Phycobacteria Biodiversity, Selected Isolation, and Bioactivity Elucidation of New Bacterial Species of Highly Toxic Marine Dinoflagellate Alexandrium minutum amtk4
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Strains and Culture
2.2. 16S rRNA Pyrosequencing Analysis
2.3. Isolation of Cultivable Phycobacteria Using the CECS Procedure
2.4. Phylogenetic Analysis of the 16S rRNA Gene of Strain ABI-6-9
2.5. Phylogenomic Analysis by ANI, AAI, and dDDH Calculations
2.6. Bacterial Growth Measurement
2.7. Characterization of the Monosaccharides of Bacterial EPSs
2.8. Evaluation of Bioflocculation and MGP Bioactivity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the PM Compositions of Six Alexandrium spp.
3.2. Analysis of Bacterial Associations in the PMs of Six Alexandrium spp.
3.3. CECS-Based Selected Isolation of Cultivable Phycobacterial Strains
3.4. Phylogenetic Analysis of Strain ABI-6-9
3.5. Phylogenomic Characterization of Strain ABI-6-9
3.6. Microalgae Growth-Promoting Potential Analysis of Strain ABI-6-9
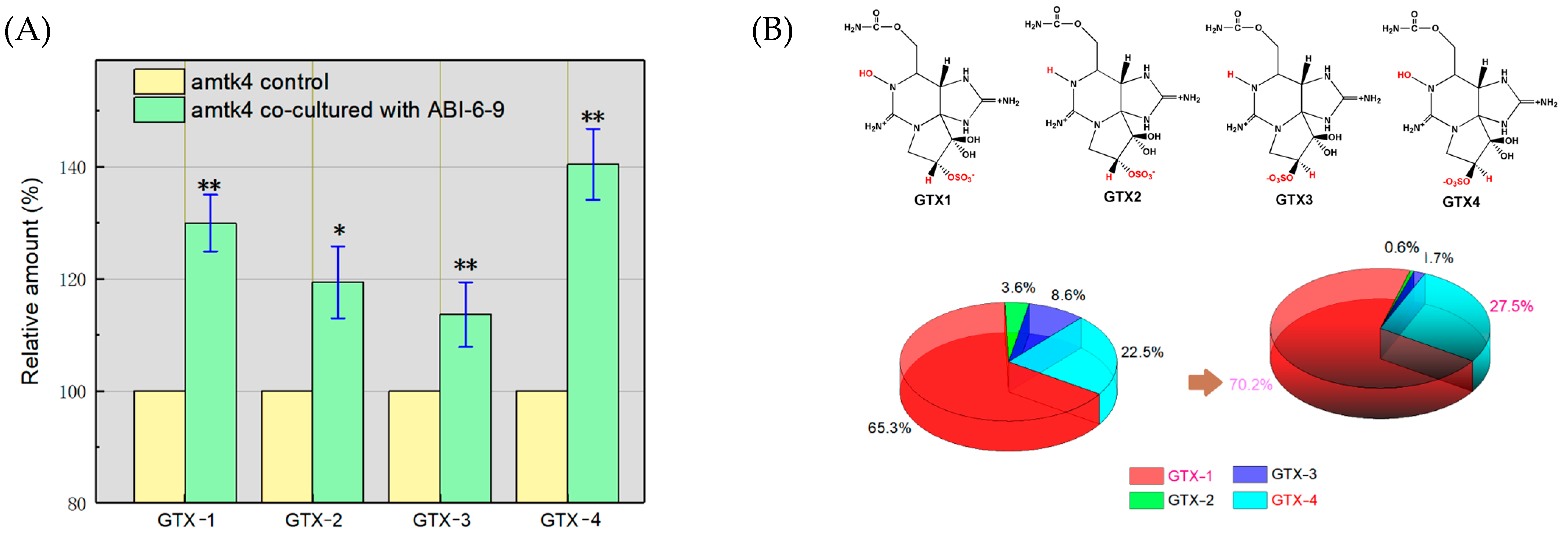
3.7. Promotion of the Accumulation of Algal GTXs by Algal–Bacterial Co-Culture
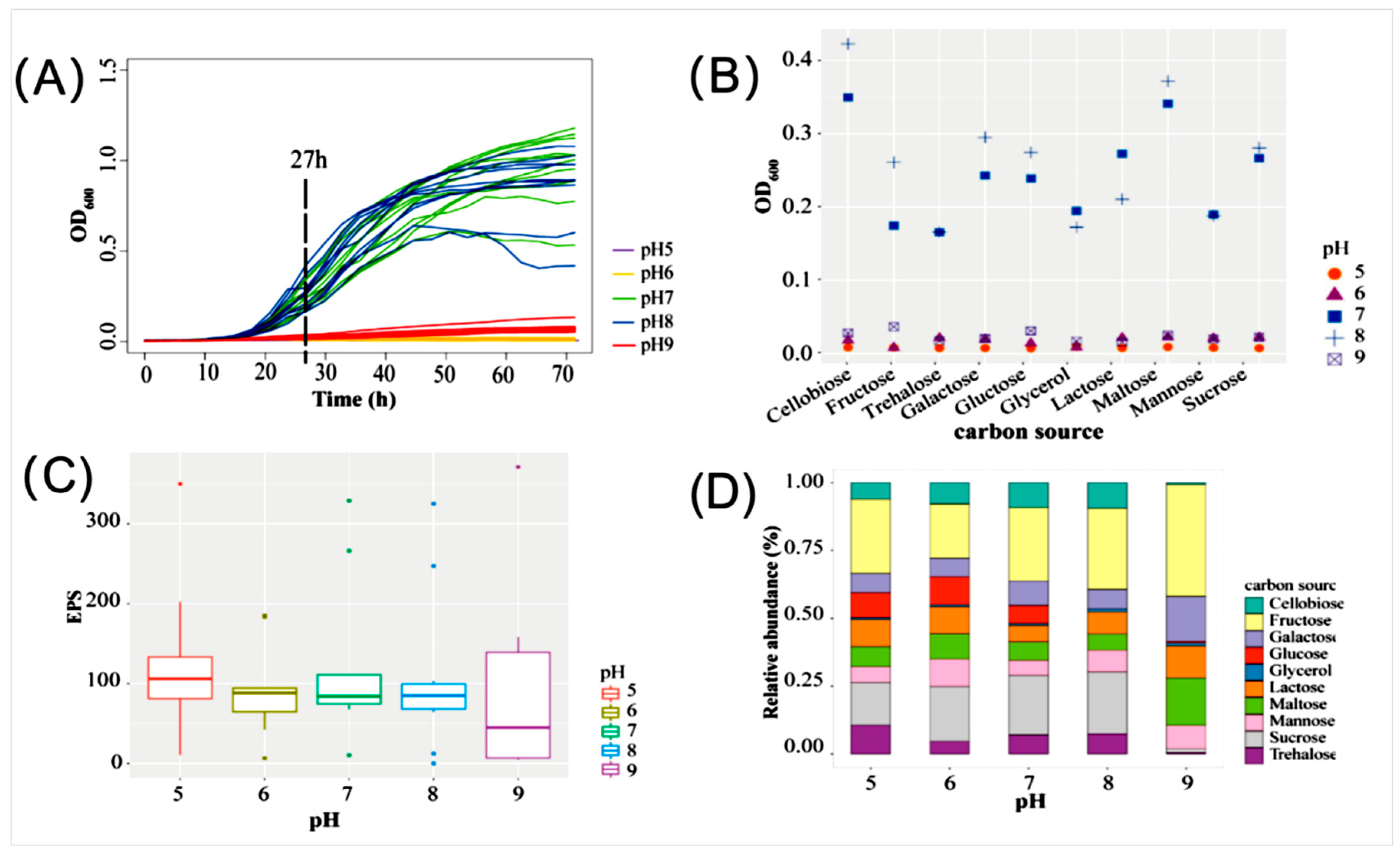
3.8. Culture Optimization of EPS Production by Strain ABI-6-9
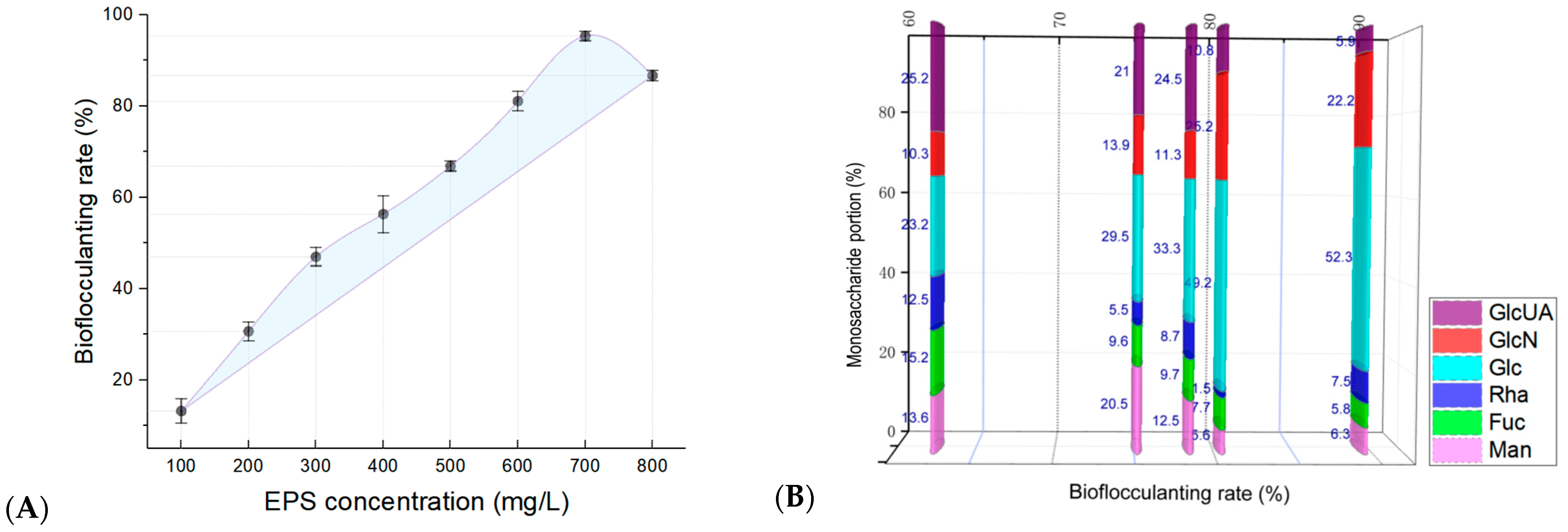
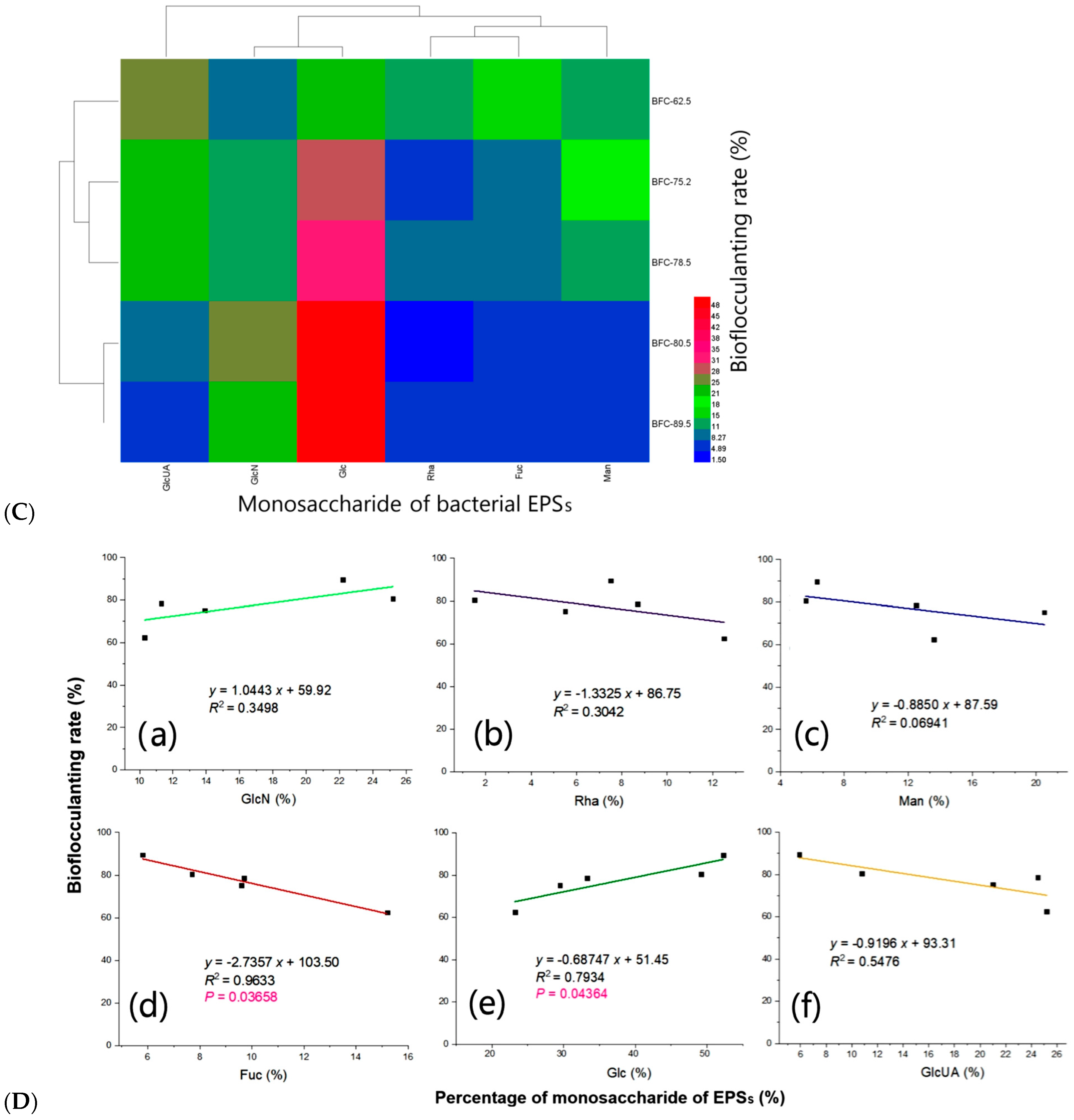
3.9. Bioflocculanting Bioactivity of EPSs Produced by Strain ABI-6-9
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAI | average amino acid Identity |
| ABI | algae-bacteria interactions |
| ANI | average nucleotide identity |
| CECS | combinational enhanced cultivation strategy |
| dDDH | digital DNA-DNA hybridization |
| DCG | designed composite gel |
| ECM | extracellular matrix |
| EPS | exopolysaccharide |
| GTAs | gene transfer agents |
| GTX | gonyautoxin |
| HABs | harmful algal blooms |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| IAA | indole-3-acetic acid |
| MA | marine agar |
| MDM | microbial dark matter |
| MGP | microalgae growth-promoting |
| MGPB | microalgae growth-promoting bacterium |
| OD | optical density |
| OTUs | operational taxonomic units |
| PM | phycosphere microbiota |
| PMP | Phycosphere Microbiome Project |
| PSP | paralytic shellfish poison |
| PSTs | paralytic shellfish poisoning toxins |
| UBCG | up-to-date bacterial core gene set |
References
- Shimizu, Y. Microalgal metabolites. Curr. Opin. Microbiol. 2003, 6, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.R.; Simó, R.; Ahmed, T.; Stocker, R. Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science 2010, 329, 342–345. [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Muylaert, K.; Bertram, M.G.; Brodin, T. Microalgae. Curr. Biol. 2023, 33, R91–R95. [Google Scholar] [CrossRef]
- Scott, G.D. Lichen terminology. Nature 1957, 179, 486–487. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Li, S.N.; Zhang, C.; Li, F.; Ren, N.Q.; Ho, S.H. Recent advances of algae-bacteria consortia in aquatic remediation. Crit. Rev. Environ. Sci. Technol. 2023, 53, 315–339. [Google Scholar] [CrossRef]
- Seymour, J.R.; Amin, S.A.; Raina, J.B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton-bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef]
- Cirri, E.; Pohnert, G. Algae-bacteria interactions that balance the planktonic microbiome. New Phytol. 2019, 223, 100–106. [Google Scholar] [CrossRef]
- Yang, Q.; Ge, Y.M.; Iqbal, N.M.; Yang, X.; Zhang, X.L. Sulfitobacter alexandrii sp. nov.; a new microalgae growth-promoting bacterium with exopolysaccharides bioflocculanting potential isolated from marine phycosphere. Antonie Van Leeuwenhoek 2021, 114, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Landry, Z.C.; Vergin, K.; Mannenbach, C.; Block, S.; Yang, Q.; Blainey, P.; Carlson, C.; Giovannoni, S.J. Optofluidic Single-Cell Genome Amplification of Sub-Micron Bacteria in the Ocean Subsurface. Front. Microbiol. 2018, 9, 1152. [Google Scholar] [CrossRef]
- Ramanan, R.; Kang, Z.; Kim, B.H.; Cho, D.H.; Jin, L.; Oh, H.M.; Kim, H.S. Phycosphere bacterial diversity in green algae reveals an apparent similarity across habitats. Algal Res. 2015, 8, 140–144. [Google Scholar] [CrossRef]
- Chen, Z.; Zakrzewska, S.; Hajare, H.S.; Alvarez-Buylla, A.; Abderemane-Ali, F.; Bogan, M.; Ramirez, D.; O’Connell, L.A.; Du Bois, J.; Minor, D.L., Jr. Definition of a saxitoxin (STX) binding code enables discovery and characterization of the anuran saxiphilin family. Proc. Natl. Acad. Sci. USA 2022, 119, e2210114119. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, D.; Nohynek, L.; Rischer, H. Algae-bacteria association inferred by 16S rDNA similarity in established microalgae cultures. MicrobiologyOpen 2014, 3, 356–368. [Google Scholar] [CrossRef]
- Rinke, C.; Schwientek, P.; Sczyrba, A.; Ivanova, N.N.; Anderson, I.J.; Cheng, J.F.; Darling, A.; Malfatti, S.; Swan, B.K.; Gies, E.A.; et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 2013, 499, 431–437. [Google Scholar] [CrossRef]
- Zhang, X.L.; Tian, X.Q.; Ma, L.Y.; Feng, B.; Liu, Q.H.; Yuan, L.D. Biodiversity of the symbiotic bacteria associated with toxic marine dinoflagellate Alexandrium tamarense. J. Biosci. Med. 2015, 3, 23–28. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Case, R.J.; Kolter, R.; Clardy, J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 2011, 3, 331–335. [Google Scholar] [CrossRef]
- Zhang, X.L.; Ma, L.Y.; Tian, X.Q.; Huang, H.L.; Yang, Q. Biodiversity study of intracellular bacteria closely associated with paralytic shellfish poisoning dinoflagellates Alexandrium tamarense and A. minutum. Int. J. Environ. Resour. 2015, 4, 23–27. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Ge, Y.M.; Dai, J.; Zhang, X.L.; Yang, Q. Alexandriicola marinus gen. nov.; sp. nov.; a new member of the family Rhodobacteraceae isolated from marine phycosphere. Antonie van Leeuwenhoek 2022, 115, 473–486. [Google Scholar] [CrossRef]
- Feng, X.M.; Mo, Y.X.; Han, L.; Nogi, Y.; Zhu, Y.H.; Lv, J. Mameliella sediminis gen. nov.; sp. nov.; a member of the family Erythrobacteraceae isolated from subterrestrial sediment. Int. J. Syst. Evol. Microbiol. 2015, 65, 3658–3665. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, G.X.; Ge, Y.M.; Iqbal, N.M.; Yang, X.; Cui, Z.D.; Yang, Q. Sphingopyxis microcysteis sp. nov.; a novel bioactive exopolysaccharides-bearing Sphingomonadaceae isolated from the Microcystis phycosphere. Antonie Van Leeuwenhoek 2021, 114, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.S. Intracellular bacteria: The origin of dinoflagellate toxicity. J. Environ. Pathol. Toxicol. Oncol. 1990, 10, 124–128. [Google Scholar]
- Ren, C.Z.; Gao, H.M.; Dai, J.; Zhu, W.Z.; Xu, F.F.; Ye, Y.; Zhang, X.L.; Yang, Q. Taxonomic and Bioactivity Characterizations of Mameliella alba Strain LZ-28 Isolated from Highly Toxic Marine Dinoflagellate Alexandrium catenella amtk4. Mar. Drugs 2022, 20, 321. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Ge, Y.; Yang, Q.; Sun, J.; Yu, X. Acute effects of CH3NH3PbI3 perovskite on Scenedesmus obliquus and Daphnia magana in aquatic environment. Ecotoxicol. Environ. Saf. 2021, 208, 111677. [Google Scholar] [CrossRef]
- Singh, R.P.; Reddy, C.R.K. Seaweed-microbial interactions: Key functions of seaweed-associated bacteria. FEMS Microbiol. Ecol. 2014, 88, 213–230. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W.; Yang, W.; Zhang, H.; Qian, X.; Sun, K.; Yang, Q.; Shen, X.; Zhang, L. Autoinducer-2 enhances defence of Vibrio furnissii against oxidative stress and DNA damage by modulation of c-di-GMP signaling via a two-component system. mBio 2025, 16, 02922–02924. [Google Scholar] [CrossRef]
- Li, S.; Liu, Q.; Duan, C.; Li, J.; Sun, H.; Xu, L.; Yang, Q.; Wang, Y.; Shen, X.; Zhang, L. c-di-GMP inhibits the DNA binding activity of H-NS in Salmonella. Nat. Commun. 2023, 14, 7502. [Google Scholar] [CrossRef]
- Sokolovskaya, O.M.; Shelton, A.N.; Taga, M.E. Sharing vitamins: Cobamides unveil microbial interactions. Science 2020, 369, eaba0165. [Google Scholar] [CrossRef]
- Thume, K.; Gebser, B.; Chen, L.; Meyer, N.; Kieber, D.J.; Pohnert, G. The metabolite dimethylsulfoxonium propionate extends the marine organosulfur cycle. Nature 2018, 563, 412–415. [Google Scholar] [CrossRef]
- Gao, H.M.; Xie, P.F.; Zhang, X.L.; Yang, Q. Isolation, Phylogenetic and Gephyromycin Metabolites Characterization of New Exopolysaccharides-Bearing Antarctic Actinobacterium from Feces of Emperor Penguin. Mar. Drugs 2021, 19, 458. [Google Scholar] [CrossRef] [PubMed]
- Tschitschko, B.; Esti, M.; Philippi, M.; Kidane, A.T.; Littmann, S.; Kitzinger, K.; Speth, D.R.; Li, S.; Kraberg, A.; Tienken, D.; et al. Rhizobia-diatom symbiosis fixes missing nitrogen in the ocean. Nature 2024, 630, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Magdouli, S.; Brar, S.K.; Blais, J.F. Co-culture for lipid production: Advances and challenges. Biomass Bioenerg. 2016, 92, 20–30. [Google Scholar] [CrossRef]
- Yang, Q.; Feng, Q.; Zhang, B.P.; Gao, J.J.; Sheng, Z.; Xue, Q.P.; Zhang, X.L. Marinobacter alexandrii sp. nov.; a novel yellow-pigmented and algae growth-promoting bacterium isolated from marine phycosphere microbiota. Antonie Van Leeuwenhoek 2021, 114, 709–718. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Z.; Zhou, X.; Zhang, R.; Xie, Z.; Zhang, S.; Wu, Y.; Ge, Y.; Zhang, X. Haliea alexandrii sp. nov.; isolated from phycosphere microbiota of the toxin-producing dinoflagellate Alexandrium catenella. Int. J. Syst. Evol. Microbiol. 2020, 70, 1133–1138. [Google Scholar] [CrossRef]
- Duan, Y.; Jiang, Z.; Wu, Z.; Sheng, Z.; Yang, X.; Sun, J.; Zhang, X.; Yang, Q.; Yu, X.; Yan, J. Limnobacter alexandrii sp. nov.; a thiosulfate-oxidizing, heterotrophic and EPS-bearing Burkholderiaceae isolated from cultivable phycosphere microbiota of toxic Alexandrium catenella amtk4. Antonie Van Leeuwenhoek 2020, 13, 1689–1698. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Q.; Luo, X.; Fang, C.; Zhang, Q.; Tang, Y. Knockout of crtB or crtI gene blocks the carotenoid biosynthetic pathway in Deinococcus radiodurans R1 and influences its resistance to oxidative DNA-damaging agents due to change of free radicals scavenging ability. Arch. Microbiol. 2007, 188, 411–419. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Z.; Zhou, X.; Zhang, R.; Wu, Y.; Lou, L.; Ma, Z.; Wang, D.; Ge, Y.; Zhang, X.; et al. Nioella ostreopsis sp. nov.; isolated from toxic dinoflagellate, Ostreopsis lenticularis. Int. J. Syst. Evol. Microbiol. 2020, 70, 759–765. [Google Scholar] [CrossRef]
- Siddharth, T.; Sridhar, P.; Vinila, V.; Tyagi, R.D. Environmental applications of microbial extracellular polymeric substance (EPS): A review. J. Environ. Manag. 2021, 287, 112307. [Google Scholar] [CrossRef]
- Zhang, X.L.; Qi, M.; Li, Q.H.; Cui, Z.D.; Yang, Q. Maricaulis alexandrii sp. nov.; a novel active bioflocculants-bearing and dimorphic prosthecate bacterium isolated from marine phycosphere. Antonie Van Leeuwenhoek 2021, 114, 1195–1203. [Google Scholar] [CrossRef]
- Wang, X.; Ye, Y.; Xu, F.; Duan, Y.; Xie, P.; Yang, Q.; Zhang, X. Maritimibacter alexandrii sp. nov., a New Member of Rhodobacteraceae Isolated from Marine Phycosphere. Curr. Microbiol. 2021, 78, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, Z.; Zhou, X.; Xie, Z.; Wang, Y.; Wang, D.; Feng, L.; Yang, G.; Ge, Y.; Zhang, X. Saccharospirillum alexandrii sp. nov.; isolated from the toxigenic marine dinoflagellate Alexandrium catenella amtk4. Int. J. Syst. Evol. Microbiol. 2020, 70, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Boysen, A.K.; Carlson, L.T.; Groussman, R.D.; Heal, K.R.; Cain, K.R.; Morales, R.L.; Coesel, S.N.; Morris, R.M.; Ingalls, A.E.; et al. Sulfonate-based networks between eukaryotic phytoplankton and heterotrophic bacteria in the surface ocean. Nat. Microbiol. 2019, 4, 1706–1715. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, R.; Iqbal, M.N.; Duan, Y.; Zhang, X.; Wang, L.; Yu, L.; Li, J.; Sun, M.; Yang, Q.; et al. Marinobacter shengliensis subsp. alexandrii subsp. nov., isolated from cultivable phycosphere microbiota of highly toxic dinoflagellate Alexandrium catenella LZT09 and description of Marinobacter shengliensis subsp. shengliensis subsp. nov. Curr. Microbiol. 2021, 78, 1648–1655. [Google Scholar]
- Yang, X.; Jiang, Z.W.; Zhang, J.; Zhou, X.; Zhang, X.L.; Wang, L.; Yu, T.; Wang, Z.; Bei, J.; Dong, B. Mesorhizobium alexandrii sp. nov.; isolated from phycosphere microbiota of PSTs-producing marine dinoflagellate Alexandrium minutum amtk4. Antonie Van Leeuwenhoek 2020, 113, 907–917. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Z.W.; Huang, C.H.; Zhang, R.N.; Li, L.Z.; Yang, G.; Feng, L.J.; Yang, G.F.; Zhang, H.; Zhang, X.L. Hoeflea prorocentri sp. nov.; isolated from a culture of the marine dinoflagellate Prorocentrum mexicanum PM01. Antonie Van Leeuwenhoek 2018, 111, 1845–1853. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, B.; Yang, Y.; Yi, X.; Wei, M.; Ecklu-Mensah, G.; Buschmann, M.M.; Liu, H.; Gao, J.; Liang, W.; et al. Multi-omics analyses of airway host-microbe interactions in chronic obstructive pulmonary disease identify potential therapeutic interventions. Nat. Microbiol. 2022, 7, 1361–1375. [Google Scholar] [CrossRef]
- Guérin, H.; Kulakauskas, S.; Chapot-Chartier, M.P. Structural variations and roles of rhamnose-rich cell wall polysaccharides in Gram-positive bacteria. J. Biol. Chem. 2022, 298, 102488. [Google Scholar] [CrossRef]
- Qin, L.; Liu, L.; Wang, Z.; Chen, W.; Wei, D. The mixed culture of microalgae Chlorella pyrenoidosa and yeast Yarrowia lipolytica for microbial biomass production. Bioprocess Biosyst. Eng. 2019, 42, 1409–1419. [Google Scholar] [CrossRef]
- Nakayama, T.; Nomura, M.; Takano, Y.; Tanifuji, G.; Shiba, K.; Inaba, K.; Inagaki, Y.; Kawata, M. Single-cell genomics unveiled a cryptic cyanobacterial lineage with a worldwide distribution hidden by a dinoflagellate host. Proc. Natl. Acad. Sci. USA 2019, 116, 15973–15978. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yang, X.; Zheng, T.; Hong, H. An efficient method to obtain axenic cultures of Alexandrium tamarense—a PSP-producing dinoflagellate. J. Microbiol Methods 2007, 69, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Hambleton, E.A.; DeNofrio, J.C.; Pringle, J.R.; Grossman, A.R. Isolation of clonal axenic strains of the symbiotic dinoflagellate Symbiodinium and their growth and host specificity. J. Phycol. 2013, 49, 447–458. [Google Scholar] [CrossRef]
- Ge, Y.; Xue, Y.; Yang, Q.; Xing, W.; Zhu, S.; Jiang, W.; Liu, J. Benzalkonium chloride disinfection increases the difficulty of controlling foodborne pathogens identified in aquatic product processing. J. Hazard. Mater. 2025, 488, 137140. [Google Scholar] [CrossRef]
- Pennisi, E. SYMBIOSIS. A lichen ménage à trois. Science 2016, 353, 337. [Google Scholar] [CrossRef]
- Jiang, Z.; Duan, Y.; Yang, X.; Yao, B.; Zeng, T.; Wang, X.; Feng, Q.; Qi, M.; Yang, Q.; Zhang, X.L. Nitratireductor alexandrii sp. nov.; from phycosphere microbiota of toxic marine dinoflagellate Alexandrium tamarense. Int. J. Syst. Evol. Microbiol. 2020, 70, 4390–4397. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Dai, J.; Zhang, L.; Feng, L.; Tian, X.; Yang, Q. Taxonomic, Phylogenomic and Bioactivity Profiling of Novel Phycosphere Bacterium from Model Cyanobacterium Synechococcus elongatus PCC 7942. Mar. Drugs. 2024, 22, 36. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yang, Q. Phycosphere Microbiology: An emerging interdiscipline comprehensively promoting sustainable development. Acta Microbiol. Sin. 2025, 65, 1831–1848. [Google Scholar]
- Zhou, J.; Lyu, Y.; Richlen, M.L.; Anderson, D.M.; Cai, Z. Quorum sensing is a language of chemical signals and plays an ecological role in algal-bacterial interactions. Crit. Rev. Plant Sci. 2016, 35, 81–105. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Bao, X.; Lu, H.; Zhang, W.; Wu, W.; Miao, H.; Jiao, B. Single cell determination of nitric oxide release using capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. A 2008, 1201, 120–127. [Google Scholar] [CrossRef]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef]
- Zipfel, C.; Oldroyd, G. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef]
- Segev, E.; Wyche, T.P.; Kim, K.H.; Petersen, J.; Ellebrandt, C.; Vlamakis, H.; Barteneva, N.; Paulson, J.N.; Chai, L.; Clardy, J.; et al. Dynamic metabolic exchange governs a marine algal-bacterial interaction. eLife 2016, 5, e17473. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Hansen, M.F.; Burmølle, M.; Heyndrickx, M.; Flint, S.; Lu, W.; Chen, W.; Zhang, H. Trans-kingdom interactions in mixed biofilm communities. FEMS Microbiol. Rev. 2022, 46, fuac024. [Google Scholar] [CrossRef]
- Moran, M.A.; Belas, R.; Schell, M.A.; González, J.M.; Sun, F.; Sun, S.; Binder, B.J.; Edmonds, J.; Ye, W.; Orcutt, B.; et al. Ecological genomics of marine Roseobacters. Appl. Environ. Microbiol. 2007, 3, 4559–4569. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, C.; Yan, X.; Wang, Y.; Zeng, Y.; Hao, L.; He, W.; Jiao, N. Mameliella alba gen. nov., sp. nov., a marine bacterium of the Roseobacter clade in the order Rhodobacterales. Int. J. Syst. Evol. Microbiol. 2010, 60, 953–957. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Martin, J.L.; Giewat, M.W.; Rooney-Varga, J.N. Microbial community diversity in the phycosphere of natural populations of the toxic alga, Alexandrium fundyense. Environ. Microbiol. 2007, 9, 3108–3121. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Martin, F.M.; Uroz, S.; Barker, D.G. Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science 2017, 356, eaad4501. [Google Scholar] [CrossRef]
- Kuhlisch, C.; Shemi, A.; Barak-Gavish, N.; Schatz, D.; Vardi, A. Algal blooms in the ocean: Hot spots for chemically mediated microbial interactions. Nat. Rev. Microbiol. 2024, 22, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, P.; Dai, J.; Zhang, L.; Yang, Q.; Zhang, X.; Yang, X. Variation in Structure and Functional Diversity of Surface Bacterioplankton Communities in the Eastern East China Sea. J. Mar. Sci. Eng. 2024, 12, 69. [Google Scholar] [CrossRef]
- Kiers, E.T.; West, S.A. Evolutionary biology. Evolving new organisms via symbiosis. Science 2015, 348, 392–394. [Google Scholar] [CrossRef]
- Fu, H.; Uchimiya, M.; Gore, J.; Moran, M.A. Ecological drivers of bacterial community assembly in synthetic phycospheres. Proc. Natl. Acad. Sci. USA 2020, 117, 3656–3662. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Pan, Z.; Zhang, J.; Liu, B.; Yang, Q. Phycobacteria Biodiversity, Selected Isolation, and Bioactivity Elucidation of New Bacterial Species of Highly Toxic Marine Dinoflagellate Alexandrium minutum amtk4. Microorganisms 2025, 13, 1198. https://doi.org/10.3390/microorganisms13061198
Zhang X, Pan Z, Zhang J, Liu B, Yang Q. Phycobacteria Biodiversity, Selected Isolation, and Bioactivity Elucidation of New Bacterial Species of Highly Toxic Marine Dinoflagellate Alexandrium minutum amtk4. Microorganisms. 2025; 13(6):1198. https://doi.org/10.3390/microorganisms13061198
Chicago/Turabian StyleZhang, Xiaoling, Zekang Pan, Jinkai Zhang, Bingqian Liu, and Qiao Yang. 2025. "Phycobacteria Biodiversity, Selected Isolation, and Bioactivity Elucidation of New Bacterial Species of Highly Toxic Marine Dinoflagellate Alexandrium minutum amtk4" Microorganisms 13, no. 6: 1198. https://doi.org/10.3390/microorganisms13061198
APA StyleZhang, X., Pan, Z., Zhang, J., Liu, B., & Yang, Q. (2025). Phycobacteria Biodiversity, Selected Isolation, and Bioactivity Elucidation of New Bacterial Species of Highly Toxic Marine Dinoflagellate Alexandrium minutum amtk4. Microorganisms, 13(6), 1198. https://doi.org/10.3390/microorganisms13061198





