristics of a neurotropic teschovirus variant belonging to genotype 1 in northeast China. Arch. Virol. 2021, 166, 1355–1370. [Google Scholar] [CrossRef]
- Kaku, Y.; Murakami, Y.; Sarai, A.; Wang, Y.; Ohashi, S.; Sakamoto, K. Antigenic properties of porcine teschovirus 1 (PTV-1) Talfan strain and molecular strategy for serotyping of PTVs. Arch. Virol. 2007, 152, 929–940. [Google Scholar] [CrossRef]
- Yang, T.; Yu, X.; Luo, B.; Yan, M.; Li, R.; Qu, T.; Ren, X. Epidemiology and molecular characterization of porcine teschovirus in Hunan. China Transbound. Emerg. Dis. 2018, 65, 480–490. [Google Scholar] [CrossRef]
- Liang, W.; Wu, X.; Ding, Z.; Zhong, S.; Qian, X.; Ye, P.; Liu, H.; Chen, Z.; Zhang, J.; Cao, H.; et al. Identification of a novel porcine Teschovirus 2 strain as causative agent of encephalomyelitis in suckling piglets with high mortality in China. BMC Vet. Res. 2023, 19, 2. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.D.; Lamb, A.; Chen, L.F. Posttranslational modifications of NF-kappaB: Another layer of regulation for NF-kappaB signaling pathway. Cell. Signal. 2010, 22, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.J.; Davis, M.E.; Gack, M.U. Regulation of RIG-I-like receptor signaling by host and viral proteins. Cytokine Growth Factor Rev. 2014, 25, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- Burke, J.D.; Sonenberg, N.; Platanias, L.C.; Fish, E.N. Antiviral effects of interferon-β are enhanced in the absence of the translational suppressor 4E-BP1 in myocarditis induced by Coxsackievirus B3. Antivir. Ther. 2011, 16, 577–584. [Google Scholar] [CrossRef]
- Yin, M.; Wen, W.; Wang, H.; Zhao, Q.; Zhu, H.; Chen, H.; Li, X.; Qian, P. Porcine Sapelovirus 3Cpro Inhibits the Production of Type I Interferon. Front. Cell. Infect. Microbiol. 2022, 12, 852473. [Google Scholar] [CrossRef]


| Primer | Sequence |
|---|---|
| NF-κB-F | gatgacgacgataaggaattcATGGACGACCTCTTCCCCC |
| NF-κB-R | attaagatctgctagctcgagTTAGGAGCTGATCTGACTCAGAAGG |
| PTV-3C-F | tctgaagaggacttggaattcGGACCAAAGGGACAAGCTAACA |
| PTV-3C-R | ccgcggccgcggtacctcgagTTGAAATTCCAAAAATCCCTCAA |
| Primer | Sequence |
|---|---|
| Q-GAPDH-F | AGCAACAGGGTGGTGGACCT |
| Q-GAPDH-R | CTGGGATGGAAACTGGAAGT |
| Q-NF-κB-F | CCTGAGGCTATAACTCGCTTGG |
| Q-NF-κB-R | GTCCGCAATGGAGGAGAAGT |
| Q-IFN-β-F | CATCCTCCAAATCGCTCTCC |
| Q-IFN-β-R | ACATGCCAAATTGCTGCTCC |
| Primer | Sequence |
|---|---|
| PTV-H49A-F | TCTGATCAATACTgcTATTTTGACAGGTATAAA |
| PTV-H49A-R | TTTATACCTGTCAAAATAgcAGTATTGATCAGA |
| PTV-C158A-F | ACGGCTATgcCGGCTCTGTGATGGTTGCGGATGCTGGAG |
| PTV-C158A-R | CTCCAGCATCCGCAACCATCACAGAGCCGgcATAGCCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-Y.; Li, Y.-Y.; Zhou, Y.-M.; Chen, W.; Xie, L.-L.; Hu, Y.-Q.; Qin, Y.; Huang, H.-X.; Zhou, L.; Lan, T.; et al. Porcine Teschovirus 2 3Cpro Evades Host Antiviral Innate Immunity by Inhibiting the IFN-β Signaling Pathway. Microorganisms 2025, 13, 1209. https://doi.org/10.3390/microorganisms13061209
Zhang X-Y, Li Y-Y, Zhou Y-M, Chen W, Xie L-L, Hu Y-Q, Qin Y, Huang H-X, Zhou L, Lan T, et al. Porcine Teschovirus 2 3Cpro Evades Host Antiviral Innate Immunity by Inhibiting the IFN-β Signaling Pathway. Microorganisms. 2025; 13(6):1209. https://doi.org/10.3390/microorganisms13061209
Chicago/Turabian StyleZhang, Xin-Yu, Yu-Ying Li, Yi-Min Zhou, Wei Chen, Lu-Lu Xie, Yan-Qing Hu, Yan Qin, Hai-Xin Huang, Lin Zhou, Tian Lan, and et al. 2025. "Porcine Teschovirus 2 3Cpro Evades Host Antiviral Innate Immunity by Inhibiting the IFN-β Signaling Pathway" Microorganisms 13, no. 6: 1209. https://doi.org/10.3390/microorganisms13061209
APA StyleZhang, X.-Y., Li, Y.-Y., Zhou, Y.-M., Chen, W., Xie, L.-L., Hu, Y.-Q., Qin, Y., Huang, H.-X., Zhou, L., Lan, T., & Sun, W.-C. (2025). Porcine Teschovirus 2 3Cpro Evades Host Antiviral Innate Immunity by Inhibiting the IFN-β Signaling Pathway. Microorganisms, 13(6), 1209. https://doi.org/10.3390/microorganisms13061209






