Genetic Diversity in Phytoplasmas from X-Disease Group Based in Analysis of idpA and imp Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Identification of imp and idpA Homologues
2.3. PCR Amplification of imp and idpA Genes
2.4. Cloning and Sequencing of imp and idpA Genes
2.5. Genetic Diversity
2.6. Phylogenetic Analysis of X-Disease Phytoplasmas
3. Results
3.1. Identification of imp and idpA ORFs in X-Disease Genomes
3.2. PCR Amplifications and Sequencing
3.3. Sequences Homology and Predicted Protein Structure
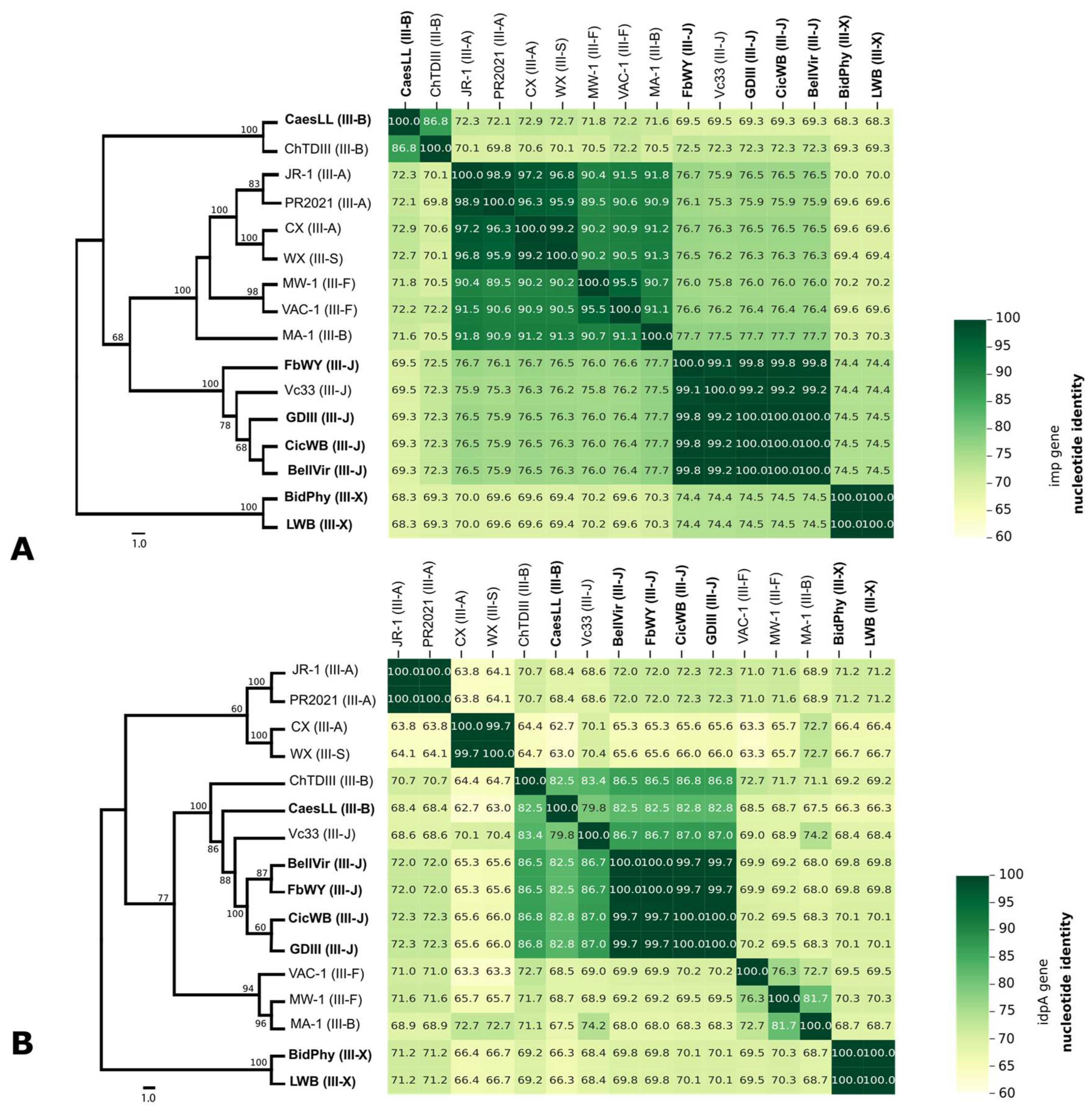
3.4. Genetic Diversity
3.5. Phylogeny Based on 16S rRNA, idpA, and imp Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maejima, K.; Oshima, K.; Namba, S. Exploring the phytoplasmas, plant pathogenic bacteria. J. Gen. Plant Pathol. 2014, 80, 210–221. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, Y. Phytoplasma Taxonomy: Nomenclature, Classification, and Identification. Biology 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Arocha-Rosete, Y.; Contaldo, N.; Duduk, B.; Fiore, N.; Montano, H.G.; Kube, M.; Kuo, C.H.; Martini, M.; Oshima, K.; et al. Revision of the ‘Candidatus Phytoplasma’ species description guidelines. Int. J. Syst. Bacteriol. 2022, 72, 005353. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, D.E.; Lee, I.M.; Schaff, D.A.; Harrison, N.A.; Chang, C.J.; Davis, R.E.; Kingsbury, D.T. Genomic diversity and differentiation among phytoplasma strains in 16S rRNA groups I (aster yellows and related phytoplasmas) and III (X-disease and related phytoplasmas). Int. J. Syst. Evol. Microbiol. 1996, 46, 64–75. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, W.; Lee, I.M.; Shao, J.; Suo, X.; Davis, R.E. Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII). Int. J. Syst. Evol. Microbiol. 2009, 59, 2582–2593. [Google Scholar] [CrossRef]
- Liefting, L.W.; Andersen, M.T.; Beever, R.E.; Gardner, R.C.; Forster, R.L. Sequence heterogeneity in the two 16S rRNA genes of Phormium yellow leaf phytoplasma. Appl. Environ. Microbiol. 1996, 62, 3133–3139. [Google Scholar] [CrossRef]
- Zhao, Y.; Davis, R.E. Criteria for phytoplasma 16Sr group/subgroup delineation and the need of a platform for proper registration of new groups and subgroups. Int. J. Syst. Evol. Microbiol. 2016, 66, 2121–2123. [Google Scholar] [CrossRef]
- Cho, S.T.; Kung, H.J.; Huang, W.; Hogenhout, S.A.; Kuo, C.H. Species boundaries and molecular markers for the classification of 16SrI phytoplasmas inferred by genome analysis. Front. Microbiol. 2020, 11, 1531. [Google Scholar] [CrossRef]
- Arnaud, G.; Malembic-Maher, S.; Salar, P.; Bonnet, P.; Maixner, M.; Marcone, C.; Boudon-Padieu, E.; Foissac, X. Multilocus sequence typing confirms the close genetic interrelatedness of three distinct flavescence dorée phytoplasma strain clusters and group 16SrV phytoplasmas infecting grapevine and alder in Europe. Appl. Environ. Microbiol. 2007, 73, 4001–4010. [Google Scholar] [CrossRef]
- Davis, R.E.; Zhao, Y.; Dally, E.L.; Lee, I.M.; Jomantiene, R.; Douglas, S.M. ‘Candidatus Phytoplasma pruni’, a novel taxon associated with X-disease of stone fruits, Prunus spp.: Multilocus characterization based on 16S rRNA, secY, and ribosomal protein genes. Int. J. Syst. Evol. Microbiol. 2013, 63, 766–776. [Google Scholar] [CrossRef]
- Abeysinghe, S.; Abeysinghe, P.D.; Kanatiwela-de Silva, C.; Udagama, P.; Warawichanee, K.; Aljafar, N.; Dickinson, M. Refinement of the taxonomic structure of 16SrXI and 16SrXIV phytoplasmas of gramineous plants using multilocus sequence typing. Plant Dis. 2016, 100, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, S.; Kamagata, Y. A multiplex-PCR method for strain identification and detailed phylogenetic analysis of AY-group phytoplasmas. Plant Dis. 2014, 98, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.; Ilic, A.M.; Huettel, B.; Duduk, B.; Kube, M. Divergence within the Taxon ‘Candidatus Phytoplasma asteris’ Confirmed by Comparative Genome Analysis of Carrot Strains. Microorganisms 2024, 12, 1016. [Google Scholar] [CrossRef]
- Hugenholtz, P.; Chuvochina, M.; Oren, A.; Parks, D.H.; Soo, R.M. Prokaryotic taxonomy and nomenclature in the age of big sequence data. ISME J. 2021, 15, 1879–1892. [Google Scholar] [CrossRef]
- Riesco, R.; Trujillo, M.E. Update on the Proposed Minimal Standards for the Use of Genome Data for the Taxonomy of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2024, 74, 006300. [Google Scholar] [CrossRef] [PubMed]
- García-López, M.; Meier-Kolthoff, J.P.; Tindall, B.J.; Gronow, S.; Woyke, T.; Kyrpides, N.C.; Göker, M. Analysis of 1,000 Type-Strain Genomes Improves Taxonomic Classification of Bacteroidetes. Front. Microbiol. 2019, 10, 2083. [Google Scholar] [CrossRef]
- Galdeano, E.; Guzmán, F.; Fernández, F.; Conci, L. Genetic diversity of 16SrIII group phytoplasmas in Argentina. Predominance of subgroups 16SrIII-J and B and two new subgroups 16SrIII-W and X. Eur. J. Plant Pathol. 2013, 137, 753–764. [Google Scholar] [CrossRef]
- Perez-Lopez, E.; Luna-Rodríguez, M.; Olivier, C.Y.; Dumonceaux, T.J. The underestimated diversity of phytoplasmas in Latin America. Int. J. Syst. Evol. Microbiol. 2016, 66, 492–513. [Google Scholar] [CrossRef]
- Montano, H.G.; Bertaccini, A.; Fiore, N. Phytoplasma-Associated Diseases in South America: Thirty Years of Research. Microorganisms 2024, 12, 1311. [Google Scholar] [CrossRef]
- Amaral Mello, A.P.O.; Eckstein, B.; Flôres, D.; Kreyci, P.F.; Bedendo, I.P. Identification by computer-simulated RFLP of phytoplasmas associated with eggplant giant calyx representative of two subgroups, a lineage of 16SrIII-J and the new subgroup 16SrIII-U. Int. J. Syst. Evol. Microbiol. 2011, 61, 1454–1461. [Google Scholar] [CrossRef]
- Gonzalez, F.; Zamorano, A.; Pino, A.M.; Paltrinieri, S.; Bertaccini, A.; Fiore, N. Identification of phytoplasma belonging to X-disease group in cherry in Chile. Bull. Insectol. 2011, 64 (Suppl.), S235–S236. [Google Scholar]
- Rappussi, M.C.C.; Eckstein, B.; Flôres, D.; Haas, I.C.R.; Amorim, L.; Bedendo, I.P. Cauliflower stunt associated with a phytoplasma of subgroup 16SrIII-J and the spatial pattern of disease. Eur. J. Plant Pathol. 2012, 133, 829–840. [Google Scholar] [CrossRef]
- Fernández, F.; Uset, A.; Baumgratz, G.; Conci, L. Detection and identification of a 16SrIII-J phytoplasma affecting cassava (Manihot esculenta Crantz) in Argentina. Australas. Plant Dis. Notes 2018, 13, 24. [Google Scholar] [CrossRef]
- Fernández, F.D.; Guzmán, F.A.; Baffoni, P.; Reinoso, L.; Kiehr, M.; Delhey, R.; Conci, L.R. Phytoplasmas of subgroup 16SrIII-J associated with Beta vulgaris in Argentina. Trop. Plant Pathol. 2020, 45, 143–147. [Google Scholar] [CrossRef]
- Arneodo, J.D.; Galdeano, E.; Orrego, A.; Stauffer, A.; Nome, S.F.; Conci, L.R. Identification of two phytoplasmas detected in China-trees with decline symptoms in Paraguay. Australas. Plant Pathol. 2005, 34, 583–585. [Google Scholar] [CrossRef]
- Flôres, D.; Haas, I.C.; Canale, M.C.; Bedendo, I.P. Molecular identification of a 16SrIII-B phytoplasma associated with cassava witches’ broom disease. Eur. J. Plant Pathol. 2013, 137, 237–242. [Google Scholar] [CrossRef]
- Galdeano, E.; Torres, L.E.; Meneguzzi, N.; Guzmán, F.; Gomez, G.G.; Docampo, D.M.; Conci, L.R. Molecular characterization of 16S ribosomal DNA and phylogenetic analysis of two X-disease group phytoplasmas affecting China-tree (Melia azedarach L.) and garlic (Allium sativum L.) in Argentina. J. Phytopathol. 2004, 152, 174–181. [Google Scholar] [CrossRef]
- Fernandez, F.D.; Carloni, E.; Alessio, F.; Bongiorno, V.; Conci, L.R. First report of a 16SrIII-X phytoplasma associated with Lactuca sativa witches’ broom in Argentina. New Dis. Rep. 2022, 46, e12103. [Google Scholar] [CrossRef]
- Alvarez, E.; Mejía, J.F.; Llano, G.; Loke, J.; Calari, A.; Duduk, B.; Bertaccini, A. Characterization of a phytoplasma associated with frogskin disease in cassava. Plant Dis. 2009, 93, 1139–1145. [Google Scholar] [CrossRef]
- Konnerth, A.; Krczal, G.; Boonrod, K. Immunodominant membrane proteins of phytoplasmas. Microbiology 2016, 162, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, S.; Oshima, K.; Jung, H.Y.; Suzuki, S.; Nishigawa, H.; Arashida, R.; Lee, J.T.; Miyata, S.; Ugaki, M.; Namba, S. Positive selection acting on a surface membrane protein of the plant-pathogenic phytoplasmas. J. Bacteriol. 2006, 188, 3424–3428. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, S.; Oshima, K.; Ishii, Y.; Hoshi, A.; Maejima, K.; Jung, H.Y.; Yamaji, Y.; Namba, S. Cloning of immunodominant membrane protein genes of phytoplasmas and their in planta expression. FEMS Microbiol. Lett. 2009, 293, 92–101. [Google Scholar] [CrossRef]
- Galetto, L.; Siampour, M.; Marzachì, C. Preparation of phytoplasma membrane recombinant proteins. Methods Mol. Biol. 2013, 938, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Deng, S.; Hiruki, C. Amplification of 16S rRNA genes from culturable and unculturable Mollicutes. J. Microbiol. Methods 1991, 14, 53–61. [Google Scholar] [CrossRef]
- Lee, I.M.; Hammond, R.W.; Davis, R.E.; Gundersen, D.E. Universal amplification and analysis of pathogen 16S rDNA for classification and identification of MLOs. Phytopathology 1993, 83, 834–842. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000; p. 174. [Google Scholar]
- Pond, S.L.K.; Frost, S.D.W.; Muse, S.V. HyPhy: Hypothesis testing using phylogenies. Bioinformatics 2005, 21, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Villamor, D.E.V.; Eastwell, K.C. Multilocus characterization, gene expression analysis of putative immunodominant protein coding regions, and development of recombinase polymerase amplification assay for detection of ‘Candidatus Phytoplasma pruni’ in Prunus avium. Phytopathology 2019, 109, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Siampour, M.; Izadpanah, K.; Galetto, L.; Salehi, M.; Marzachi, C. Molecular characterization, phylogenetic comparison and serological relationship of the Imp protein of several ‘Candidatus Phytoplasma aurantifolia’ strains. Plant Pathol. 2013, 62, 452–459. [Google Scholar] [CrossRef]
- Blomquist, C.L.; Barbara, D.J.; Davies, D.L.; Clark, M.F.; Kirkpatrick, B.C. An immunodominant membrane protein gene from the Western X-disease phytoplasma is distinct from those of other phytoplasmas. Microbiology 2001, 147, 571–580. [Google Scholar] [CrossRef]
- Neriya, Y.; Sugawara, K.; Maejima, K.; Hashimoto, M.; Komatsu, K.; Minato, N.; Miura, C.; Kakizawa, S.; Yamaji, Y.; Oshima, K.; et al. Cloning, expression analysis, and sequence diversity of genes encoding two different immunodominant membrane proteins in poinsettia branch-inducing phytoplasma (PoiBI). FEMS Microbiol. Lett. 2011, 324, 38–47. [Google Scholar] [CrossRef]
- Martini, M.; Quaglino, F.; Bertaccini, A. Multilocus genetic characterization of phytoplasmas. In Phytoplasmas: Plant Pathogenic Bacteria-III: Genomics, Host Pathogen Interactions and Diagnosis; Bertaccini, A., Oshima, K., Kube, M., Rao, G.P., Eds.; Springer: Singapore, 2019; pp. 161–200. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Wang, X.Y.; Shan, H.L.; Li, J.; Li, Y.H.; Li, W.F.; Huang, Y.K. Multilocus sequence typing reveals two distinct populations of “Candidatus Phytoplasma sacchari” in China. Trop. Plant Pathol. 2023, 48, 199–206. [Google Scholar] [CrossRef]
- Liu, C.Y.; Cheng, H.P.; Lin, C.P.; Liao, Y.T.; Ko, T.P.; Lin, S.J.; Wang, H.C. Structural insights into the molecular mechanism of phytoplasma immunodominant membrane protein. IUCrJ 2024, 11, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Bohunická, M.; Valentová, L.; Suchá, J.; Nečas, T.; Eichmeier, A.; Kiss, T.; Cmejla, R. Identification of 17 ‘Candidatus Phytoplasma pyri’ genotypes based on the diversity of the imp gene sequence. Plant Pathol. 2018, 67, 971–977. [Google Scholar] [CrossRef]
- Pusz-Bochenska, K.; Perez-Lopez, E.; Wist, T.J.; Bennypaul, H.; Sanderson, D.; Green, M.; Dumonceaux, T.J. Multilocus sequence typing of diverse phytoplasmas using hybridization probe-based sequence capture provides high resolution strain differentiation. Front. Microbiol. 2022, 13, 959562. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.D.; Guzmán, F.A.; Conci, L.R. Draft genome sequence of Cicuta witches’ broom phytoplasma, subgroup 16SrIII-J: A subgroup with phytopathological relevance in South America. Trop. Plant Pathol. 2024, 49, 558–565. [Google Scholar] [CrossRef]
- Soto, N.; Helmick, E.E.; Harrison, N.A.; Bahder, B.W. Genetic Variability of Palm Lethal Decline Phytoplasmas in the Caribbean Basin and Florida, USA, Based on a Multilocus Analysis. Phytopathology 2021, 111, 2203–2212. [Google Scholar] [CrossRef]
- Filippin, L.; Trivellone, V.; Galetto, L.; Marzachì, C.; Elicio, V.; Angelini, E. Development of an anti-Imp serological assay for the detection of “flavescence dorée” phytoplasmas in grapevine, insect vectors and host plants. Phytopathogenic Mollicutes 2019, 9, 75–76. [Google Scholar] [CrossRef]
- Tan, C.M.; Lin, Y.C.; Li, J.R.; Chien, Y.Y.; Wang, C.J.; Chou, L.; Yang, J.Y. Accelerating complete phytoplasma genome assembly by immunoprecipitation-based enrichment and MinION-based DNA sequencing for comparative analyses. Front. Microbiol. 2021, 12, 766221. [Google Scholar] [CrossRef]

| Phytoplasma Strain | 16SrIII * | Host | imp PCR (+) | idpA PCR (+) | #Accession (imp/idpA) |
|---|---|---|---|---|---|
| Bellis virescence (BellVir) | III-J | Bellis perennis | 2/2 | 2/2 | MG435348.1/MG435349.1 |
| Garlic Decline (GDIII) | III-J | Allium sativum | 2/2 | 2/2 | PQ429243.1/PQ429237.1 |
| Fodder Beet Wilting-Yellowing (FbWY) | III-J | Beta vulgaris var. rapacea | 2/2 | 2/2 | PQ429242.1/PQ429236.1 |
| Sugar Beet Wilting-Yellowing (SugBeetWY) | III-J | Beta vulgaris var. altissima | 0/3 | 0/3 | - |
| Cicuta Witches Broom (CicWB) | III-J | Conium maculatum | 2/2 | 2/2 | PQ429241.1/PQ429238.1 |
| China tree decline (ChTDIII) | III-B | Melia azedarach | 3/3 | 3/3 | NWN45603.1/NWN45596.1 |
| Caesalpinia little leaf (CaesLL) | III-B | Caesalpinia gilliesii | 2/2 | 2/2 | PQ429239.1/PQ429233.1 |
| Argentinean Peach Yellows (ArPY) | III-B | Prunus persica | 0/3 | 0/3 | - |
| Lettuce Witches’ Broom (LWB) | III-X | Lactuca sativa | 2/2 | 2/2 | PQ871563/PQ429235.1 |
| Bidens Phyllody (BidPhy) | III-X | Bidens subalternans | 1/3 | 1/3 | PQ429240.1/PQ429234.1 |
| Heterosperma Phyllody (HetPhy) | III-X | Heterosperma ovatifolium | 0/3 | 0/3 | - |
| Phytoplasma [Strain] | 16SrIII * | Host | Location | GenBank Accession |
|---|---|---|---|---|
| Ca. Phytoplasma pruni [WX] | III-S | Prunus avium | USA | AF533231.1 |
| Ca. Phytoplasma pruni [CX] | III-A | Prunus domestica | USA | LHCF00000000.1 |
| Ca. Phytoplasma pruni [PR2021] | III-A | Euphorbia pulcherrima | Taiwan | CP119306.1 |
| Poinsettia branch-inducing [JR-1] | III-A | Euphorbia pulcherrima | USA | AKIK00000000.1 |
| Clover Phyllody [MA-1] | III-B | Chrysanthemum leuchantemum | Italy | AKIM00000000.1 |
| Vaccinium Witches’ Broom [VAC-1] | III-F | Vaccinium myrtillus | Italy | AKIN00000000.1 |
| Milkweed Yellows [MW-1] | III-F | Asclepias syriaca | USA | AKIL00000000.1 |
| Ca. Phytoplasma sp. [Vc33] | III-J | Catharanthus roseus | Chile | LLKK00000000.1 |
| Chinaberry tree decline [ChTDIII] | III-B | Melia azedarach | Argentina | JABUOH000000000.1 |
| Dataset | N° | #nt | S | P | dN/dS | p-Value | Normalized dN/dS > 0 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| TM | HD | #Codons | % | |||||||
| imp | 16 | 516 | 258 | 0.18272 | 3474 | 0.01 | 10 | 70 | 157 | 50.955 |
| idpA | 16 | 857 | 268 | 0.10407 | −3090 | 0.002 | 11 | 104 | 253 | 45.454 |
| 16S rRNA | 16 | 1163 | 94 | 0.01354 | NA | NA | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessio, F.I.; Bongiorno, V.A.; Marcone, C.; Conci, L.R.; Fernandez, F.D. Genetic Diversity in Phytoplasmas from X-Disease Group Based in Analysis of idpA and imp Genes. Microorganisms 2025, 13, 1170. https://doi.org/10.3390/microorganisms13051170
Alessio FI, Bongiorno VA, Marcone C, Conci LR, Fernandez FD. Genetic Diversity in Phytoplasmas from X-Disease Group Based in Analysis of idpA and imp Genes. Microorganisms. 2025; 13(5):1170. https://doi.org/10.3390/microorganisms13051170
Chicago/Turabian StyleAlessio, Florencia Ivette, Vanina Aylen Bongiorno, Carmine Marcone, Luis Rogelio Conci, and Franco Daniel Fernandez. 2025. "Genetic Diversity in Phytoplasmas from X-Disease Group Based in Analysis of idpA and imp Genes" Microorganisms 13, no. 5: 1170. https://doi.org/10.3390/microorganisms13051170
APA StyleAlessio, F. I., Bongiorno, V. A., Marcone, C., Conci, L. R., & Fernandez, F. D. (2025). Genetic Diversity in Phytoplasmas from X-Disease Group Based in Analysis of idpA and imp Genes. Microorganisms, 13(5), 1170. https://doi.org/10.3390/microorganisms13051170







