Identification of Pyrrole-2-Carboxylic Acid from the Biocontrol Agent Lysobacter Involved in Interactions with Fusarial Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Strains, and Growth Conditions
2.2. Lysobacter-Fungal Co-Culture
2.3. HPLC Analysis of Metabolites
2.4. Isolation and Structure Elucidation of Pyrrole-2-Carboxylic Acid
2.5. Chitin Supplementation to Bacterial Cultures
2.6. Antimicrobial Assays
2.7. Microtiter Plate Biofilm Formation Assay
2.8. Microscopic Images of Biofilm
2.9. Statistical Analysis
3. Results and Discussion
3.1. Co-Culture of Lysobacter and Fungus Led to Drastic Change of a Lysobacter Metabolite
3.2. Structural Determination of the Lysobacter Metabolite
3.3. Components of Fungal Cell Walls Contributed to P2C Suppression in Lysobacter
3.4. Antimicrobial Activity Assays for P2C
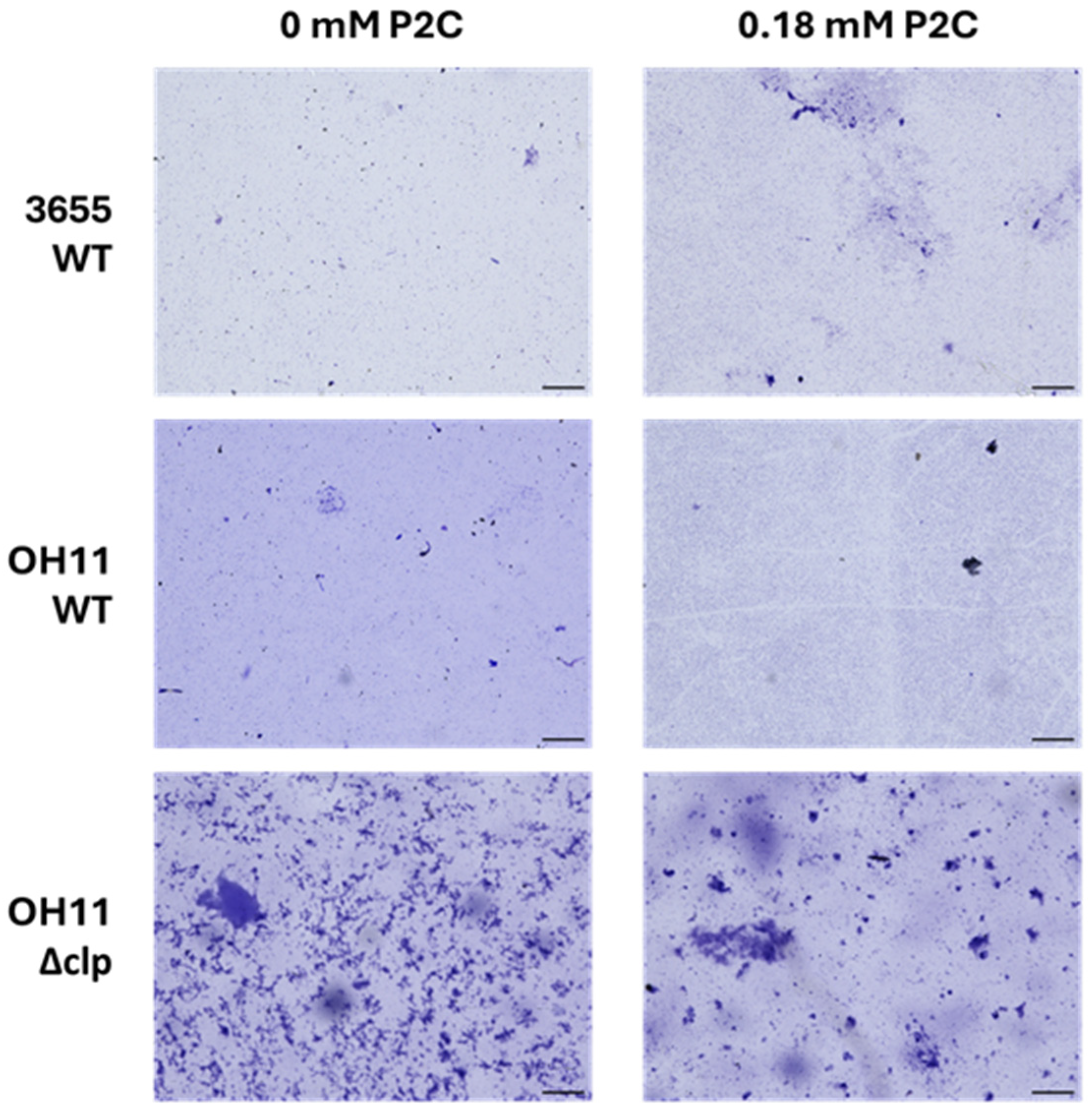
3.5. Role of P2C in Biofilm Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, H.; Miller, A.L.; Khetrapal, V.; Jayaseker, V.; Wright, S.; Du, L. Biosynthesis, regulation, and engineering of natural products from Lysobacter. Nat. Product. Rep. 2022, 39, 842–874. [Google Scholar] [CrossRef] [PubMed]
- Panthee, S.; Hamamoto, H.; Paudel, A.; Sekimizu, K. Lysobacter species: A potential source of novel antibiotics. Arch. Microbiol. 2016, 198, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Brescia, F.; Pertot, I.; Puopolo, G. Lysobacter. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 313–338. [Google Scholar]
- Yang, M.M.; Ren, S.S.; Shen, D.Y.; Yang, N.D.; Wang, B.X.; Han, S.; Shen, X.; Chou, S.H.; Qian, G.L. An intrinsic mechanism for coordinated production of the contact-dependent and contact-independent weapon systems in a soil bacterium. PLoS Pathog. 2020, 16, e1008967. [Google Scholar] [CrossRef]
- Lin, L.; Shen, D.Y.; Shao, X.L.; Yang, Y.C.; Li, L.; Zhong, C.H.; Jiang, J.D.; Wang, M.C.; Qian, G.L. Soil microbiome bacteria protect plants against filamentous fungal infections via intercellular contacts. Proc. Natl. Acad. Sci. USA 2025, 122, e2418766122. [Google Scholar] [CrossRef]
- Shen, X.; Wang, B.X.; Yang, N.D.; Zhang, L.L.; Shen, D.Y.; Wu, H.J.; Dong, Y.; Niu, B.; Chou, S.H.; Puopolo, G.; et al. Lysobacter enzymogenes antagonizes soilborne bacteria using the type IV secretion system. Environ. Microbiol. 2021, 23, 4673–4688. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Huffman, J.; Gerber, R.; Du, L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers 2010, 93, 764–776. [Google Scholar] [CrossRef]
- Gao, Z.C.; Luo, K.X.; Zhu, Q.X.; Peng, J.H.; Liu, C.; Wang, X.Y.; Li, S.J.; Zhang, H.Y. The natural occurrence, toxicity mechanisms and management strategies of Fumonisin B1: A review. Environ. Pollut. 2023, 320, 121065. [Google Scholar] [CrossRef]
- Braun, M.S.; Wink, M. Exposure, occurrence, and chemistry of fumonisins and their cryptic derivatives. Compr. Rev. Food Sci. Food Saf. 2018, 17, 769–791. [Google Scholar] [CrossRef]
- Chen, Y.; Kistler, H.C.; Ma, Z.H. Trichothecene mycotoxins: Biosynthesis, regulation, and management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Q.H.; Wang, G.H.; Zhang, X.; Liu, H.Q.; Jiang, C. Combatting Fusarium head blight: Advances in molecular interactions between Fusarium graminearum and wheat. Phytopathol. Res. 2022, 4, 37. [Google Scholar] [CrossRef]
- Li, S.; Jochum, C.C.; Yu, F.; Zaleta-Rivera, K.; Du, L.; Harris, S.D.; Yuen, G.Y. An antibiotic complex from Lysobacter enzymogenes strain C3: Antimicrobial activity and role in plant disease control. Phytopathology 2008, 98, 695–701. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cheng, C.; Jiang, T.P.; Xu, H.Y.; Chen, Y.; Ma, Z.H.; Qian, G.L.; Liu, F.Q. Control of wheat Fusarium head blight by heat-stable antifungal factor (HSAF) from Lysobacter enzymogenes. Plant Dis. 2019, 103, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Tang, B.; Hou, R.X.; Sun, W.B.; Han, C.Y.; Guo, B.D.; Zhao, Y.Y.; Li, C.H.; Sheng, C.; Zhao, Y.C.; et al. The natural polycyclic tetramate macrolactam HSAF inhibit Fusarium graminearum through altering cell membrane integrity by targeting FgORP1. Int. J. Biol. Macromol. 2024, 261, 129744. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Jiang, J.S.; Taylor, A.J.; Leite, A.D.; Dodds, E.D.; Du, L. Outer membrane vesicle-mediated codelivery of the antifungal HSAF metabolites and lytic polysaccharide monooxygenase in the predatory Lysobacter enzymogenes. ACS Chem. Biol. 2021, 16, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Nian, J.N.; Yu, M.H.; Bradley, C.A.; Zhao, Y.F. Lysobacter enzymogenes strain C3 suppresses mycelium growth and spore germination of eight soybean fungal and oomycete pathogens and decreases disease incidences. Biol. Control 2021, 152, 104424. [Google Scholar] [CrossRef]
- Chen, X.S.; Li, S.R.; Yu, L.J.; Miller, A.; Du, L. Systematic optimization for production of the anti-MRSA antibiotics WAP-8294A in an engineered strain of Lysobacter enzymogenes. Microb. Biotechnol. 2019, 12, 1430–1440. [Google Scholar] [CrossRef]
- Meers, P.R.; Liu, C.; Chen, R.; Bartos, W.; Davis, J.; Dziedzic, N.; Orciuolo, J.; Kutyla, S.; Pozo, M.J.; Mithrananda, D.; et al. Vesicular delivery of the antifungal antibiotics of Lysobacter enzymogenes C3. Appl. Environ. Microbiol. 2018, 84, e01353-18. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuen, G.Y.; Sarath, G.; Penheiter, A.R. Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology 2001, 91, 204–211. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuen, G.Y. Effects of culture fluids and preinduction of chitinase production on biocontrol of Bipolaris leaf spot by Stenotrophomonas maltophilia C3. Biol. Control 2000, 18, 277–286. [Google Scholar] [CrossRef]
- Yi, H.; Bojja, R.S.; Fu, J.; Du, L. Direct evidence for the function of FUM13 in 3-ketoreduction of mycotoxin fumonisins in Fusarium verticillioides. J. Agric. Food Chem. 2005, 53, 5456–5460. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Du, F.Y.; Chen, X.S.; Zheng, Y.B.; Morton, M.; Liu, F.Q.; Du, L. Identification of the biosynthetic gene cluster for the anti-MRSA lysocins through gene cluster activation using strong promoters of housekeeping genes and production of new analogs in Lysobacter sp. 3655. ACS Synth. Biol. 2020, 9, 1989–1997. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Jiang, L.; Zheng, Y.; Yu, L.; Du, L. Production of the siderophore lysochelin in rich media through maltose-promoted high-density growth of Lysobacter sp. 3655. Front. Microbiol. 2024, 15, 1433983. [Google Scholar] [CrossRef]
- Xu, K.W.; Wang, L.M.; Xiong, D.; Chen, H.J.; Tong, X.R.; Shao, X.L.; Li, T.; Qian, G.L. The Wsp chemosensory system modulates c-di-GMP-dependent biofilm formation by integrating DSF quorum sensing through the WspR-RpfG complex in Lysobacter. Npj Biofilms Microbiomes 2022, 8, 97. [Google Scholar] [CrossRef]
- Niu, C.; Gilbert, E.S. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl. Environ. Microb. 2004, 70, 6951–6956. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.Y.; Reedy, R.M.; Palumbo, J.D.; Zhou, J.M.; Yuen, G.Y. A clp gene homologue belonging to the Crp gene family globally regulates lytic enzyme production, antimicrobial activity, and biological control activity expressed by Lysobacter enzymogenes strain C3. Appl. Environ. Microb. 2005, 71, 261–269. [Google Scholar] [CrossRef]
- Yue, Y.; Zhong, K.; Mu, Y.; Gao, H. Insight into the antibacterial activity and membrane-damage mechanism of pyrrole-2-carboxylic acid against Listeria monocytogenes. LWT-Food Sci. Technol. 2023, 184, 114999. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.J.; Li, F.Q.; Du, L. Spermidine-Regulated Biosynthesis of Heat-Stable Antifungal Factor (HSAF) in Lysobacter enzymogenes OH11. Front. Microbiol. 2018, 9, 2984. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Zhang, J.; Zhao, Y.; Shen, Y.; Su, Z.; Xu, G.; Du, L.; Huffman, J.M.; Venturi, V.; et al. Transcriptomic analysis reveals new regulatory roles of Clp signaling in secondary metabolite biosynthesis and surface motility in Lysobacter enzymogenes OH11. Appl. Microbiol. Biotechnol. 2014, 98, 9009–9020. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Wang, H.K.; Xu, S.D.; Liu, K.; Qi, H.; Wang, M.C.; Chen, X.Y.L.; Berg, G.; Ma, Z.H.; Cernava, T.; et al. Bacterial-fungal interactions under agricultural settings: From physical to chemical interactions. Stress. Biol. 2022, 2, 22. [Google Scholar] [CrossRef]
- Kobayashi, D.Y.; Crouch, J.A. Bacterial/Fungal Interactions: From Pathogens to Mutualistic Endosymbionts. Annu. Rev. Phytopathol. 2009, 47, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Li, Z.M.; Huang, C.Y.; Yang, H.J. Self-digestive solution of Lysobacter enzymogenes LE16 as a biofungicide to control plant powdery mildew. Arch. Agron. Soil. Sci. 2023, 69, 2898–2910. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Wang, T.; Pu, X.J.; Su, Y.L.; Shi, T.T.; Zhao, P.J.; Yang, Z.X.; Li, G.H. Metabolites from Lysobacter gummosus YMF3.00690 against Meloidogyne javanica. Phytopathology 2024, 114, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.H.; Naing, K.W.; Lee, Y.S.; Kim, Y.H.; Moon, J.H.; Kim, K.Y. Antagonism of antifungal metabolites from. J. Basic. Microb. 2015, 55, 45–53. [Google Scholar] [CrossRef]
- Yue, Y.X.; Chen, C.; Zhong, K.; Wu, Y.P.; Gao, H. Purification, fermentation optimization, and antibacterial activity of pyrrole-2-carboxylic acid produced by an endophytic bacterium, ZBE, Isolated from. Ind. Eng. Chem. Res. 2022, 61, 1267–1276. [Google Scholar] [CrossRef]
- He, H.; Hao, X.; Zhou, W.; Shi, N.; Feng, J.; Han, L. Identification of antimicrobial metabolites produced by a potential biocontrol Actinomycete strain A217. J. Appl. Microbiol. 2020, 128, 1143–1152. [Google Scholar] [CrossRef]
- Yue, Y.X.; Zhong, K.; Wu, Y.P.; Gao, H. Pyrrole-2-carboxylic acid inhibits biofilm formation and suppresses the virulence of Listeria monocytogenes. Biofouling 2023, 39, 527–536. [Google Scholar] [CrossRef]
- Hassan, R.; Shaaban, M.I.; Bar, F.M.A.; El-Mahdy, A.M.; Shokralla, S. Quorum sensing inhibiting activity of Streptomyces coelicoflavus isolated from soil. Front. Microbiol. 2016, 7, 659. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasekera, V.; Han, Y.; Du, L. Identification of Pyrrole-2-Carboxylic Acid from the Biocontrol Agent Lysobacter Involved in Interactions with Fusarial Fungi. Microorganisms 2025, 13, 1202. https://doi.org/10.3390/microorganisms13061202
Jayasekera V, Han Y, Du L. Identification of Pyrrole-2-Carboxylic Acid from the Biocontrol Agent Lysobacter Involved in Interactions with Fusarial Fungi. Microorganisms. 2025; 13(6):1202. https://doi.org/10.3390/microorganisms13061202
Chicago/Turabian StyleJayasekera, Vishakha, Yong Han, and Liangcheng Du. 2025. "Identification of Pyrrole-2-Carboxylic Acid from the Biocontrol Agent Lysobacter Involved in Interactions with Fusarial Fungi" Microorganisms 13, no. 6: 1202. https://doi.org/10.3390/microorganisms13061202
APA StyleJayasekera, V., Han, Y., & Du, L. (2025). Identification of Pyrrole-2-Carboxylic Acid from the Biocontrol Agent Lysobacter Involved in Interactions with Fusarial Fungi. Microorganisms, 13(6), 1202. https://doi.org/10.3390/microorganisms13061202






